Abstract
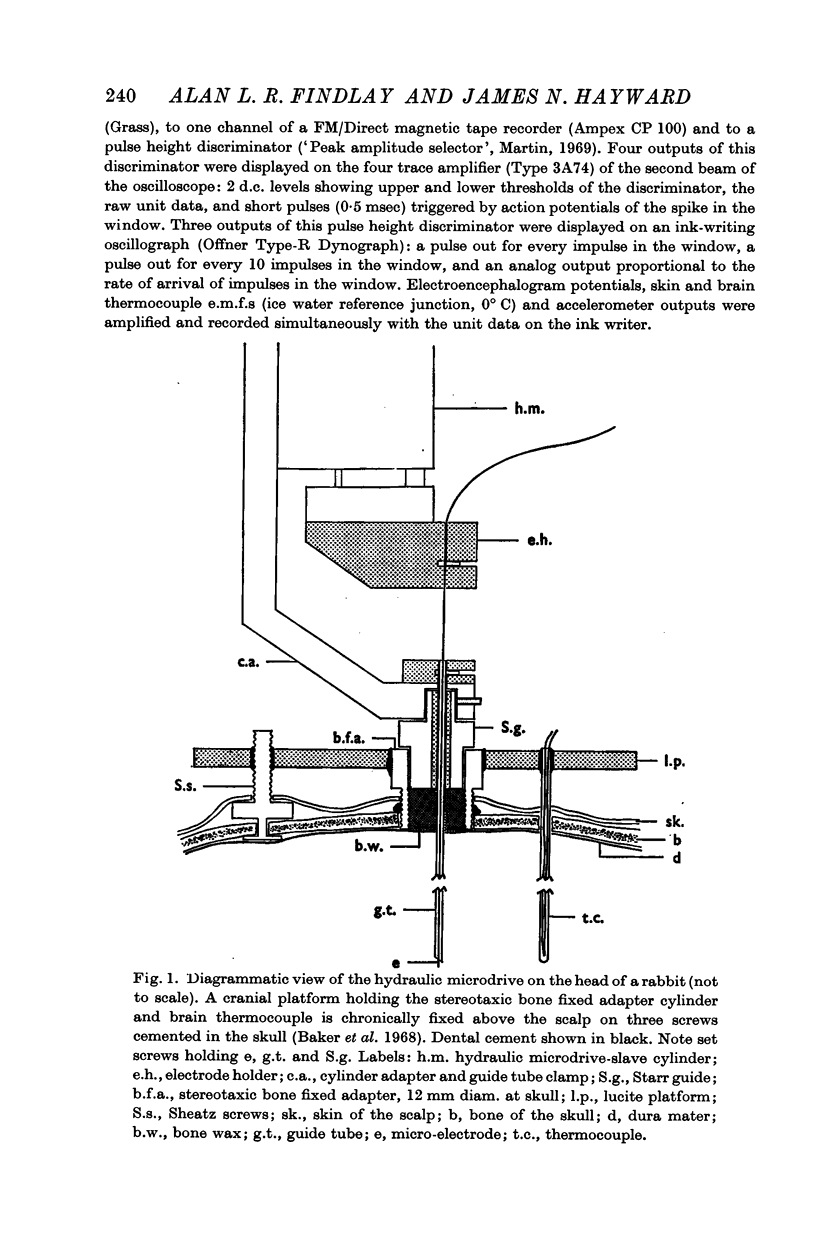
1. A method is described for recording from single cells in the hypothalamus of unanaesthetized freely moving rabbits. Behaviour, bodily movement, skin and brain temperatures and e.e.g. were monitored.
2. Patterns of unit firing during slow sleep, paradoxical sleep and waking were studied in several regions of the hypothalamus, thalamus and in the septum.
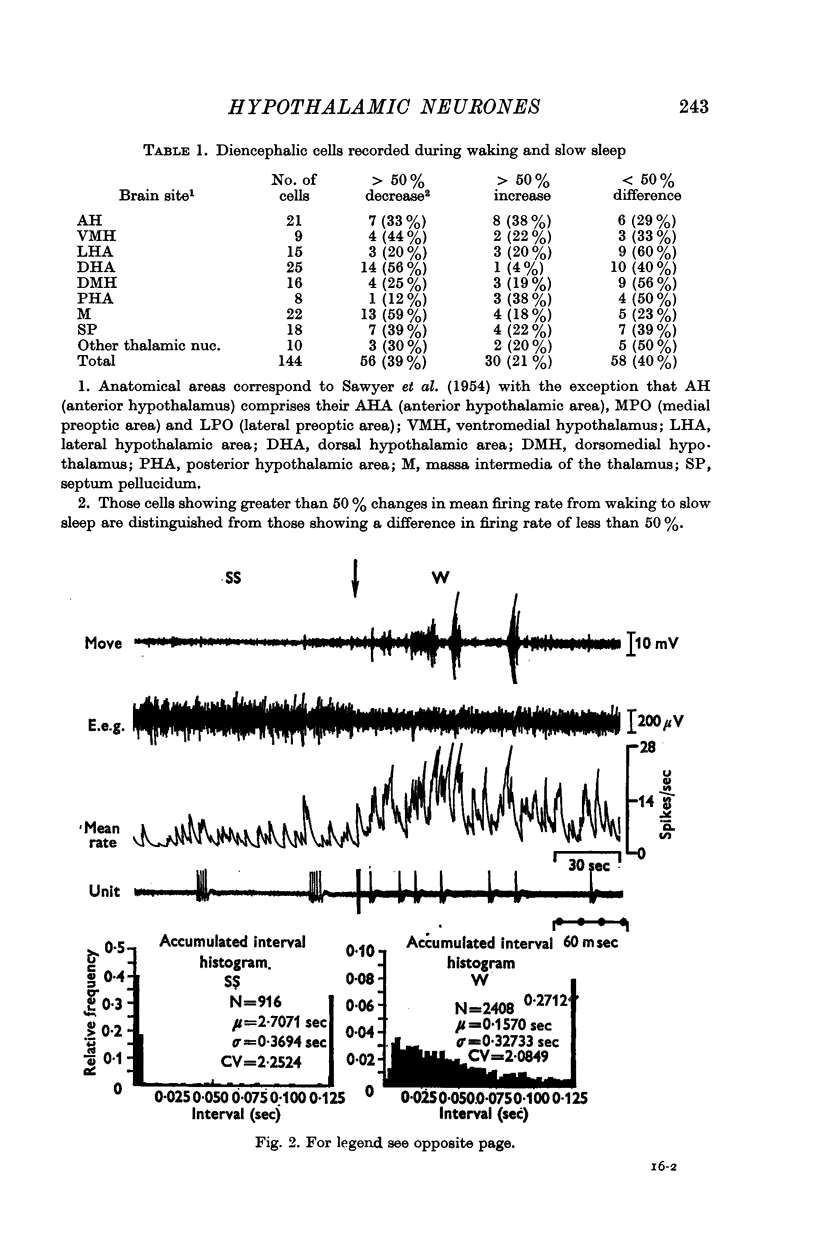
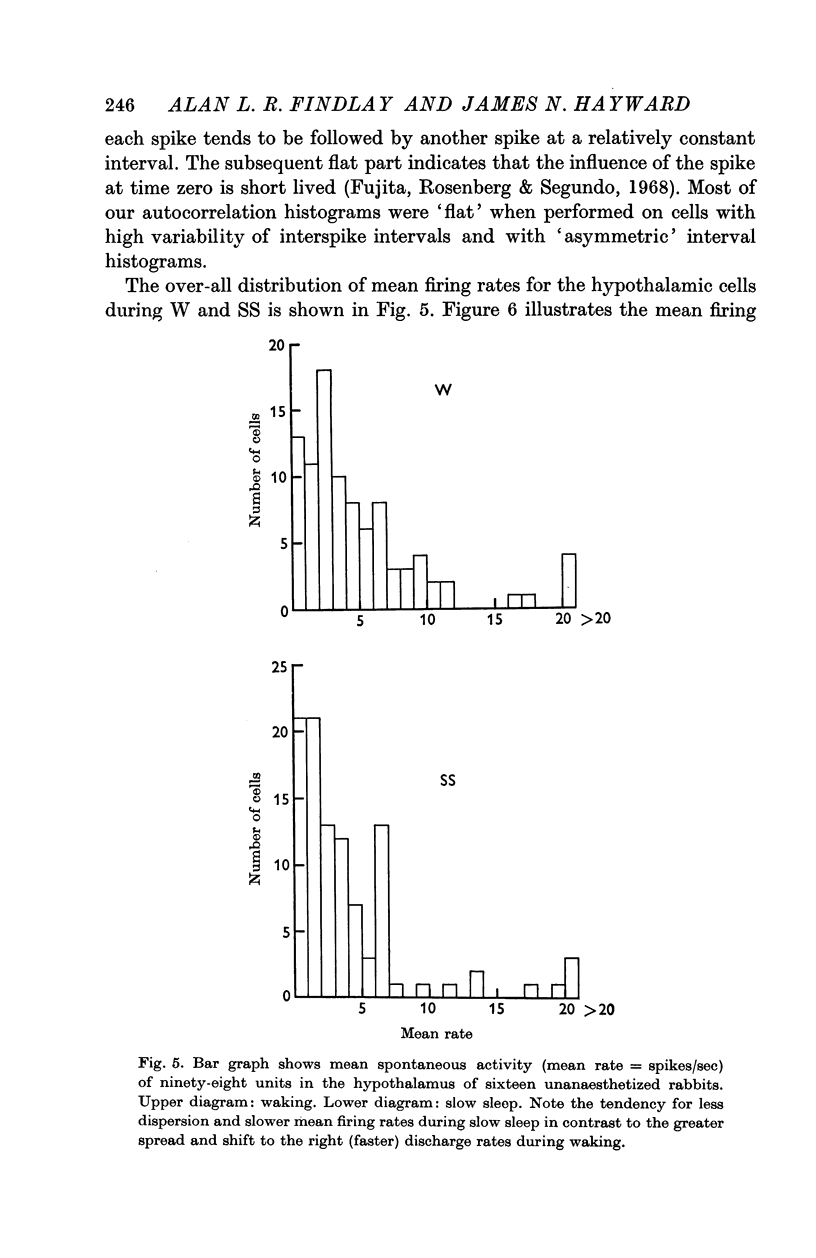
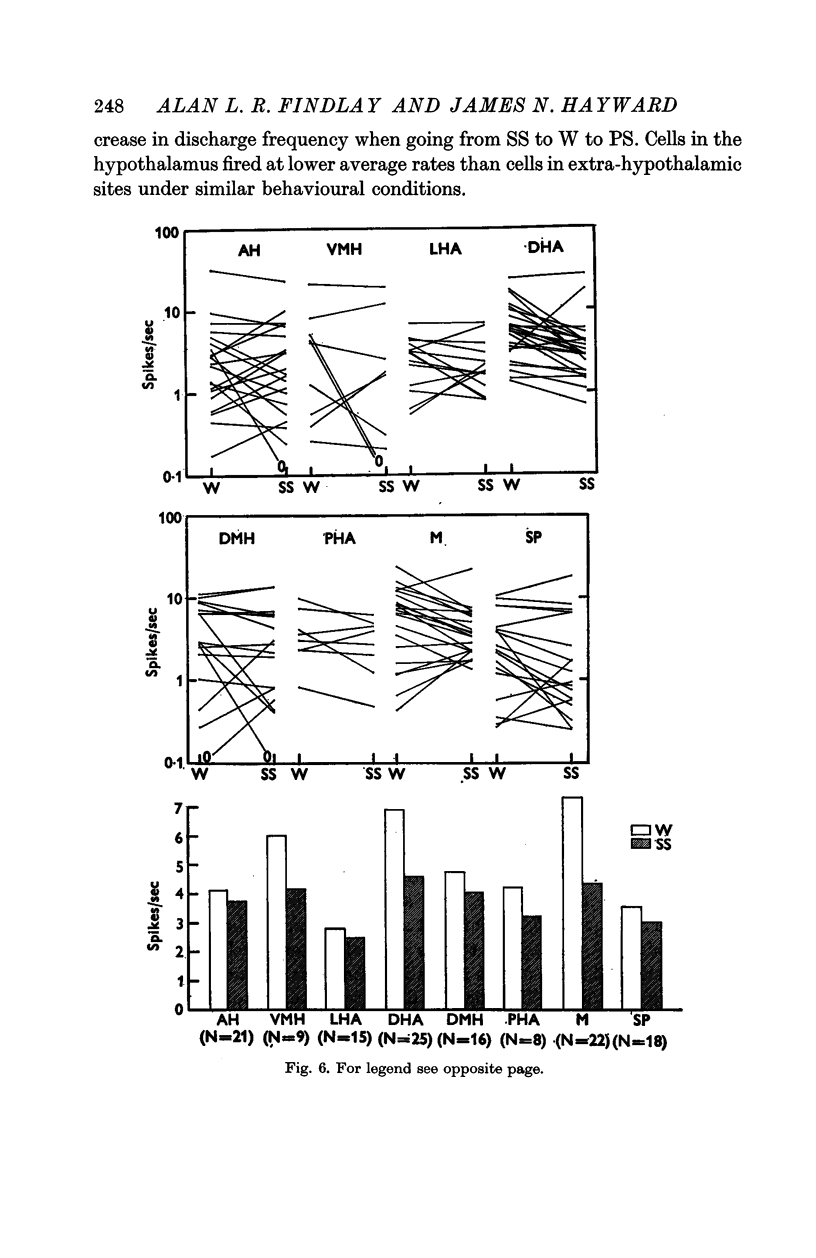
3. Of the 144 cells analysed from waking to slow sleep, fifty-six (39%) decreased mean firing rates, thirty (21%) increased spike discharges and fifty-eight (40%) showed no marked change. Dorsal hypothalamic and massa intermedia thalamic cells fired in brief high frequency clusters during slow sleep with a characteristic `bimodal' interspike interval histogram. Waking and paradoxical sleep abolished these cluster discharges with a concomitant change to an `asymmetric' histogram.
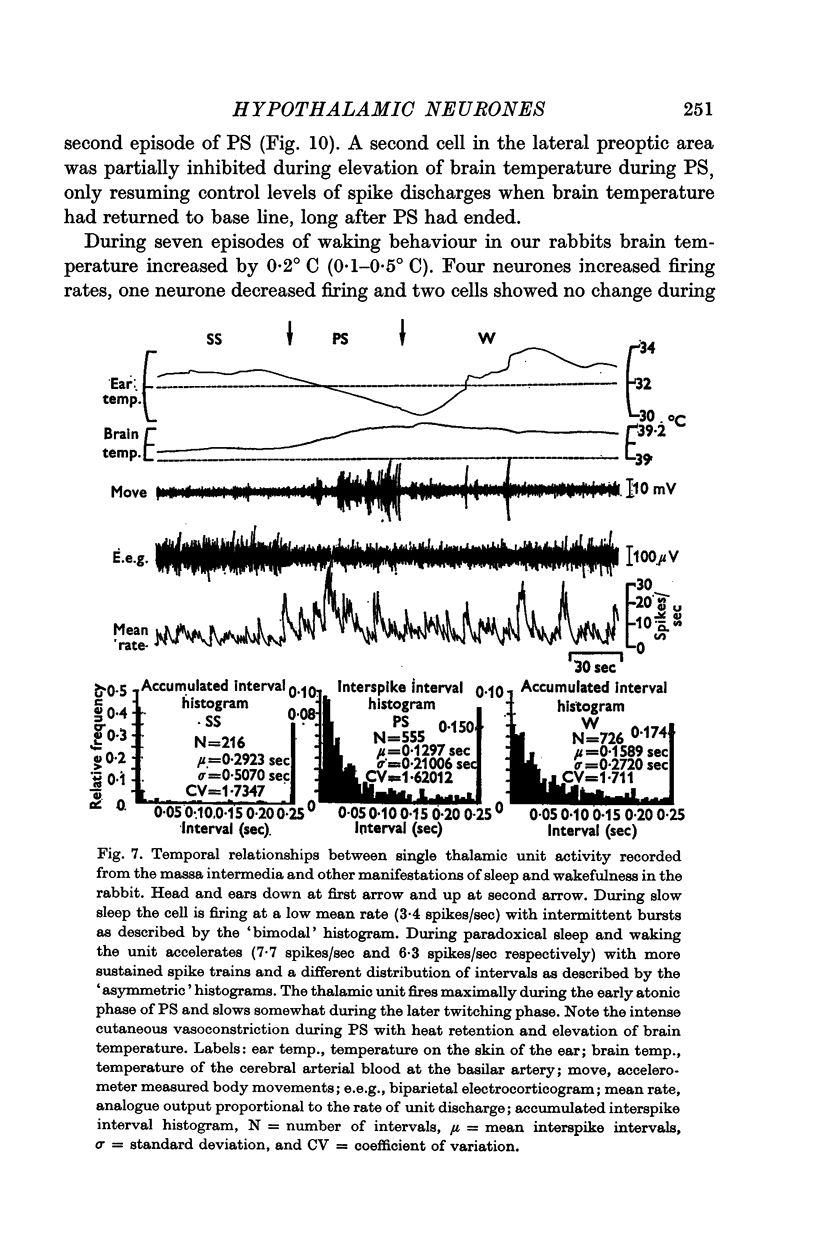
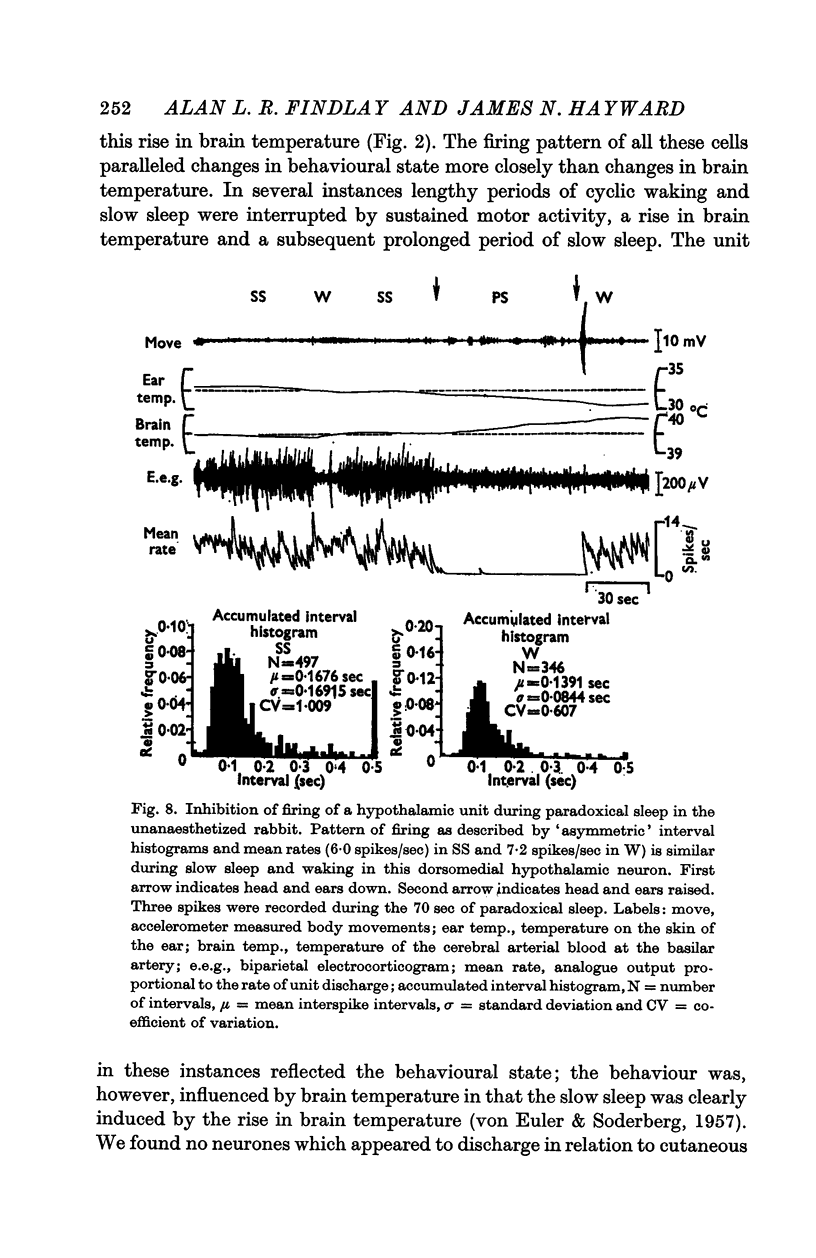
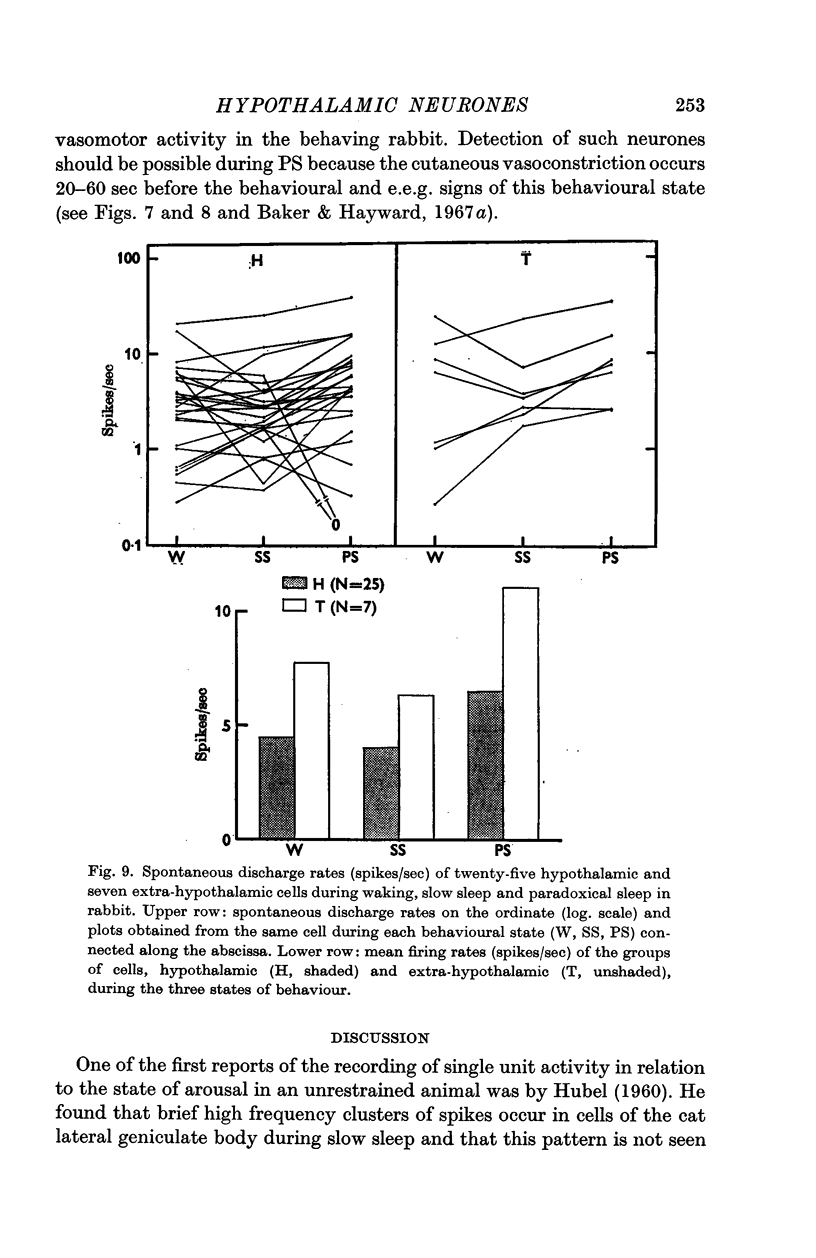
4. Of the thirty-two cells observed during the three states of waking, slow sleep and paradoxical sleep, a majority (twenty-five or 78%) showed their highest rates of spontaneous discharge during paradoxical sleep. Discharge rates of cells sometimes changed in the course of paradoxical sleep according to the presence or absence of phasic events such as myoclonic motor activity. Two hypothalmic cells were almost totally arrested during paradoxical sleep.
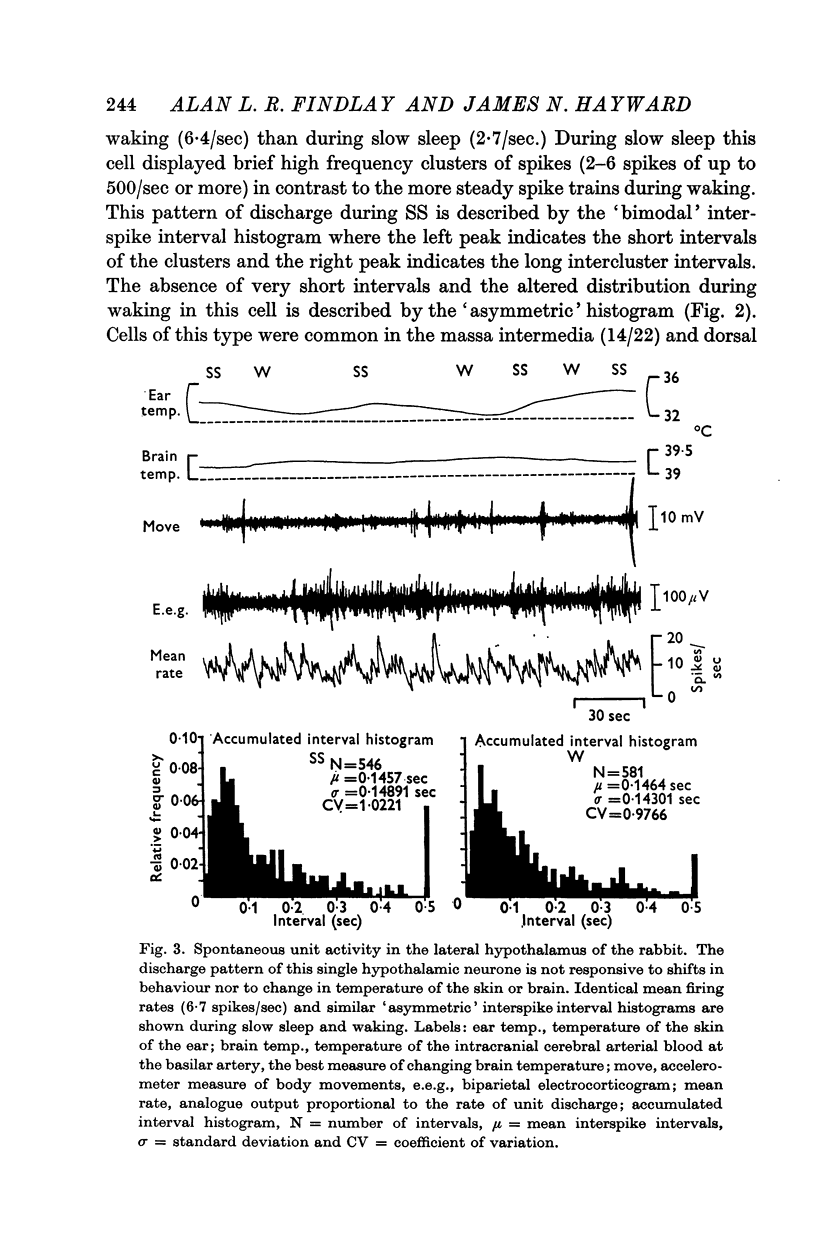
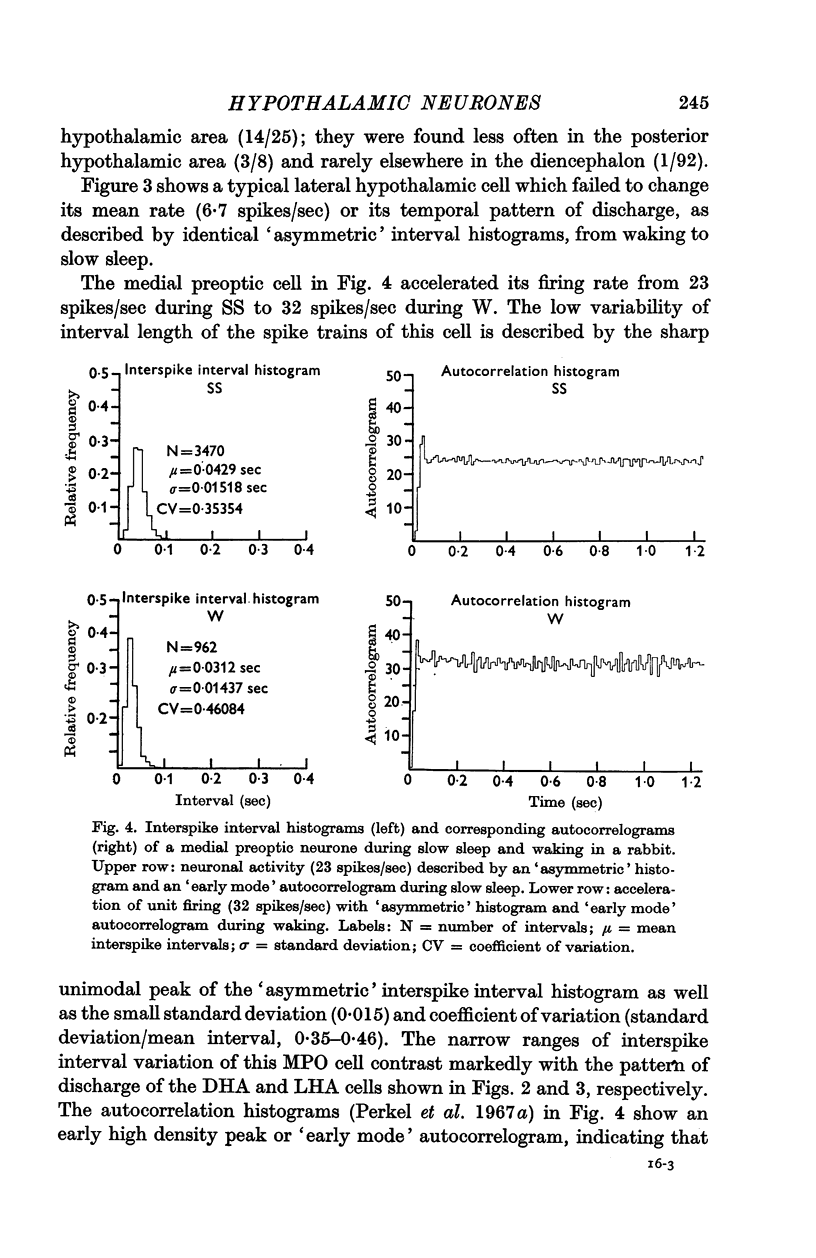
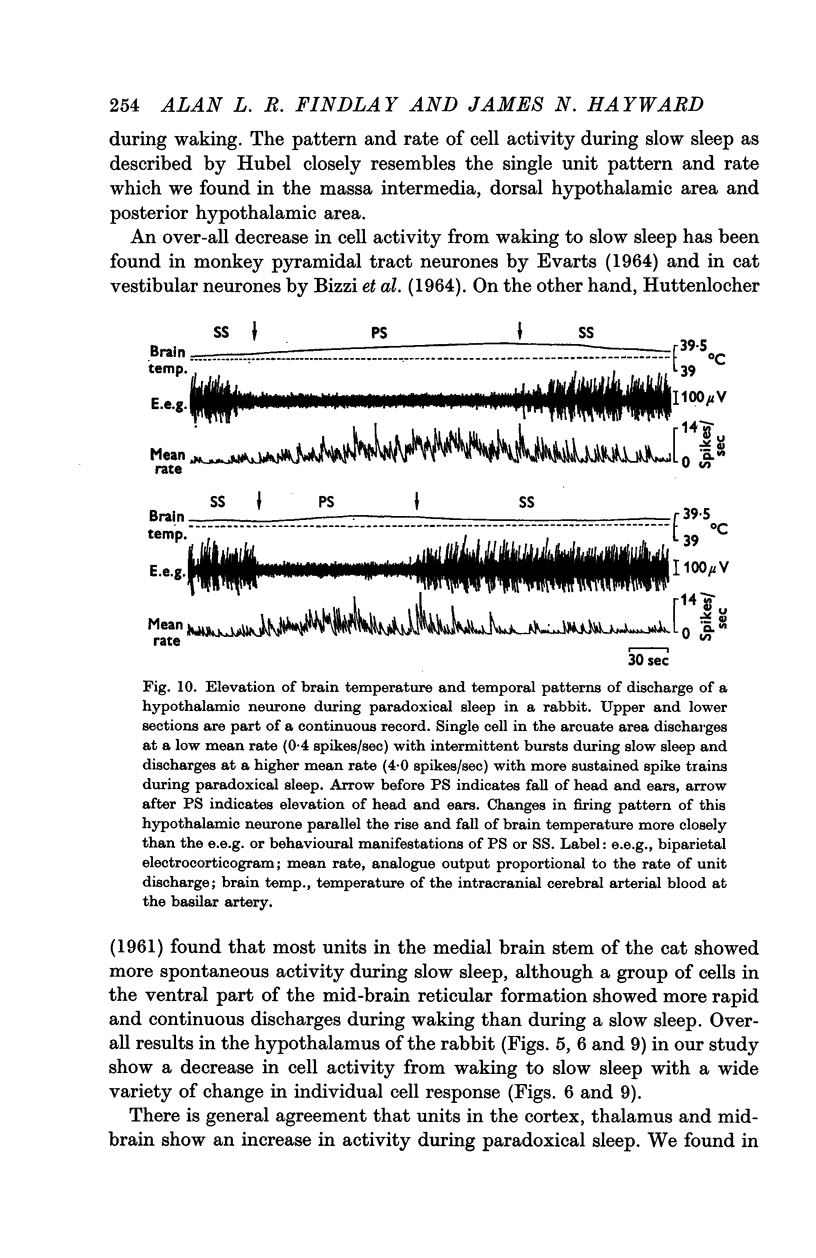
5. Analysis of unit firing rates during spontaneous rises in brain temperature during waking and paradoxical sleep revealed that a majority of the neurones (22/24) changed their discharge rates in relation to behaviour rather than to brain temperature. Two cells did appear to respond specifically to the central thermal stimulus.
6. Hypothalamic cells do not behave as a homogeneous population in relation to changes in the state of arousal of the rabbit. Spontaneous changes in cell discharge related to sleep-waking behaviour must be considered in any interpretation of hypothalamic unit activity as related to neuroendocrine or autonomic mechanisms.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLUCCHI G., MORUZZI G., SALVI G., STRATA P. PUPIL BEHAVIOR AND OCULAR MOVEMENTS DURING SYNCHRONIZED AND DESYNCHRONIZED SLEEP. Arch Ital Biol. 1964 Apr 18;102:230–244. [PubMed] [Google Scholar]
- BIZZI E., POMPEIANO O., SOMOGYI I. SPONTANEOUS ACTIVITY OF SINGLE VESTIBULAR NEURONS OF UNRESTRAINED CATS DURING SLEEP AND WAKEFULNESS. Arch Ital Biol. 1964 Apr 18;102:308–330. [PubMed] [Google Scholar]
- Baker M. A., Burrell E., Penkhus J., Hayward J. N. Capping and stabilizing chronic intravascular cannulae. J Appl Physiol. 1968 Apr;24(4):577–579. doi: 10.1152/jappl.1968.24.4.577. [DOI] [PubMed] [Google Scholar]
- Baker M. A., Hayward J. N. Carotid rete and brain temperature of cat. Nature. 1967 Oct 14;216(5111):139–141. doi: 10.1038/216139a0. [DOI] [PubMed] [Google Scholar]
- Baker M. A., Hayward J. N. The influence of the nasal mucosa and the carotid rete upon hypothalamic temperature in sheep. J Physiol. 1968 Oct;198(3):561–579. doi: 10.1113/jphysiol.1968.sp008626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS B. A., GREEN J. D. Activity of single neurones in the hypothalamus: effect of osmotic and other stimuli. J Physiol. 1959 Oct;148:554–569. doi: 10.1113/jphysiol.1959.sp006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B. A., Silver I. A. Electrophysiological studies on the hypothalamus. Br Med Bull. 1966 Sep;22(3):254–260. doi: 10.1093/oxfordjournals.bmb.a070483. [DOI] [PubMed] [Google Scholar]
- EVARTS E. V. TEMPORAL PATTERNS OF DISCHARGE OF PYRAMIDAL TRACT NEURONS DURING SLEEP AND WAKING IN THE MONKEY. J Neurophysiol. 1964 Mar;27:152–171. doi: 10.1152/jn.1964.27.2.152. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Rosenberg J., Segundo J. P. Activity of cells in the lateral vestibular nucleus as a function of head position. J Physiol. 1968 May;196(1):1–18. doi: 10.1113/jphysiol.1968.sp008490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASSEL M. M., GHELARDUCCI B., MARCHIAFAVA P. L., POMPEIANO O. PHASIC CHANGES IN BLOOD PRESSURE AND HEART RATE DURING THE RAPID EYE MOVEMENT EPISODES OF DESYNCHRONIZED SLEEP IN UNRESTRAINED CATS. Arch Ital Biol. 1964 Jul;102:530–544. [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J. N., Baker M. A. Diuretic and thermoregulatory responses to preoptic cooling in the monkey. Am J Physiol. 1968 Apr;214(4):843–850. doi: 10.1152/ajplegacy.1968.214.4.843. [DOI] [PubMed] [Google Scholar]
- Hayward J. N., Baker M. A. Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am J Physiol. 1968 Aug;215(2):389–403. doi: 10.1152/ajplegacy.1968.215.2.389. [DOI] [PubMed] [Google Scholar]
- Hayward J. N. Brain temperature regulation during sleep and arousal in the dog. Exp Neurol. 1968 Jun;21(2):201–212. doi: 10.1016/0014-4886(68)90138-6. [DOI] [PubMed] [Google Scholar]
- Hayward J. N., Fairchild M. D., Stuart D. G. Hypothalamic and cortical D-C potential changes induced by stimulation of the midbrain reticular formation. Exp Brain Res. 1966;1(3):205–219. doi: 10.1007/BF00234342. [DOI] [PubMed] [Google Scholar]
- Hellon R. F. Thermal stimulation of hypothalamic neurones in unanaesthetized rabbits. J Physiol. 1967 Nov;193(2):381–395. doi: 10.1113/jphysiol.1967.sp008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet M. Neurophysiology of the states of sleep. Physiol Rev. 1967 Apr;47(2):117–177. doi: 10.1152/physrev.1967.47.2.117. [DOI] [PubMed] [Google Scholar]
- Kinnard M. A., MacLean P. D. A platinum micro-electrode for intracerebral exploration with a chronically fixed stereotaxic device. Electroencephalogr Clin Neurophysiol. 1967 Feb;22(2):183–186. doi: 10.1016/0013-4694(67)90161-7. [DOI] [PubMed] [Google Scholar]
- Komisaruk B. R., McDonald P. G., Whitmoyer D. I., Sawyer C. H. Effects of progesterone and sensory stimulation on EEG and neuronal activity in the rat. Exp Neurol. 1967 Dec;19(4):494–507. doi: 10.1016/0014-4886(67)90169-0. [DOI] [PubMed] [Google Scholar]
- MALLIANI A., RUDOMIN P., ZANCHETTI A. CONTRIBUTION OF LOCAL ACTIVITY AND ELECTRIC SPREAD TO SOMATICALLY EVOKED POTENTIALS IN DIFFERENT AREAS OF THE HYPOTHALAMUS. Arch Ital Biol. 1965 Feb 20;103:119–135. [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J. 1967 Jul;7(4):391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. II. Simultaneous spike trains. Biophys J. 1967 Jul;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER C. H., EVERETT J. W., GREEN J. D. The rabbit diencephalon in stereotaxic coordinates. J Comp Neurol. 1954 Dec;101(3):801–824. doi: 10.1002/cne.901010307. [DOI] [PubMed] [Google Scholar]
- VON EULER C., SODERBERG U. The influence of hypothalamic thermoceptive structures on the electroencephalogram and gamma motor activity. Electroencephalogr Clin Neurophysiol. 1957 Aug;9(3):391–408. doi: 10.1016/0013-4694(57)90029-9. [DOI] [PubMed] [Google Scholar]


