Abstract
1. The effects of adrenaline and noradrenaline on neuromuscular transmission and the question of whether these catecholamines act on the presynaptic nerve terminal or on the post-synaptic membrane were investigated using the fin muscles of the silver carp.
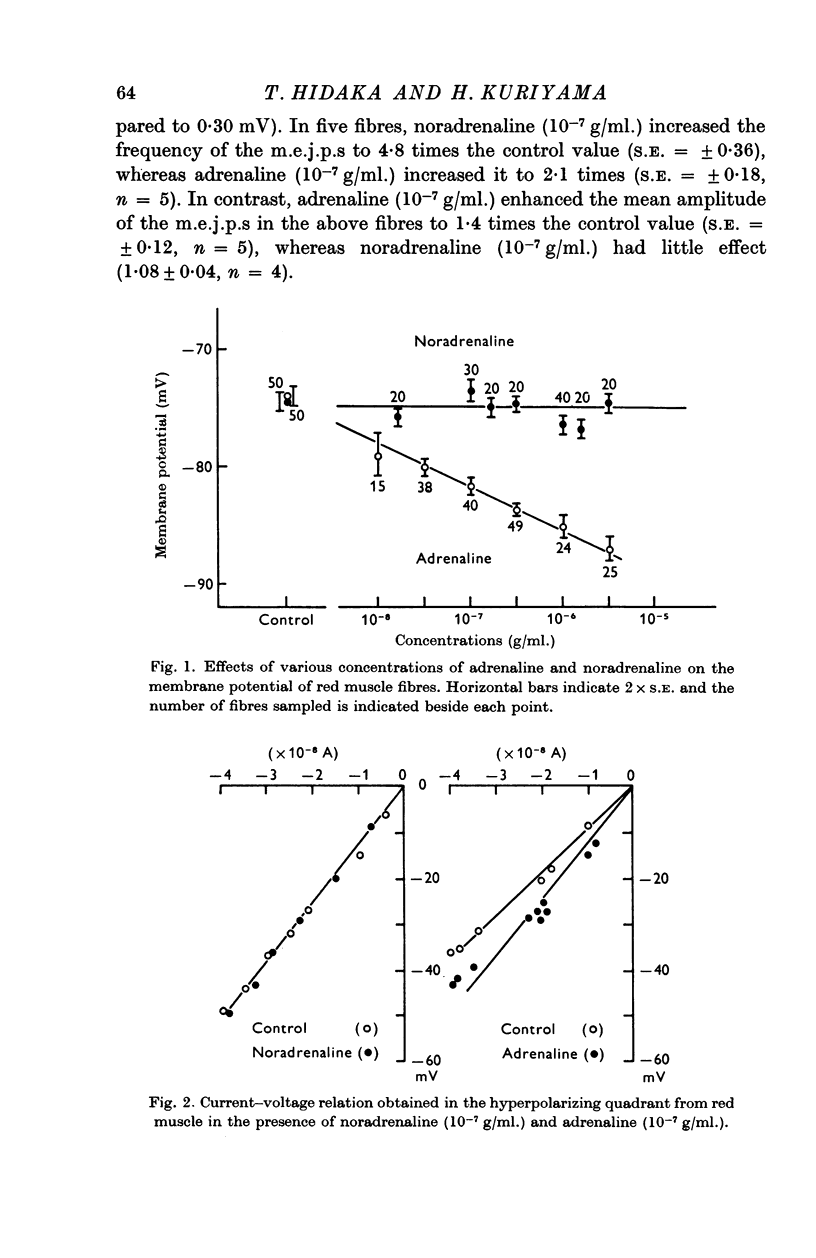
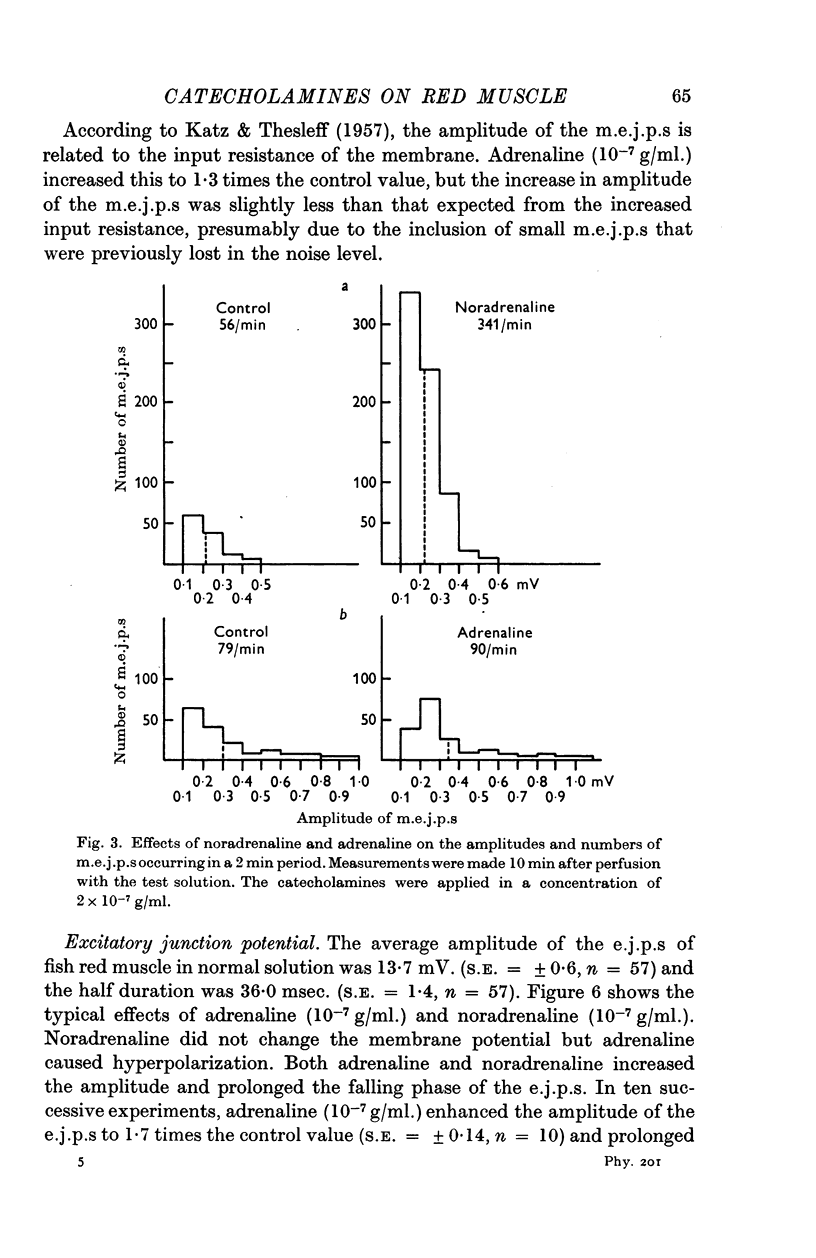
2. Noradrenaline (10-8-10-6 g/ml.) had no effect on membrane potential or input resistance of the membrane. In contrast, adrenaline (10-8-10-6 g/ml.) hyperpolarized the membrane and increased the input resistance.
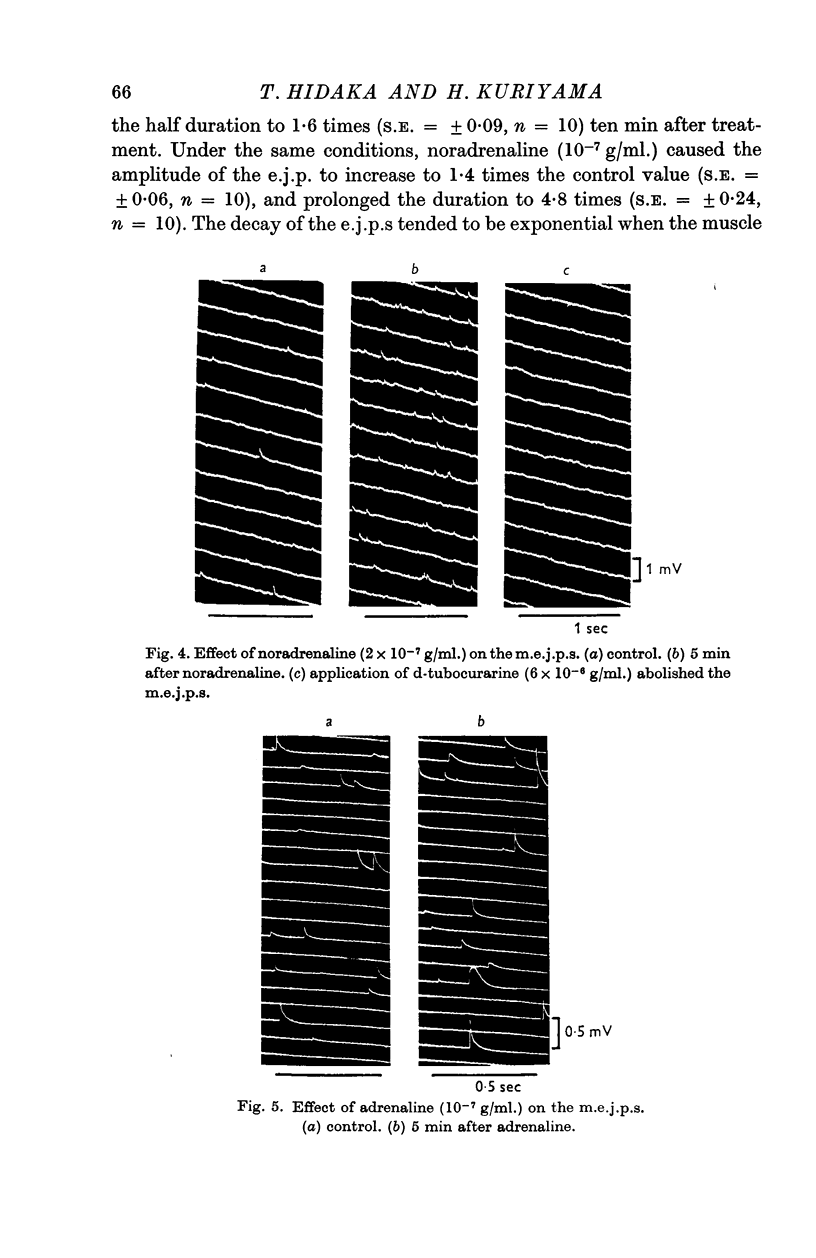
3. Noradrenaline had no effect on the amplitude of the miniature junction potentials but caused them to become more frequent.
4. Adrenaline enhanced the amplitude of the miniature junction potentials and slightly increased their frequency. These effects of adrenaline were partly due to the increased input resistance of the post-synaptic membrane, and probably did not involve the presynaptic terminals.
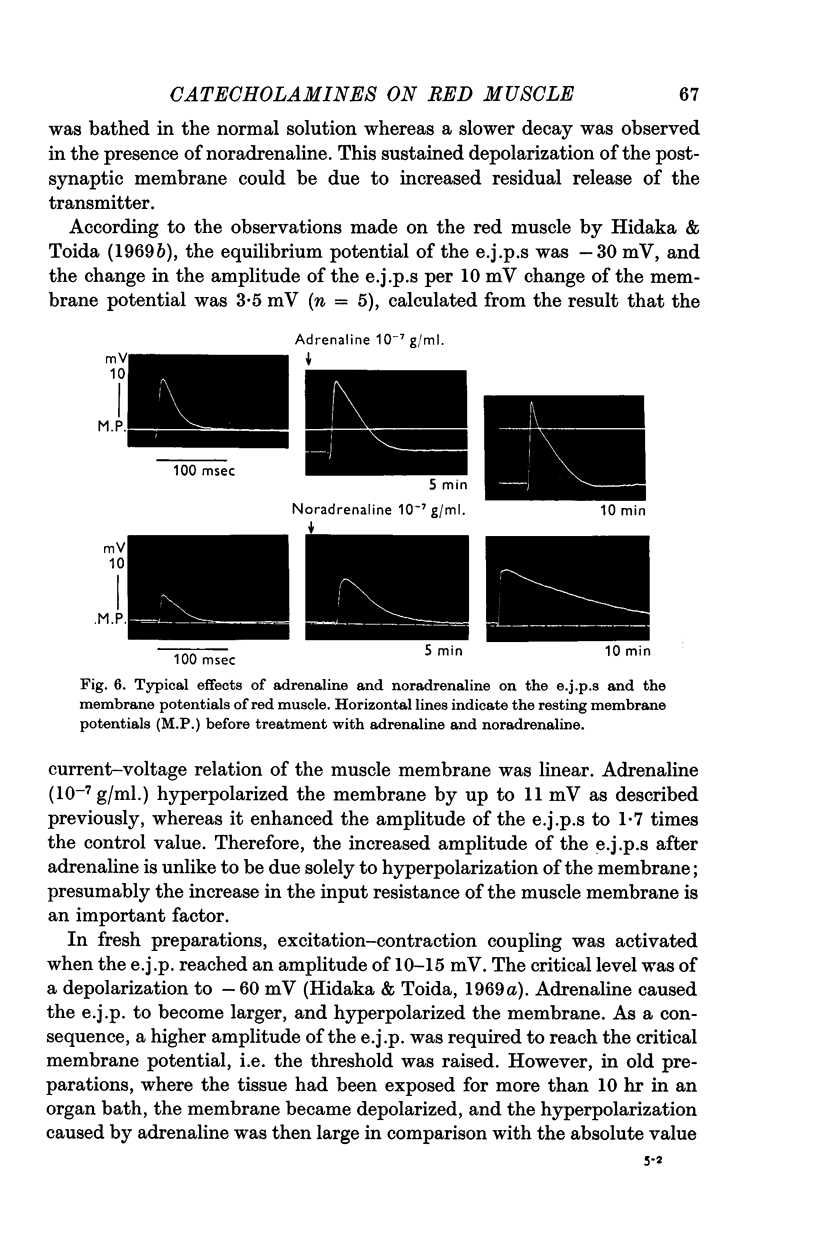
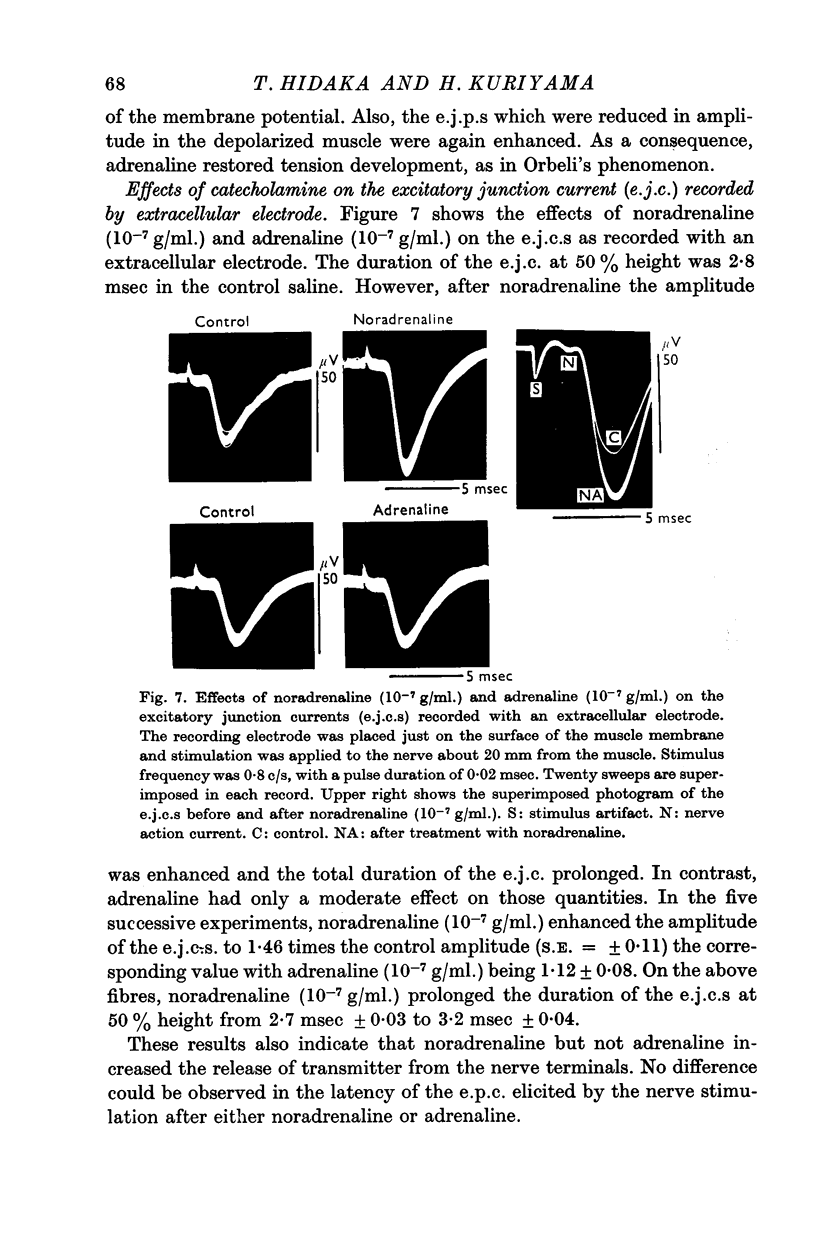
5. Both noradrenaline and adrenaline increased the amplitude and prolonged the falling phase of the excitatory junction potentials (e.j.p.s).
6. Noradrenaline enhanced the amplitude and prolonged the duration of the extracellularly recorded excitatory junction current. No such effect was observed with adrenaline.
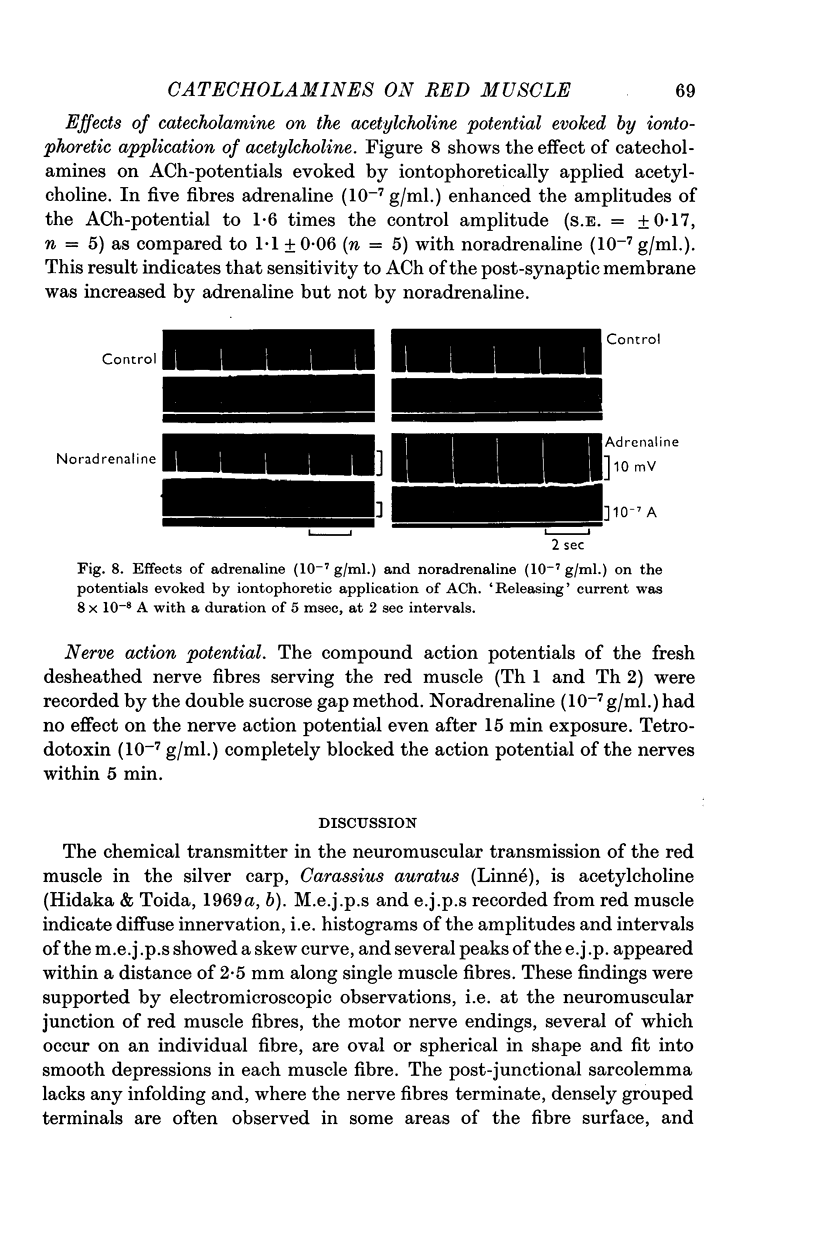
7. Adrenaline but not noradrenaline enhanced the amplitudes of acetylcholine potentials evoked by iontophoretic application of acetylcholine.
8. Noradrenaline and adrenaline had no effect on the compound action potentials of the nerve supplying the red muscle, recorded by the sucrose gap method.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman W. C., Raper C. Adrenotropic receptors in skeletal muscle. Ann N Y Acad Sci. 1967 Feb 10;139(3):741–753. doi: 10.1111/j.1749-6632.1967.tb41241.x. [DOI] [PubMed] [Google Scholar]
- GOFFART M. Recherches relatives à l'action de l'adrénaline sur le muscle strié de mammifère. I. Potentiation par l'adrénaline de la contraction maximale du muscle non fatigué. Arch Int Physiol. 1952 Sep;60(3):318–349. doi: 10.3109/13813455209145098. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., LOEWENSTEIN W. R. Nature of neuromuscular facilitation by sympathetic stimulation in the frog. J Physiol. 1955 Dec 29;130(3):559–571. doi: 10.1113/jphysiol.1955.sp005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka T., Toida N. Biophysical and mechanical properties of red and white muscle fibres in fish. J Physiol. 1969 Mar;201(1):49–59. doi: 10.1113/jphysiol.1969.sp008741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Failure of neuromuscular propagation in rats. J Physiol. 1958 Mar 11;140(3):440–461. [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Some effects produced by adrenaline upon neuromuscular propagation in rats. J Physiol. 1958 Apr 30;141(2):291–304. doi: 10.1113/jphysiol.1958.sp005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASTUK W. L. The electrical activity of the muscle cell membrane at the neuromuscular junction. J Cell Physiol. 1953 Oct;42(2):249–272. doi: 10.1002/jcp.1030420206. [DOI] [PubMed] [Google Scholar]
- Nishihara H. Studies on the fine structure of red and white fin muscles of the fish (Carassius auratus). Arch Histol Jpn. 1967 Sep;28(4):425–447. doi: 10.1679/aohc1950.28.425. [DOI] [PubMed] [Google Scholar]


