Abstract
1. Synaptic responses of triceps surae motoneurones of the cat following stimulation of single afferent fibres were examined by intracellular recording techniques.
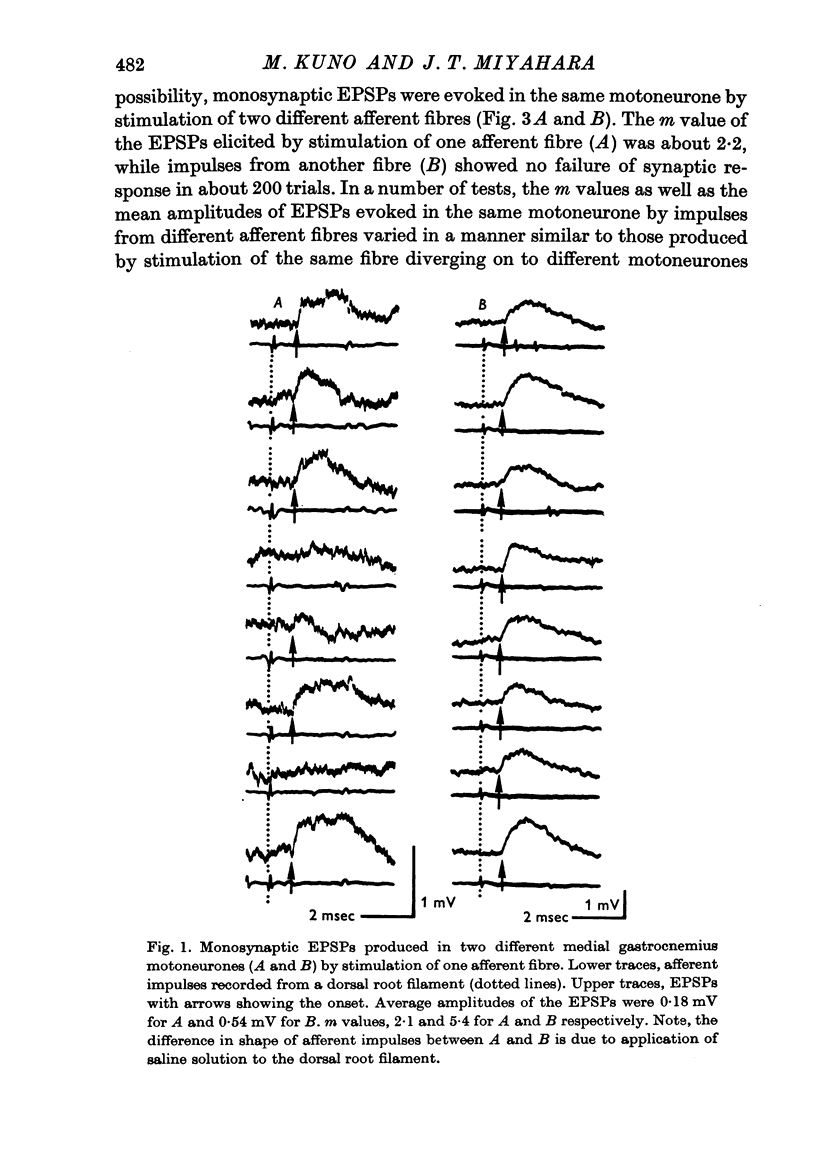
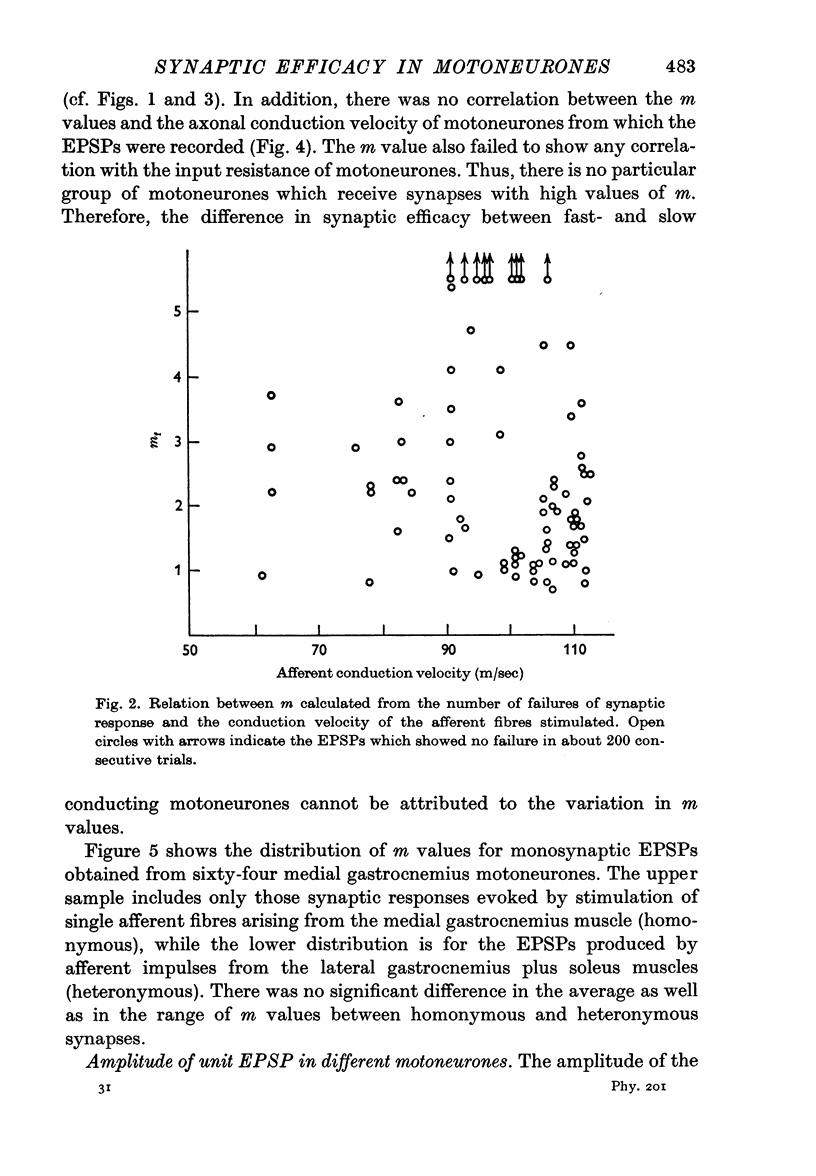
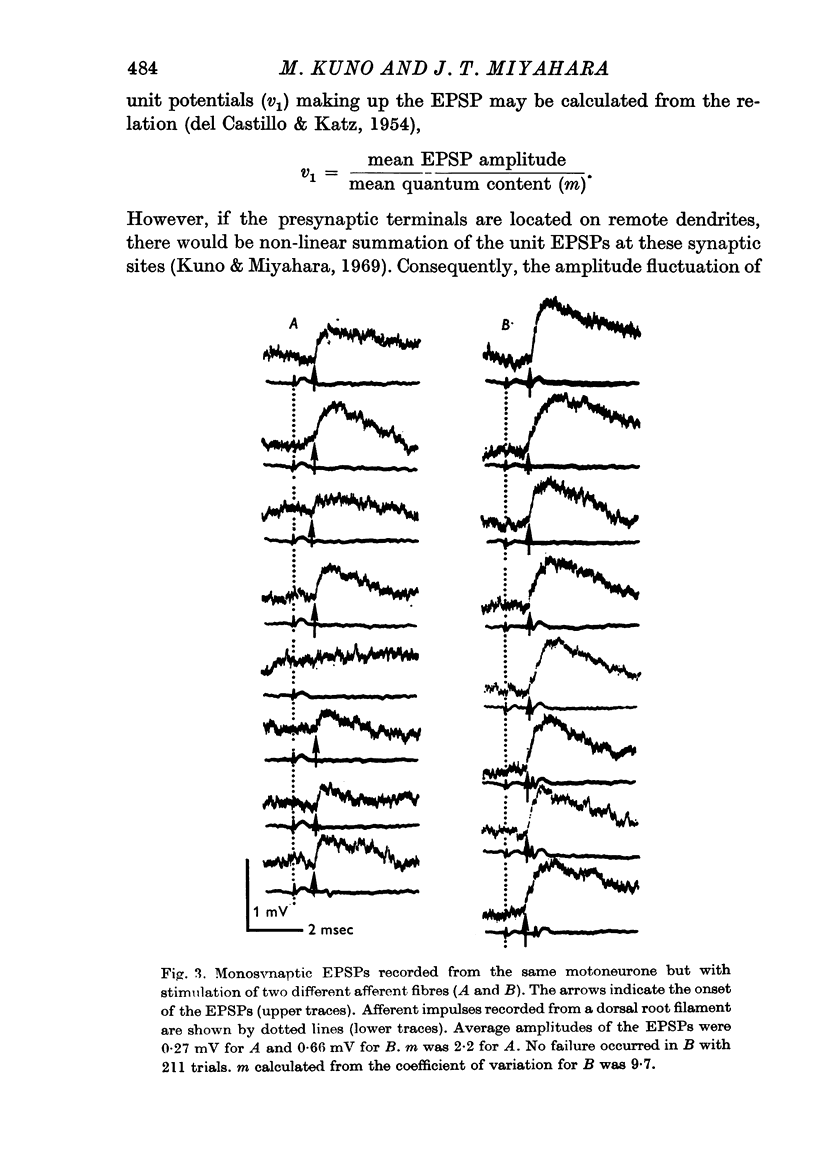
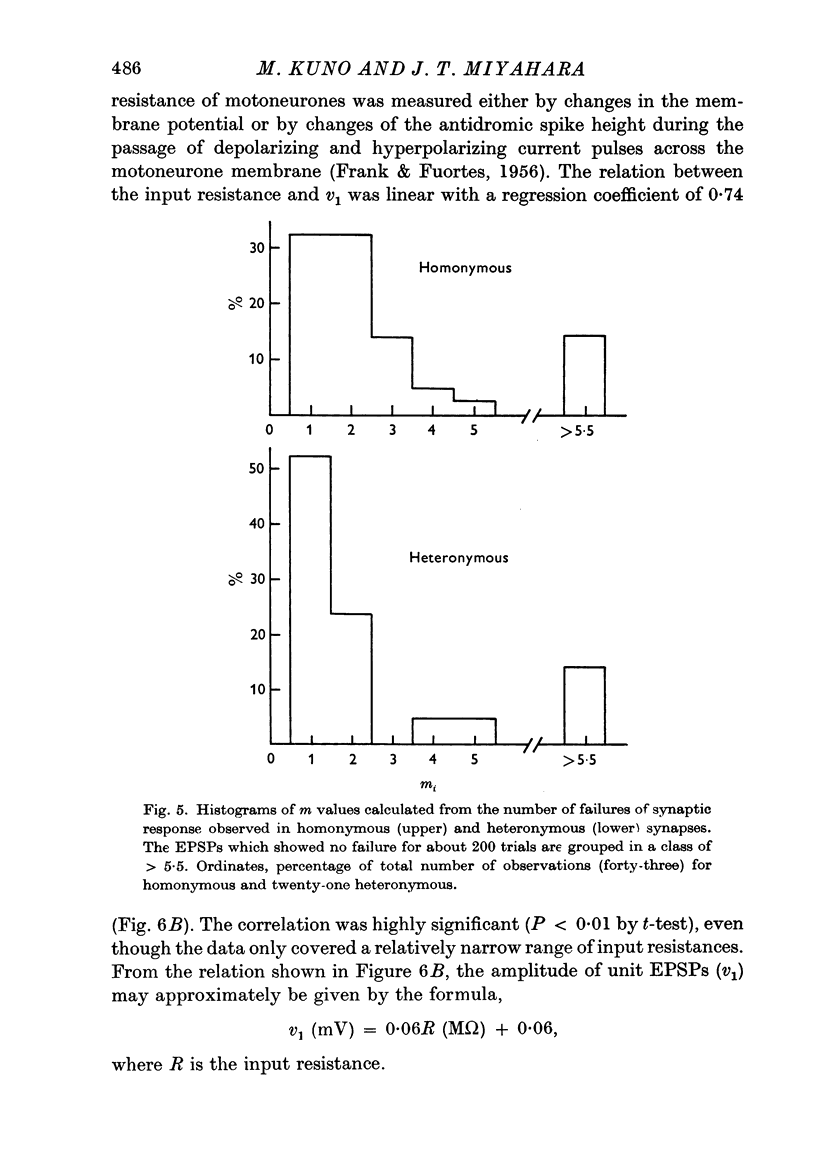
2. The mean quantum content (m) of monosynaptic excitatory postsynaptic potentials (EPSPs) was independent of the type of motoneurone recorded and of the afferent fibre stimulated. There was no significant difference in m value between homonymous and heteronymous synapses.
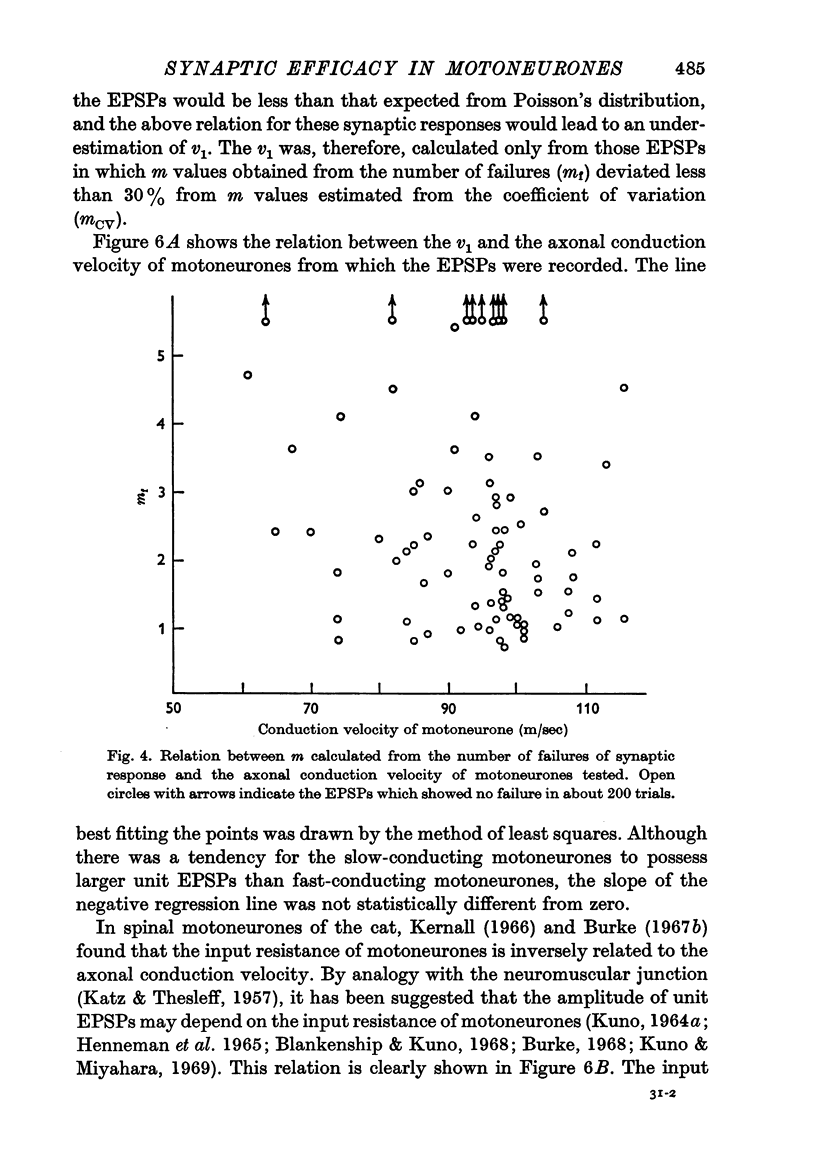
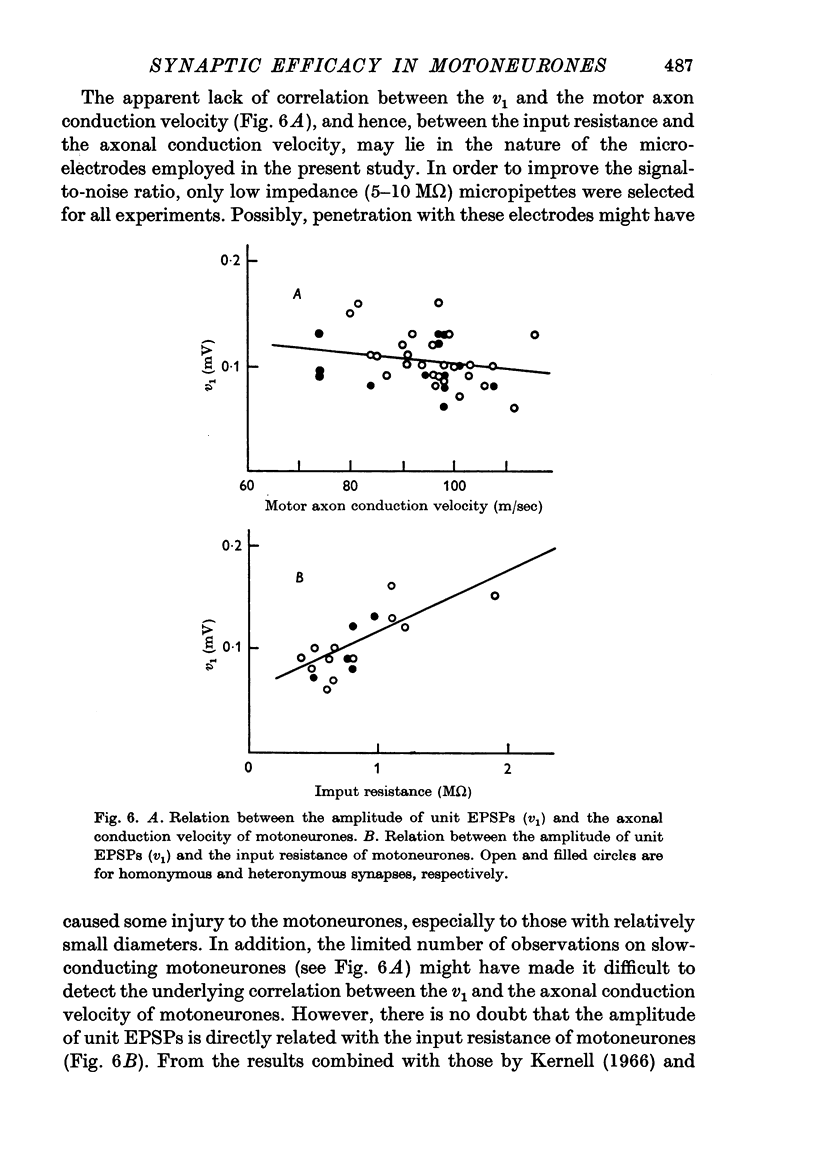
3. A positive correlation was found between the amplitude of unit EPSPs and the input resistance of motoneurones. The difference in the amplitude of unit EPSPs appears to be responsible for the higher synaptic efficacy in slow-conducting motoneurones than in fast-conducting motoneurones.
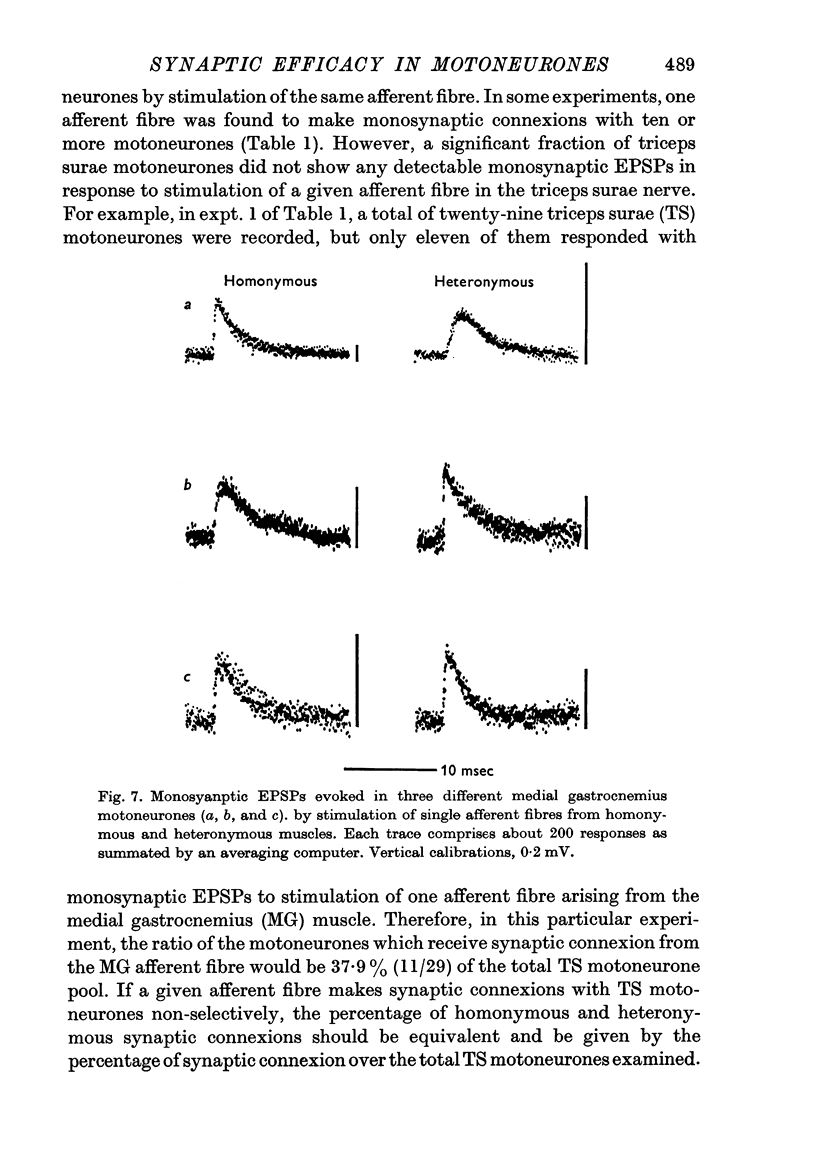
4. There was no significant difference in the time course of monosynaptic EPSPs evoked by impulses from homonymous and heteronymous afferent fibres.
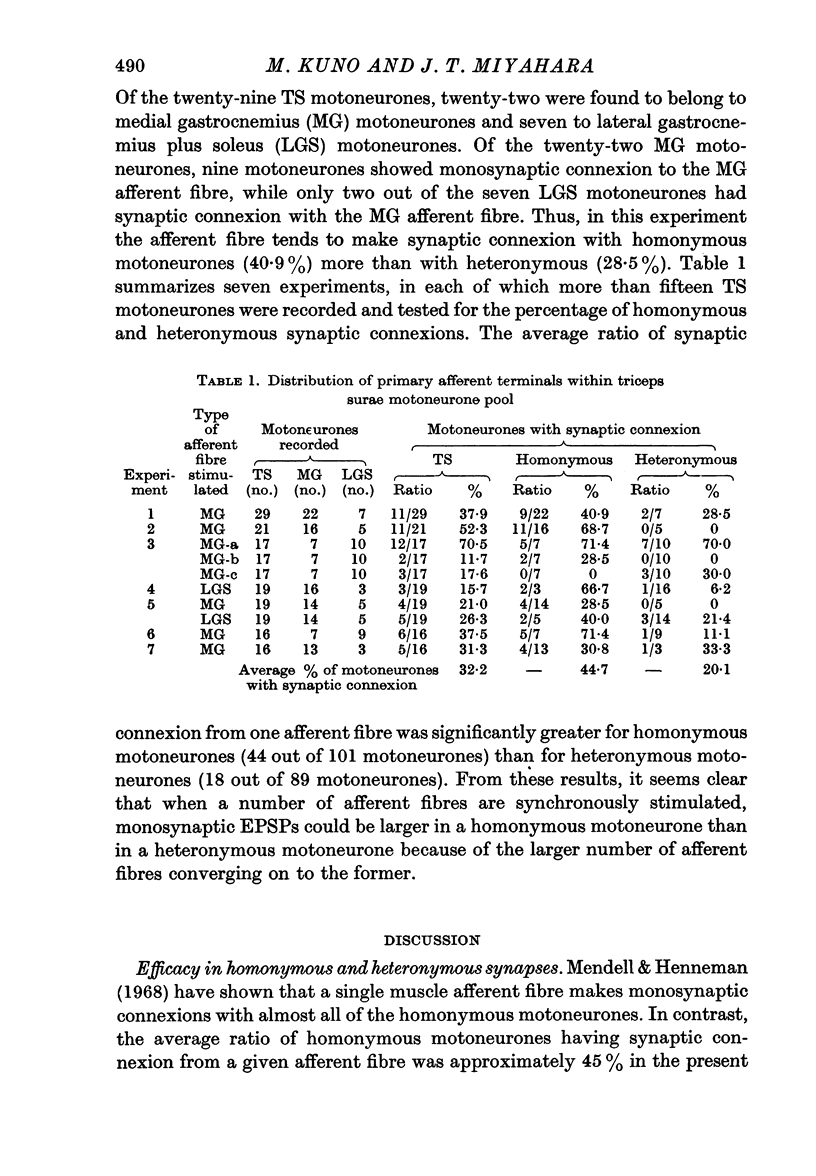
5. The ratio of monosynaptic connexions from a given afferent fibre was significantly greater on to homonymous than to heteronymous motoneurones. It is concluded that the difference in efficacy between homonymous and heteronymous synaptic transmission is due to the difference in the number of afferent fibres converging upon these motoneurones.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship J. E., Kuno M. Analysis of spontaneous subthreshold activity in spinal motoneurons of the cat. J Neurophysiol. 1968 Mar;31(2):195–209. doi: 10.1152/jn.1968.31.2.195. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Group Ia synaptic input to fast and slow twitch motor units of cat triceps surae. J Physiol. 1968 Jun;196(3):605–630. doi: 10.1113/jphysiol.1968.sp008526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E. Motor unit types of cat triceps surae muscle. J Physiol. 1967 Nov;193(1):141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Nelson P. G. Synaptic activity in motoneurons during natural stimulation of muscle spindles. Science. 1966 Mar 4;151(3714):1088–1091. doi: 10.1126/science.151.3714.1088. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957 Jun 18;137(1):22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Urinary excitatory postsynaptic potentials in Clarke's column neurons. Nature. 1967 Sep 9;215(5106):1176–1177. doi: 10.1038/2151176a0. [DOI] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELFAN S., RAPISARDA A. F. SYNAPTIC DENSITY ON SPINAL NEURONS OF NORMAL DOGS AND DOGS WITH EXPERIMENTAL HIND-LIMB RIGIDITY. J Comp Neurol. 1964 Aug;123:73–96. doi: 10.1002/cne.901230108. [DOI] [PubMed] [Google Scholar]
- GRANIT R., HENATSCH H. D., STEG G. Tonic and phasic ventral horn cells differentiated by post-tetanic potentiation in cat extensors. Acta Physiol Scand. 1956 Sep 26;37(2-3):114–126. doi: 10.1111/j.1748-1716.1956.tb01347.x. [DOI] [PubMed] [Google Scholar]
- HAGGAR R. A., BARR M. L. Quantitative data on the size of synaptic end-bulbs in the cat's spinal cord. J Comp Neurol. 1950 Aug;93(1):17–35. doi: 10.1002/cne.900930103. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. Monosynaptic reflex response of spinal motoneurons to graded afferent stimulation. J Gen Physiol. 1955 Jul 20;38(6):813–852. doi: 10.1085/jgp.38.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILLIS L. SPINAL CORD SYNAPSES IN THE CAT: THE NORMAL APPEARANCES BY THE LIGHT MICROSCOPE. Brain. 1964 Sep;87:543–554. doi: 10.1093/brain/87.3.543. [DOI] [PubMed] [Google Scholar]
- Jack J. J., Porter R. Rapidly and slowly rising components of monosynaptic excitatory post-synaptic potentials in spinal motoneurones. J Physiol. 1966 Oct;186(2):106P–107P. [PubMed] [Google Scholar]
- KATZ B., MILEDI R. A STUDY OF SPONTANEOUS MINIATURE POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1963 Sep;168:389–422. doi: 10.1113/jphysiol.1963.sp007199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. QUANTAL COMPONENTS OF EXCITATORY SYNAPTIC POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:81–99. doi: 10.1113/jphysiol.1964.sp007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D. Input resistance, electrical excitability, and size of ventral horn cells in cat spinal cord. Science. 1966 Jun 17;152(3729):1637–1640. doi: 10.1126/science.152.3729.1637. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T. Factors responsible for multiple discharge of neurons in Clarke's column. J Neurophysiol. 1968 Jul;31(4):624–638. doi: 10.1152/jn.1968.31.4.624. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T. Non-linear summation of unit synaptic potentials in spinal motoneurones of the cat. J Physiol. 1969 Apr;201(2):465–477. doi: 10.1113/jphysiol.1969.sp008767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LLOYD D. P., McINTYRE A. K. Transmitter potentiality of homonymous and heteronymous monosynaptic reflex connections of individual motoneurons. J Gen Physiol. 1955 Jul 20;38(6):789–799. doi: 10.1085/jgp.38.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: distribution within a pool of 300 homonymous motor neurons. Science. 1968 Apr 5;160(3823):96–98. doi: 10.1126/science.160.3823.96. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J., ALBERT A. The synaptology of Clarke's column. Acta Morphol Acad Sci Hung. 1955;5(1-2):43–51. [PubMed] [Google Scholar]
- WYCKOFF R. W., YOUNG J. Z. The motorneuron surface. Proc R Soc Lond B Biol Sci. 1956 Mar 13;144(917):440–450. doi: 10.1098/rspb.1956.0002. [DOI] [PubMed] [Google Scholar]


