Abstract
Sinorhizobium meliloti, a gram-negative soil bacterium, forms a nitrogen-fixing symbiotic relationship with members of the legume family. To facilitate our studies of transcription in S. meliloti, we cloned and characterized the gene for the α subunit of RNA polymerase (RNAP). S. meliloti rpoA encodes a 336-amino-acid, 37-kDa protein. Sequence analysis of the region surrounding rpoA identified six open reading frames that are found in the conserved gene order secY (SecY)-adk (Adk)-rpsM (S13)-rpsK (S11)-rpoA (α)-rplQ (L17) found in the α-proteobacteria. In vivo, S. meliloti rpoA expressed in Escherichia coli complemented a temperature sensitive mutation in E. coli rpoA, demonstrating that S. meliloti α supports RNAP assembly, sequence-specific DNA binding, and interaction with transcriptional activators in the context of E. coli. In vitro, we reconstituted RNAP holoenzyme from S. meliloti α and E. coli β, β′, and σ subunits. Similar to E. coli RNAP, the hybrid RNAP supported transcription from an E. coli core promoter and responded to both upstream (UP) element- and Fis-dependent transcription activation. We obtained similar results using purified RNAP from S. meliloti. Our results demonstrate that S. meliloti α functions are conserved in heterologous host E. coli even though the two α subunits are only 51% identical. The ability to utilize E. coli as a heterologous system in which to study the regulation of S. meliloti genes could provide an important tool for our understanding and manipulation of these processes.
The α-proteobacterium Sinorhizobium meliloti is able to live either as a soil saprophyte or in a symbiotic relationship with members of the legume family, such as alfalfa. Recent studies have focused on understanding how rhizobia adapt to these unique environments, especially at the level of gene expression (6, 13, 62). For the symbiosis to occur, expression of a subset of genes, such as the nod and nif genes, must be tightly regulated (reviewed in reference 22). As is the case with other bacteria, much of the gene regulation occurs at the level of initiation of transcription (28). To facilitate our studies of transcription and its regulation in S. meliloti, we must understand RNA polymerase (RNAP) structure and function.
Previous work demonstrated that RNAP from S. meliloti displays the characteristic α2ββ′ core subunit structure found in most bacteria (23, 45). In addition σ70, σ54, and σ 72 homologs have been cloned from S. meliloti (47, 48, 52, 55). These results are consistent with the evidence that bacterial RNAPs display overall sequence and functional similarities, although they can exhibit some differences in individual steps during transcription such as promoter recognition and promoter escape (4). Since only a limited number of S. meliloti promoters have been characterized, the cis-acting elements are not yet as well defined as in Escherichia coli promoters (7, 23, 55). Nevertheless, S. meliloti RNAP can initiate transcription at typical E. coli promoters (19, 23). However, most S. meliloti promoters that have been characterized are not transcribed by E. coli RNAP in vivo or in vitro (5, 19), perhaps because the S. meliloti Eσ70 homolog recognizes these promoters slightly differently from E. coli Eσ70 or because these promoters utilize σ factors or transcription activators not found in E. coli.
In the past decade, based primarily on work with E. coli, RNAP α has emerged as a key player in both basal transcription and in transcriptional activation (reviewed in references 20 and 29). RNAP α consists of two independently folded domains connected by a flexible linker (9, 64). The amino-terminal domain (αNTD) is required for α dimerization, for RNAP assembly, and for interaction with a subset of transcription factors (32, 51); the carboxy-terminal domain (αCTD) is required for binding to the upstream (UP) element, an A+T-rich sequence found upstream of the −35 hexamer, and for interaction with a number of transcription factors (35, 53). Several screens for α mutants have identified residues required for the activation of transcription. These α-activator contacts help recruit RNAP to promoters and/or stimulate the isomerization of the RNAP-promoter complex from the closed to the open state (46). Furthermore, the αCTD may also interact with the σCTD during transcription initiation at some promoters (29).
In some cases, α-activator contacts appear be species specific, suggesting that α and activators have coevolved. For example, Agrobacterium tumefaciens α is required for VirG-activated transcription of the virB promoter in E. coli (39). Similarly, transcription activation from the Bacillus subtilis phage A3 promoter requires RNAP containing B. subtilis α and is not supported by E. coli RNAP (43). In both cases, the species specificity of the α-activator contact was mapped to the αCTD (40, 43). Interestingly, Bordetella pertussis α reconstituted into E. coli RNAP does not support transcription at the E. coli CAP-dependent lac promoter (58), suggesting that different activator-α specificities may exist, despite striking sequence homologies in the αCTDs of B. pertussis and E. coli.
Our ultimate goal is to understand how α interacts with transcription factors to initiate transcription at S. meliloti promoters. In this paper we describe the cloning and characterization of the S. meliloti RNAP α subunit. Furthermore, we establish that S. meliloti α can functionally replace E. coli α in vivo and that S. meliloti α reconstituted into E. coli RNAP holoenzyme can support both UP element- and Fis-dependent transcription in vitro. These results suggest that the study of transcription activation in S. meliloti may be facilitated by utilizing tools developed for E. coli RNAP.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains and plasmids used in this work are listed in Table 1. S. meliloti and E. coli were grown in Luria broth at 30 and 37°C, respectively, unless otherwise noted. Ampicillin (50 to 100 μg/ml) was used to select and maintain plasmids.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 56 |
| HN317ts112 | F−aroE thi Su− Str rpoA112 | 36 |
| XL1-Blue MRA | Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 gyrA96 relA1 lac | Stratagene |
| S. meliloti JM57 | 1021, pSym nodC-lacZ | 44 |
| Plasmids | ||
| pBluescript SK(−) | Cloning vector, Apr | Stratagene |
| pBAD/HisB | Cloning vector, arabinose promoter, six-His tag, Apr | Invitrogen |
| pREIIα | ori-pBR322; lppP-′lacPUV5-E. coli rpoA, Apr | 9 |
| pCR2.1-TOPO | Cloning vector, Apr | Invitrogen |
| pMP1 | 2.1-kb BamHI fragment containing rpoA in pBluescript SK(−) | This study |
| pMP2 | 3.3-kb HindIII fragment containing rpoA in pBluescript SK(−) | This study |
| pMP3 | Full-length rpoA in EcoRI site of pBluescript SK(−) | This study |
| pMP16 | rpoA cloned into pCR2.1-TOPO | This study |
| pMP18 | rpoA (1-254) plus adjacent stop codon in pCR2.1-TOPO | This study |
| pMP19a | rpoA cloned into pBAD/HisB | This study |
| pMP21 | rpoA (1-254) in pBAD/HisB | This study |
| pMP22 | E. coli rpoA replaced by S. meliloti rpoA with six-His tag in pREIIα | This study |
| pMP25 | Upstream stop codons and Shine-Dalgarno sequence, rpoA with six-His tag in pCR2.1-TOPO | This study |
| pHTT7α | ori-pBR322; φ10P-E. coli rpoA, Apr | 9 |
| pRLG770 | Vector containing E. coli rrnB P1 terminator, RNA I promoter | 54 |
| pRLG593 | pRLG770 with lacUV5 (−59-+38) | 54 |
| pRLG862 | pRLG770 with E. coli rrnB P1 (−88-+1) | 54 |
| pLR14 | pRLG770 with E. coli rrnB P1 (−88-+1, SUB) | 50 |
| pRmE65 | nodD3 expressed under trp promoter in pTE3, IncP Tcr | 24 |
Plasmid construction.
Plasmid constructions in E. coli were carried out as described previously (56). Full-length rpoA was amplified by PCR from pMP3 and cloned into pCR2.1-TOPO to create pMP16. The region of rpoA encoding amino acids 1 to 254 plus an added adjacent stop codon was amplified by PCR from pMP3 and cloned into pCR2.1-TOPO to create pMP18, encoding αΔ254. EcoRI fragments containing full-length rpoA from pMP16 or the coding sequence for αΔ254 from pMP18 were cloned into EcoRI-digested pBAD/HisB to create pMP19a and pMP21, respectively. PMP19a and pMP21 contain sequences encoding an additional 62 amino acids at the N terminus of the product of rpoA representing a six-His tag, an anti-Xpress epitope, an enterokinase cleavage site, and a Shine-Dalgarno-like sequence upstream of the rpoA product initiating methionine. rpoA encoding a full-length protein with a six-His tag was amplified by PCR from pMP19a and cloned into pCR2.1-TOPO to create pMP25. To create pMP22 an XbaI-EcoRV insert from pMP25 was cloned into pREIIα digested with BamHI, filled in with the Klenow fragment of DNA polymerase I, and digested with XbaI.
DNA manipulation, sequencing, and sequence analysis.
Plasmid DNA was isolated with the Miniprep kit (Qiagen, Inc.) or the Wizard DNA plasmid kit (Promega) or by CsCl banding. Genomic S. meliloti DNA was isolated with the Puregene DNA isolation kit (Gentra Systems).
Generation of λ-ZAP phage lysates and infection of XL1-Blue MRA DNA were performed as described previously (56). To purify phage DNA, lysed E. coli cultures were centrifuged for 5 min at 5,000 × g. Supernatants were incubated at 37°C for 1 h after addition of 1 μg each of pancreatic DNase I and RNase H (Sigma)/ml and centrifuged for 5 min at 5,000 × g, and then they were recentrifuged for 90 min at 100,000 × g. Pellets were resuspended in a solution containing 500 μl of 50 mM Tris-HCl, pH 8.0, and 500 μl of phenol and incubated (with vortexing every 5 min) for 15 min at room temperature to lyse the phage. They were extracted twice with phenol-CHCl3 and once with CHCl3, and the phage DNA was precipitated with ethanol, air dried, and resuspended in 200 μl of H20. Alternatively, λ-ZAP DNA was purified with the Qiagen phage purification kit.
Restriction digestions were performed according to manufacturer's directions. DNA was gel purified with the Gene-Clean kit (Bio 101) or the Qiaex II kit (Qiagen, Inc.). Nucleotide analysis of both strands was by fluorescence sequencing with an Applied Biosystems Prism 310. The DNA sequences were assembled with the Sequencher, version 3.0, computer program (Gene Codes Corporation). Alignment of nucleotide sequences was performed with the Lasergene computer program (DNASTAR Inc.). Database searches were performed with BLAST (2).
Purification of RNAP from S. meliloti.
S. meliloti RNAP was purified by the method of Burgess and Jendrisak with the following modifications (12). JM57/pRmE65 cell paste, frozen at −70°C, was the source of S. meliloti RNAP. Cells were broken by passage through a French press at 10,000 to 14,000 lb/in2 in lieu of lysozyme-sodium deoxycholate lysis. A cocktail of protease inhibitors (3 μM chymotrypsin, 16 μM leupeptin, 3.6 μM pepstatin, and 130 μM phenylmethylsulfonyl fluoride [final concentrations]) was added to the crude extract. A DNA-agarose column prepared by the method of Schaller et al. (57) was used in place of DNA-cellulose. Bio-Gel A-1.5m was substituted for Bio-Gel A-5m (41). RNAP activity on a pUC derivative containing the cloned Salmonella enterica serovar Typhimurium trp promoter was assayed (18). Pooled active fractions were dialyzed against buffer (10 mM Tris-HCl [pH 8.0], 0.1 mM EDTA, 0.1 mM dithiothreitol) plus 50% glycerol. Proteins were analyzed by sodium dodecyl sulfate-12.5% polyacrylamide gel electrophoresis (SDS-12.5% PAGE) and visualized with Coomassie blue R250 (Sigma).
Isolation of rpoA and the surrounding region.
Full-length RNAP α or peptides obtained from digestion of α with Staphylococcus aureus V8 protease in the presence of SDS (17) were isolated on an SDS-polyacrylamide gel, electroblotted to an Immobilon P membrane, and subjected to Edman degradation to obtain the N-terminal sequence (Protein and Nucleic Acids Facility, Stanford University). Degenerate primers based on the polypeptide sequence (rpoA 5′2, rpoA 5′3, rpoA 7, and rpoA 8) were used to obtain 400- and 800-bp PCR fragments containing part of the S. meliloti rpoA open reading frame (ORF). Primer and peptide sequences are available from the authors upon request. A wild-type S. meliloti genomic λ-library (63) was screened with the 800-bp PCR fragment to isolate three independent (nonsibling) λ-clones that contain rpoA and flanking genes. Southern blot analysis of the λ-clones identified a 2.2-kb BamHI fragment containing rpoA, which was cloned into pBluescript SK(−) to create pMP1. To obtain the sequence of the BamHI fragment, deletion fragments of EcoRV- and KpnI-digested pMP1 were generated with exonuclease III as described previously (56) and sequenced. A primer (5′-S11) based on the 5′ sequence of the 2.2-kb BamHI insert was used in Southern blot analysis to identify a 3.3-kb HindIII fragment, and this fragment was cloned into pBluescript SK(−) to create pMP2, which contained DNA upstream of rpoA. To obtain additional upstream sequence, we used chromosome walking. Library screening and Southern blot analysis were performed using digoxigenin (DIG) detection according to the manufacturer's directions (Boehringer Mannheim).
Purification of RNAP α.
A 250-ml culture of DH5α containing pMP21 and a 150-ml culture of DH5α containing pMP19a were induced at an A600 of 0.4 with 0.002% arabinose for 3 and 4 h, respectively. Cells were harvested by centrifugation (5 min at 5,000 × g), resuspended in lysis buffer according to manufacturer's directions (Qiagen, Inc.), and broken by three passages through a BioNeb cell and DNA disrupter (Glas-Col). Six-His-tagged α and αΔ254 were purified on Ni+2-nitrilotriacetic acid Superflow in batch under native and denaturing conditions, respectively, according to the manufacturer's directions (Qiagen, Inc.). Protein fractions were concentrated in an Ultrafree-NMWL 30K centrifugal filter (Millipore) and stored with 50% glycerol (25). E. coli six-His-tagged α was purified under denaturing conditions on a Ni+2 spin column according to the manufacturer's directions (Qiagen, Inc.). Protein concentrations were determined with the Bio-Rad protein assay. Samples were fractionated by SDS-12.5% PAGE and visualized with Coomassie blue 250 (Sigma).
Reconstitution of RNAP and in vitro transcription.
Overexpression, purification, and reconstitution of RNAP holoenzymes were performed as described previously (61). E. coli α was replaced with purified S. meliloti six-His-tagged wild-type α or αΔ254. The activities of the wild-type and hybrid holoenzymes were normalized on the RNA I and lacUV5 (pRLG 593) promoters. Reaction mixtures contained supercoiled plasmid DNA (20 ng); 10 mM Tris-Cl (pH 7.9); 10 mM MgCl2; 1 mM dithiothreitol; 100 μg of bovine serum albumin/ml; 200 μM ATP, GTP, and CTP; 10 μM UTP; 4 μCi of [32P]UTP (NEN); and 100 mM KCl. Fis-dependent transcription was measured in the same buffer but containing 170 mM KCl. Fis (200 nM final concentration) was added 15 min before addition of RNAP. Transcription was initiated by addition of RNAP and terminated by addition of stop solution (53) after 15 min at 22°C. Final RNAP concentrations in reaction mixtures were 16 (E. coli RNAP), 11 (E. coli RNAP with S. meliloti α), 26 (E. coli RNAP with S. meliloti α Δ254), and 7 nM (S. meliloti RNAP). Samples were electrophoresed on 5.5% polyacrylamide-7 M urea gels and quantified by phosphorimaging.
Nucleotide sequence accession number.
The nucleotide sequence of the rpoA region has been deposited in the GenBank database under accession no. AF317474.
RESULTS AND DISCUSSION
Isolation of rpoA and surrounding macromolecular-synthesis genes.
Oligonucleotides based on the N-terminal sequences of peptides generated from S. meliloti α were used to obtain PCR fragments containing the partial ORF of S. meliloti rpoA. The PCR fragments were used to screen a wild-type S. meliloti λ-library for clones containing rpoA and surrounding genes. DNA sequencing and BLAST analysis of these clones yielded ORFs that have significant homology to the genes for SecY (secY), adenylate kinase (adk), ribosomal protein S13 (rpsM), ribosomal protein S11 (rpsK), RNAP α (rpoA), and ribosomal protein L17 (rplQ) (Fig. 1). Subsequent sequence determination of the S. meliloti chromosome confirmed our data (14, 27).
FIG. 1.
Genetic organization of the rpoA region in S. meliloti. Sites for restriction enzymes mentioned in Materials and Methods are indicated. ORFs, direction of transcription, and protein names are indicated. The stem-loop structure indicates a proposed transcriptional terminator.
The genetic organization in the immediate area of rpoA is partially conserved among several bacterial species. rpsD, which encodes the S4 protein, is present immediately upstream of rpoA in Bordetella pertussis, Pseudomonas aeruginosa, and E. coli and has been implicated in translational regulation of the operon that includes rpoA (8, 15, 16, 59). However, rpsD is not part of this operon in S. meliloti, B. subtilis, Mesorhizobium loti, or Rickettsia prowazekii (3, 11, 37), indicating that S4 may not regulate this operon in these species. The second gene in this region in S. meliloti, adk, which encodes adenylate kinase, is not found in the rpoA region in most bacterial species for which this region has been sequenced (30, 31, 49). Based both on comparisons with the DNA sequences for E. coli and B. subtilis and on the lack of a predicted transcriptional terminator between any of the genes we sequenced, we hypothesize that at least some S. meliloti rpoA transcription must be initiated from a promoter upstream of secY, which is part of the spc operon (16, 60) and which is probably cotranscribed with ribosomal protein genes. Finally, we suggest that the gene coding for L17 is the last gene in the operon, because a predicted rho-independent terminator lies 23 nucleotides downstream of the L17 gene stop codon (Fig. 1). The next predicted ORF, which lies 2.4 kb downstream, encodes a putative serine protease and is not found in the rpoA regions of other bacteria (27).
Deduced amino acid sequence of α.
Figure 2 illustrates the sequences of the α subunits from S. meliloti and E. coli. The sequence comparison indicates that the α-domain structure is conserved. RNAP αNTD residues 45, 48, and 80, which are involved in the interaction with the β subunit, and residues 86, 173, 180, and 200, which are involved in the interaction with β′, are all conserved between E. coli and S. meliloti (33). In addition, the amino acids in the αCTD essential for UP element recognition and for the interaction with transcriptional activator Fis are conserved between E. coli and S. meliloti (1, 25, 42).
FIG. 2.
Comparison of amino acid sequence of S. meliloti (S.m.) RNAP α with that from E. coli (E.c.) (49). Dashes, gaps introduced for alignment; ∼, amino acids in the linker region; −, position of the αΔ254 deletion. Amino acid residues identical in S. meliloti α and E. coli α are indicated between the two sequences; +, conservative replacements. Amino acids essential for the αCTD interaction with the UP element are in boldface, and amino acids essential for interaction with Fis are underlined.
Purification of α.
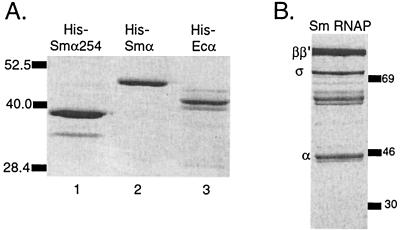
To facilitate in vitro transcription studies, we cloned the rpoA ORF into a vector containing the E. coli arabinose promoter. This vector adds 62 amino acids including a hexahistidine tag to the NH2 terminus of S. meliloti α. Previous studies have established that the N-terminal domain of E. coli α forms a stable polypeptide capable of assembling into functional RNAP (32, 34) but defective in recognizing the UP element and in interacting with certain transcriptional activators. Therefore, we also constructed a C-terminally truncated version of the product of S. meliloti rpoA in which amino acids 1 to 254 were removed (αΔ254). After induction by arabinose, six-His-tagged α and αΔ254 were purified (see Materials and Methods; Fig. 3A, lanes 1 and 2). For purposes of comparison, we also purified six-His-tagged E. coli α (61) (Fig. 3A, lane 3). Six-His-tagged S. meliloti α has an apparent molecular mass of 46 kDa, and untagged S. meliloti α has an apparent molecular mass of 43 kDa, both larger than the predicted molecular mass of 37.2 kDa and larger than six-His-tagged E. coli α (Fig. 3) (23). The purified polypeptides cross-reacted with an antibody to E. coli α (data not shown).
FIG. 3.
Analysis of six-His-tagged purified α-subunits resolved by SDS-PAGE and stained with Coomassie blue R250. (A) S. meliloti (Sm) αΔ254 purified in batch on Ni+2 resin under native conditions from cells containing pMP21 (lane 1), six-His-tagged S. meliloti α purified in batch on Ni+2 resin under denaturing conditions from cells containing pMP19a (lane 2), and six-His-tagged E. coli α purified under denaturing conditions on a Ni+2 spin column from cells containing pHTT7α (lane 3). Six-His-tagged S. meliloti α has a larger molecular mass than untagged α due to the addition of 62 amino acids at the N terminus of the six-His-tagged protein (see Materials and Methods). (B) RNAP isolated from S. meliloti. Size markers are in kilodaltons.
S. meliloti α assembles into active RNAP in E. coli.
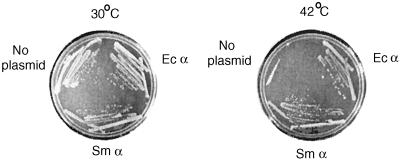
To facilitate an understanding of the role of the αCTD in transcription activation of S. meliloti RNAP, we cloned versions of S. meliloti rpoA encoding full-length and C-terminally truncated (αΔ254) proteins into a broad-host-range vector for examination in S. meliloti. Interestingly, while the full-length clone could be introduced into S. meliloti, αΔ254 was deleterious to cell growth (data not shown). Therefore, we utilized E. coli as a heterologous system to examine transcription by S. meliloti RNAP in vitro. First, we showed that S. meliloti rpoA functions in E. coli by demonstrating that it complements an E. coli rpoA temperature sensitive mutation for growth because of a defect in RNAP assembly (38). Specifically, we transformed E. coli rpoA112(Ts) with a plasmid constitutively expressing six-His-tagged S. meliloti α or wild-type E. coli α (Fig. 4). We observed growth of all of the strains at the permissive temperature, 30°C. At 42°C, the nonpermissive temperature, the strains expressing S. meliloti or E. coli α were viable, even though the two proteins are only 51% identical, while the strain with only the host-encoded α was not viable. We note that while the temperature-sensitive E. coli mutant expressing S. meliloti α plated with high efficiency, forming independent single colonies on plates, it formed smaller colonies than the cells containing the plasmid encoding wild-type α. We conclude that S. meliloti α can assemble into a fully functional holoenzyme with the other E. coli RNAP subunits and is able to replace E. coli α for all functions essential for viability.
FIG. 4.
Complementation of the E. coli rpoA mutant with S. meliloti rpoA. Strains carrying rpoA112(Ts) either alone or with plasmids coding for E. coli (Ec) α or S. meliloti (Sm) α were streaked on Luria-Bertani agar and grown for 24 h at the indicated temperatures.
S. meliloti α assembles into functional RNAP in vitro.
We next reconstituted RNAP from S. meliloti α and E. coli β, β′, and σ in order to address whether hybrid RNAP containing only S. meliloti α subunits is functional and to study transcription by RNAP containing S. meliloti α in vitro. Recovery of the active holoenzyme was completely dependent on exogenously added purified α (data not shown), indicating that potential contamination of the other E. coli subunits with E. coli α could not account for the observed transcription. Since the amount of S. meliloti α required for assembly into functional RNAP was approximately the same as for E. coli α (see Materials and Methods), the assembly determinants in S. meliloti α apparently are conserved in the two species. We assayed the reconstituted enzymes (and, as a control, S. meliloti RNAP holoenzyme) for their abilities to initiate transcription, to respond to an UP element, and to interact with well-characterized transcriptional activator Fis. Because previous studies of E. coli have demonstrated that the αCTD is essential for both UP element function and Fis-mediated transcription (10, 21, 25, 53), we also tested RNAP reconstituted with S. meliloti αΔ254. As a control, we tested the activity of RNAP purified from S. meliloti in the same assays to address whether S. meliloti α in native RNAP functioned similarly to S. meliloti α in the hybrid RNAP. We normalized the activities of the RNAP preparations on the lacUV5 and RNA I promoters, two promoters that do not require the function of the αCTD (Fig. 5B, lanes 1 to 4).
FIG. 5.
In vitro transcription with reconstituted E. coli (E), E. coli containing either six-His-tagged S. meliloti α (E/S), or αΔ254 (E/SΔ), or S. meliloti RNAP (S). (A) rrnB P1 promoter used for in vitro transcription studies contains the Fis binding site, UP element, and the core −10 and −35 elements. Insertion of promoter fragments into the plasmid vector gives rise to a 150-nucleotide RNA fragment terminating at the rrnB T1 and T2 terminators. (B) Normalization of E. coli, S. meliloti, and hybrid RNAPs on the lacUV5 and RNA I promoters. RNA I is a control promoter from the plasmid vector. (C) E. coli, S. meliloti, and hybrid RNAPs activate UP element-mediated transcription. Templates carrying rrnB P1 promoters with (+) and without (−) the UP element were transcribed, and the products were analyzed by denaturing gel electrophoresis. (D) E. coli, S. meliloti, and hybrid RNAPs are activated by Fis. The template containing the rrnB P1 UP element and Fis site was incubated with the indicated RNAP with or without Fis (200 nM). (E) Activation by Fis depends on the αCTD. Lanes 1 and 2, RNAP containing intact S. meliloti α (E/S); lanes 3 and 4, RNAP containing αΔ254 (E/SΔ). Reactions were performed under conditions in which basal transcription could be detected in the absence of αCTD (see text).
To test whether S. meliloti α supports UP element- and/or Fis-dependent transcription, we assayed transcription at the E. coli rrnB P1 promoter (Fig. 5A). The presence of the UP element stimulated transcription by E. coli RNAP containing S. meliloti α 10-fold (Fig. 5C, lanes 3 and 4) under conditions where transcription by the native E. coli and S. meliloti RNAPs was stimulated 14- and 13-fold, respectively (Fig. 5C, lanes 1, 2, 5, and 6). As observed previously with E. coli RNAP lacking the αCTD (53), transcription from the promoter containing the UP element was extremely weak with the S. meliloti αΔ254 RNAP (Fig. 5C, lanes 7 and 8). Thus, S. meliloti αCTD contains the determinants required for recognition of the rrnB P1 UP element. Since S. meliloti rrn promoters contain sequences homologous to E. coli consensus UP elements (21, 26) and since the DNA-binding determinants in E. coli αCTD are present in S. meliloti αCTD, it is likely that the UP element-α interaction is conserved in S. meliloti. Reconstituted E. coli RNAP, S. meliloti RNAP, and the hybrid RNAP carrying S. meliloti α were stimulated by Fis 5.1-, 4.8-, and 3.2-fold, respectively (Fig. 5D, lanes 1 to 6). The RNAP reconstituted with S. meliloti αΔ254 was less active than the other RNAP preparations under the high-salt conditions (170 mM KCl) used in this experiment (Fig. 5D, lanes 7 and 8), making it difficult to evaluate whether Fis can or cannot activate the enzyme lacking the αCTD. Therefore, the effect of Fis on the RNAP containing S. meliloti αΔ254 was also compared with that of the enzyme containing the full-length α under slightly more permissive conditions (100 mM KCl), where basal transcription is visible. The mutant RNAP was stimulated only 1.4-fold (Fig. 5E, lanes 3 and 4), while RNAP containing the intact S. meliloti α was stimulated 3.3-fold (Fig. 5E, lanes 1 and 2). Thus, we conclude that S. meliloti α interacts with Fis and that, as in E. coli (10), the interaction is mediated primarily by the αCTD.
These results demonstrate that S. meliloti α is competent for assembly with E. coli β, β′, and σ70; for UP element recognition; and for recognition by a transcription factor. We showed above that S. meliloti α complements E. coli α in vivo. Our results with the hybrid enzymes, formed in vitro in the absence of E. coli α, support the conclusion that complementation in vivo resulted from the function of S. meliloti α.
Summary.
We have isolated the region from S. meliloti encoding proteins SecY, Adk, S13, S11, α, and L17 and overexpressed and purified S. meliloti α and reconstituted it with the β, β′, and σ70 subunits from E. coli. In vivo assays of E. coli and in vitro assays using purified S. meliloti α demonstrate that S. meliloti α retains its critical functions as a hybrid enzyme with other E. coli RNAP subunits. The cloning and characterization of S. meliloti RNAP α thus provide new opportunities for studying S. meliloti transcription.
Acknowledgments
M.C.P. was supported by a NSF predoctoral fellowship. Additional funding for this work was provided by NIH GM30962 and by the Howard Hughes Medical Institute to S.R.L. and by GM37048 and a Hatch grant from the USDA to R.L.G.
We thank members of our laboratory for helpful discussions, D. Keating for critical reading of the manuscript, R. Ebright for his gift of E. coli anti-α antibody, and K. Severinov for his gift of HN317ts112, obtained from A. Ishihama.
REFERENCES
- 1.Aiyar, S. E., S. M. McLeod, W. Ross, C. A. Hirvonen, M. S. Thomas, R. C. Johnson, and R. L. Gourse. 2001. Architecture of Fis-activated transcription complexes at the Escherichia coli rrnB P1 and rrnE P1 promoters. J. Mol. Biol. 316:510-516. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., and D. J. Lipman. 1990. Protein database searches for multiple alignments. Proc. Natl. Acad. Sci. USA 87:5509-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, S. G., A. Zomorodipour, J. O. Andersson, T. Sicheritz-Ponten, U. C. Alsmark, R. M. Podowski, A. K. Naslund, A. S. Eriksson, H. H. Winkler, and C. G. Kurland. 1998. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396:133-140. [DOI] [PubMed] [Google Scholar]
- 4.Artsimovitch, I., V. Svetlov, L. Anthony, R. R. Burgess, and R. Landick. 2000. RNA polymerases from Bacillus subtilis and Escherichia coli differ in recognition of regulatory signals in vitro. J. Bacteriol. 182:6027-6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae, Y. M., E. Holmgren, and I. P. Crawford. 1989. Rhizobium meliloti anthranilate synthase gene: cloning, sequence, and expression in Escherichia coli. J. Bacteriol. 171:3471-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett, M. J., D. Y. Hung, A. Reisenauer, L. Shapiro, and S. R. Long. 2001. A homolog of the CtrA cell cycle regulator is present and essential in Sinorhizobium meliloti. J. Bacteriol. 183:3204-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck, C., R. Marty, S. Klausli, H. Hennecke, and M. Gottfert. 1997. Dissection of the transcription machinery for housekeeping genes of Bradyrhizobium japonicum. J. Bacteriol. 179:364-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedwell, D., G. Davis, M. Gosink, L. Post, M. Nomura, H. Kestler, J. M. Zengel, and L. Lindahl. 1985. Nucleotide sequence of the alpha ribosomal protein operon of Escherichia coli. Nucleic Acids Res. 13:3891-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blatter, E. E., W. Ross, H. Tang, R. L. Gourse, and R. H. Ebright. 1994. Domain organization of RNA polymerase alpha subunit: C-terminal 85 amino acids constitute a domain capable of dimerization and DNA binding. Cell 78:889-896. [DOI] [PubMed] [Google Scholar]
- 10.Bokal, A. J., W. Ross, T. Gaal, R. C. Johnson, and R. L. Gourse. 1997. Molecular anatomy of a transcription activation patch: FIS-RNA polymerase interactions at the Escherichia coli rrnB P1 promoter. EMBO J. 16:154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boylan, S. A., J. W. Suh, S. M. Thomas, and C. W. Price. 1989. Gene encoding the alpha core subunit of Bacillus subtilis RNA polymerase is cotranscribed with the genes for initiation factor 1 and ribosomal proteins B, S13, S11, and L17. J. Bacteriol. 171:2553-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgess, R. R., and J. J. Jendrisak. 1975. A procedure for the rapid, large-scale purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry 14:4634-4638. [DOI] [PubMed] [Google Scholar]
- 13.Cabanes, D., P. Boistard, and J. Batut. 2000. Identification of Sinorhizobium meliloti genes regulated during symbiosis. J. Bacteriol. 182:3632-3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capela, D., F. Barloy-Hubler, J. Gouzy, G. Bothe, F. Ampe, J. Batut, P. Boistard, A. Becker, M. Boutry, E. Cadieu, S. Dreano, S. Gloux, T. Godrie, A. Goffeau, D. Kahn, E. Kiss, V. Lelaure, D. Masuy, T. Pohl, D. Portetelle, A. Puhler, B. Purnelle, U. Ramsperger, C. Renard, P. Thebault, M. Vandenbol, S. Weidner, and F. Galibert. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc. Natl. Acad. Sci. USA 98:9877-9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbonetti, N. H., T. M. Fuchs, A. A. Patamawenu, T. J. Irish, H. Deppisch, and R. Gross. 1994. Effect of mutations causing overexpression of RNA polymerase alpha subunit on regulation of virulence factors in Bordetella pertussis. J. Bacteriol. 176:7267-7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerretti, D. P., D. Dean, G. R. Davis, D. M. Bedwell, and M. Nomura. 1983. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 11:2599-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cleveland, D. W., S. G. Fischer, M. W. Kirschner, and U. K. Laemmli. 1977. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J. Biol. Chem. 252:1102-1106. [PubMed] [Google Scholar]
- 18.Das, A., J. Urbanowski, H. Weissbach, J. Nestor, and C. Yanofsky. 1983. In vitro synthesis of the tryptophan operon leader peptides of Eschericia coli, Serratia marcescens, and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 80:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dusha, I., J. Schroder, P. Putnoky, Z. Banfalvi, and A. Kondorosi. 1986. A cell-free system from Rhizobium meliloti to study the specific expression of nodulation genes. Eur. J. Biochem. 160:69-75. [DOI] [PubMed] [Google Scholar]
- 20.Ebright, R. H., and S. Busby. 1995. The Escherichia coli RNA polymerase alpha subunit: structure and function. Curr. Opin. Genet. Dev. 5:197-203. [DOI] [PubMed] [Google Scholar]
- 21.Estrem, S. T., W. Ross, T. Gaal, Z. W. Chen, W. Niu, R. H. Ebright, and R. L. Gourse. 1999. Bacterial promoter architecture: subsite structure of UP elements and interactions with the carboxy-terminal domain of the RNAP polymerase α subunit. Genes Dev. 13:2134-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer, H. M. 1994. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 58:352-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher, R. F., H. L. Brierley, J. T. Mulligan, and S. R. Long. 1987. Transcription of Rhizobium meliloti nodulation genes. Identification of a nodD transcription initiation site in vitro and in vivo. J. Biol. Chem. 262:6849-6855. [PubMed] [Google Scholar]
- 24.Fisher, R. F., T. T. Egelhoff, J. T. Mulligan, and S. R. Long. 1988. Specific binding of proteins from Rhizobium meliloti cell-free extracts containing NodD to DNA sequences upstream of inducible nodulation genes. Genes Dev. 2:282-293. [DOI] [PubMed] [Google Scholar]
- 25.Gaal, T., W. Ross, E. E. Blatter, H. Tang, X. Jia, V. V. Krishnan, N. Assa-Munt, R. H. Ebright, and R. L. Gourse. 1996. DNA-binding determinants of the alpha subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev. 10:16-26. [DOI] [PubMed] [Google Scholar]
- 26.Galibert, F., F. Barloy-Hubler, D. Capela, and J. Gouzy. 2000. Sequencing the Sinorhizobium meliloti genome. DNA Seq. 11:207-210. [DOI] [PubMed] [Google Scholar]
- 27.Galibert, F., T. M. Finan, S. R. Long, A. Puhler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 28.Glass, R. E., and R. S. Hayward. 1993. Bacterial RNA polymerases: structural and functional relationships. World J. Microbiol. Biotechnol. 9:403-413. [DOI] [PubMed] [Google Scholar]
- 29.Gourse, R. L., W. Ross, and T. Gaal. 2000. UPs and downs in bacterial transcription initiation: the role of the alpha subunit of RNA polymerase in promoter recognition. Mol. Microbiol. 37:687-695. [DOI] [PubMed] [Google Scholar]
- 30.Gu, L., W. M. Wenman, M. Remacha, R. Meuser, J. Coffin, and R. Kaul. 1995. Chlamydia trachomatis RNA polymerase alpha subunit: sequence and structural analysis. J. Bacteriol. 177:2594-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gutierrez, J. A., and L. N. Csonka. 1995. Isolation and characterization of adenylate kinase (adk) mutations in Salmonella typhimurium which block the ability of glycine betaine to function as an osmoprotectant. J. Bacteriol. 177:390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayward, R. S., K. Igarashi, and A. Ishihama. 1991. Functional specialization within the alpha-subunit of Escherichia coli RNA polymerase. J. Mol. Biol. 221:23-29. [DOI] [PubMed] [Google Scholar]
- 33.Heyduk, T., E. Heyduk, K. Severinov, H. Tang, and R. Ebright. 1996. Determinants of RNA polymerase α subunit for interaction with β, β′, and σ subunits: hydroxyl-radical protein footprinting. Proc. Natl. Acad. Sci. USA 93:10162-10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Igarashi, K., and A. Ishihama. 1991. Bipartite functional map of the E. coli RNA polymerase alpha subunit: involvement of the C-terminal region in transcription activation by cAMP-CRP. Cell 65:1015-1022. [DOI] [PubMed] [Google Scholar]
- 35.Ishihama, A. 1992. Role of the RNA polymerase alpha subunit in transcription activation. Mol. Microbiol. 6:3283-3288. [DOI] [PubMed] [Google Scholar]
- 36.Ishihama, A., N. Shimamoto, H. Aiba, K. Kawakami, H. Nashimoto, A. Tsugawa, and H. Uchida. 1980. Temperature-sensitive mutations in the alpha subunit gene of Escherichia coli RNA polymerase. J. Mol. Biol. 137:137-150. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 38.Kawakami, K., and A. Ishihama. 1980. Defective assembly of ribonucleic acid polymerase subunits in a temperature-sensitive α-subunit mutant of Escherichia coli. Biochemistry 19:3491-3495. [DOI] [PubMed] [Google Scholar]
- 39.Lohrke, S. M., S. Nechaev, H. Yang, K. Severinov, and S. J. Jin. 1999. Transcriptional activation of Agrobacterium tumefaciens virulence gene promoters in Escherichia coli requires the A. tumefaciens rpoA gene, encoding the alpha subunit of RNA polymerase. J. Bacteriol. 181:4533-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lohrke, S. M., H. Yang, and S. Jin. 2001. Reconstitution of acetosyringone-mediated Agrobacterium tumefaciens virulence gene expression in the heterologous host Escherichia coli. J. Bacteriol. 183:3704-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowe, P. A., D. A. Hager, and R. R. Burgess. 1979. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry 18:1344-1352. [DOI] [PubMed] [Google Scholar]
- 42.McLeod, S. M., S. E. Aiyar, R. L. Gourse, and R. C. Johnson. 2002. The C-terminal domains of the RNA polymerase alpha subunits: contact site with Fis and localization during co-activation with CRP at the Escherichia coli proP P2 promoter. J. Mol. Biol. 316:517-529. [DOI] [PubMed] [Google Scholar]
- 43.Mencia, M., M. Monsalve, F. Rojo, and M. Salas. 1996. Transcription activation by phage phi29 protein p4 is mediated by interaction with the alpha subunit of Bacillus subtilis RNA polymerase. Proc. Natl. Acad. Sci. USA 93:6616-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulligan, J. T., and S. R. Long. 1985. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc. Natl. Acad. Sci. USA 82:6609-6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen, B. L., and L. R. Brown. 1985. Purification and subunit characterization of Rhizobium meliloti RNA polymerase. J. Bacteriol. 162:645-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niu, W., Y. Kim, G. Tau, T. Heyduk, and R. H. Ebright. 1996. Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell 87:1123-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oke, V., B. G. Rushing, E. J. Fisher, M. Moghadam-Tabrizi, and S. R. Long. 2001. Identification of the heat-shock sigma factor RpoH and a second RpoH-like protein in Sinorhizobium meliloti. Microbiology 147:2399-2408. [DOI] [PubMed] [Google Scholar]
- 48.Ono, Y., H. Mitsui, T. Sato, and K. Minamisawa. 2001. Two RpoH homologs responsible for the expression of heat shock protein genes in Sinorhizobium meliloti. Mol. Gen. Genet. 264:902-912. [DOI] [PubMed] [Google Scholar]
- 49.Post, L. E., G. D. Strycharz, M. Nomura, H. Lewis, and P. P. Dennis. 1979. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc. Natl. Acad. Sci. USA 76:1697-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rao, L., W. Ross, J. A. Appleman, T. Gaal, S. Leirmo, P. J. Schlax, M. T. Record, Jr., and R. L. Gourse. 1994. Factor independent activation of rrnB P1. An “extended” promoter with an upstream element that dramatically increases promoter strength. J. Mol. Biol. 235:1421-1435. [DOI] [PubMed] [Google Scholar]
- 51.Rhodius, V. A., and S. J. Busby. 1998. Positive activation of gene expression. Curr. Opin. Microbiol. 1:152-159. [DOI] [PubMed] [Google Scholar]
- 52.Ronson, C. W., B. T. Nixon, L. M. Albright, and F. M. Ausubel. 1987. Rhizobium meliloti ntrA (rpoN) gene is required for diverse metabolic functions. J. Bacteriol. 169:2424-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross, W., K. K. Gosink, J. Salomon, K. Igarashi, C. Zou, A. Ishihama, K. Severinov, and R. L. Gourse. 1993. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science 262:1407-1413. [DOI] [PubMed] [Google Scholar]
- 54.Ross, W., J. F. Thompson, J. T. Newlands, and R. L. Gourse. 1990. E. coli FIS protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 9:3733-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rushing, B. G., and S. R. Long. 1995. Cloning and characterization of the sigA gene encoding the major sigma subunit of Rhizobium meliloti. J. Bacteriol. 177:6952-6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 57.Schaller, H., C. Gray, and K. Herrmann. 1975. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc. Natl. Acad. Sci. USA 72:737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steffen, P., and A. Ullman. 1998. Hybrid Bordetella pertussis-Escherichia coli RNA polymerases: selectivity of promoter activation. J. Bacteriol. 180:1567-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 60.Suh, J. W., S. A. Boylan, and C. W. Price. 1986. Gene for the alpha subunit of Bacillus subtilis RNA polymerase maps in the ribosomal protein gene cluster. J. Bacteriol. 168:65-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang, H., Y. Kim, K. Severinov, A. Goldfarb, and R. H. Ebright. 1996. Escherichia coli RNA polymerase holoenzyme: rapid reconstitution from recombinant alpha, beta, beta′, and sigma subunits. Methods Enzymol. 273:130-140. [DOI] [PubMed] [Google Scholar]
- 62.Trzebiatowski, J. R., D. M. Ragatz, and F. J. de Bruijn. 2001. Isolation and regulation of Sinorhizobium meliloti 1021 loci induced by oxygen limitation. Appl. Environ. Microbiol. 67:3728-3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Willits, M. 1995. Molecular and biochemical characterizaton of sulfate activation pathways in Rhizobium meliloti. Ph.D. thesis. Stanford University, Stanford, Ca.
- 64.Zhang, G., and S. A. Darst. 1998. Structure of the Escherichia coli RNA polymerase alpha subunit amino-terminal domain. Science 281:262-266. [DOI] [PubMed] [Google Scholar]