Abstract
We found that the biosynthesis of actinorhodin (Act), undecylprodigiosin (Red), and calcium-dependent antibiotic (CDA) are dramatically activated by introducing certain mutations into the rpoB gene that confer resistance to rifampin to Streptomyces lividans 66, which produces less or no antibiotics under normal growth conditions. Activation of Act and/or Red biosynthesis by inducing mutations in the rpoB gene was shown to be dependent on the mutation's position and the amino acid species substituted in the β-subunit of the RNA polymerase. Mutation analysis identified 15 different kinds of point mutations, which are located in region I, II, or III of the rpoB gene and, in addition, two novel mutations (deletion of nucleotides 1287 to 1289 and a double substitution at nucleotides 1309 and 1310) were also found. Western blot analyses and S1 mapping analyses demonstrated that the expression of actII-ORF4 and redD, which are pathway-specific regulatory genes for Act and Red, respectively, was activated in the mutants able to produce Act and Red. The ActIV-ORF1 protein (an enzyme for Act biosynthesis) and the RedD protein were produced just after the upregulation of ActII-ORF4 and RedZ, respectively. These results indicate that the mutation in the rpoB gene of S. lividans, resulting in the activation of Act and/or Red biosynthesis, functions at the transcription level by activating directly or indirectly the key regulatory genes, actII-ORF4 and redD. We propose that the mutated RNA polymerase may function by mimicking the ppGpp-bound form in activating the onset of secondary metabolism in Streptomyces.
Members of the genus Streptomyces produce most of the natural product antibiotics used clinically today. The activation of antibiotic production, often coupled to morphological development, involves many different pathways in the same organism (reviewed by Chater [8]; also see reference 35 for a brief review). Multiple and coordinated regulatory mechanisms controlling antibiotic biosynthesis are still poorly understood. Streptomyces coelicolor A3(2), the best genetically studied streptomycete, produces four biochemically and genetically distinct antibiotics: actinorhodin (Act), undecylprodigiosin (Red), methylenomycin, and calcium-dependent antibiotic (CDA) (reviewed by Hopwood et al. [19] and Chater and Bibb [10]). The gene clusters responsible for the production of Act, Red, methylenomycin, and CDA have been cloned and characterized (9, 12, 15, 29). Linked to these gene clusters, various pathway-specific regulatory genes (actII-ORF4, redD, and redZ, etc.) have been identified, and among these actII-ORF4 and redD have been shown to regulate the Act and Red biosynthesis genes, respectively. Transcription of redD has in turn been shown to be regulated by another regulatory gene, redZ (15, 31, 43). These results taken together indicate that these pathway-specific regulatory genes act as positive regulators for their respective biosynthesis genes. For example, strains carrying mutant genes that fail to accumulate regulatory gene transcripts also fail to cosynthesize Act or Red. Moreover, upregulation of actII-ORF4 and redD resulted in the upregulation of Act and Red, respectively (4, 13). In addition, many pleiotropic regulatory genes (e.g., absA, absB, afsR, afsR2, and abaA) in S. coelicolor have been found to regulate antibiotic biosynthesis (2, 14, 20).
Streptomyces lividans 66, a species closely related to S. coelicolor A3(2), carries the entire clusters for Act and Red in its genome but normally produces less or no Act and Red throughout the whole-cell cycle, although this strain is known to produce abundant amounts of their pigmented antibiotics under certain growth conditions. Recent studies revealed that the cutRS signal transduction system and LysR-type transcriptional regulator negatively regulate Act biosynthesis in S. lividans (7, 30). It was found that afsR2, which encodes a 63-amino-acid protein, stimulates Act and Red production in S. lividans (27, 42). How to activate this silent antibiotic biosynthetic gene cluster can give some clues about the regulation system for antibiotic production. We previously reported that introduction of the str mutation, which confers resistance to streptomycin, could activate Act production in S. lividans (38). Introducing the str mutation into other Streptomyces species was also effective in improving antibiotic production (21). Moreover, the str mutation could also suppress the detrimental effect of relA and relC on antibiotic production due to the failure to accumulate ppGpp (34, 38). Recently, we found that the acquisition of resistance to rifampin confers the ability to produce Act in relA and relC mutants of S. coelicolor, which were both defective in Act production due to rel mutations (J. Xu, Y. Tozawa, and K. Ochi, unpublished data). In a previous study (22), we demonstrated that Act biosynthesis could be enhanced when a rif mutation (conferring resistance to rifampin) is introduced into the wild-type strain of S. coelicolor A3(2). A variety of mutations in the rpoB gene, which encodes the RNA polymerase β-subunit, are known to confer resistance to rifampin. Here, we report that certain mutations, when introduced into the RNA polymerase β subunit, can activate the genes for antibiotic production in S. lividans. The mechanism for this activation was investigated by Western blotting and S1 mapping analysis.
MATERIALS AND METHODS
Bacterial strains and preparation of mutants.
The wild-type strain 1326 of S. lividans 66 and the rifampin-resistant mutants used in this study are listed in Table 1. Spontaneous rifampin-resistant (rif) mutants were obtained from colonies that grew within 7 days after wild-type spores were spread on GYM agar containing 100 or 200 μg of rifampin/ml. The mutants used for subsequent study were selected after single-colony isolation was performed.
TABLE 1.
Screening and characterization of rif mutants of S. lividans 66
| Straina | Relevant genotype | Frequency of mutants with the same mutation (no. with mutation/total tested) | Resistance level (μg/ml) to rifampinb | Production of antibiotics in R4 liquid culture
|
|
|---|---|---|---|---|---|
| Red (mg/g)c | Act (OD633) | ||||
| S. lividans 66 | Prototrophic wild type | 10 | 0.84 | 0.08 | |
| HY-1 | rif-1 | 8/60 | 400 | 0.79 | 0.53 |
| HR-1 | rif-2 | 3/60 | 400 | 0.36 | 0.17 |
| HC-1 | rif-3 | 2/60 | 400 | 1.39 | 0.08 |
| HN-1 | rif-4 | 1/60 | 400 | 1.28 | 0.09 |
| HL-1 | rif-5 | 2/60 | 400 | 0.95 | 0.23 |
| CT-1 | rif-6 | 7/60 | 200 | 1.20 | 0.18 |
| NY-1 | rif-7 | 4/60 | 400 | 1.07 | 0.05 |
| LP-1 | rif-8 | 1/60 | 300 | 0.40 | 0.02 |
| EN-1 | rif-9 | 2/60 | 400 | 4.36 | 0.08 |
| DV-1 | rif-10 | 2/60 | 400 | 2.03 | 0.35 |
| DE-1 | rif-11 | 4/60 | 300 | 0.85 | 0.24 |
| DN-1 | rif-12 | 2/60 | 400 | 1.05 | 0.12 |
| DG-1 | rif-13 | 3/60 | 400 | 0.82 | 0.08 |
| SL-1 | rif-14 | 2/60 | 400 | 0.48 | 0.16 |
| SP-1 | rif-15 | 1/60 | 300 | 0.68 | 0.73 |
| RH-1 | rif-16 | 3/60 | 400 | 1.03 | 0.15 |
| RC-1 | rif-17 | 3/60 | 300 | 0.86 | 0.38 |
All mutant strains isolated in this study were spontaneously generated rifampin-resistant mutants from S. lividans 66.
Determined after 4 days of cultivation on GYM agar.
That is, one milligram of Red per gram of dry mycelia.
Media and culture conditions.
GYM, R4, ONA, ONB, and SMMS media were described previously (26, 32, 38). Culture conditions were as reported previously for S. coelicolor (22). Determination of antibiotic productivity was always performed by using triplicated culture flasks, and the mean values of the three samples were presented in Table 1 and Fig. 2. The reproducibility of the results was confirmed at least by two separate experiments (for Table 1 and Fig. 2 to 4).
FIG. 2.
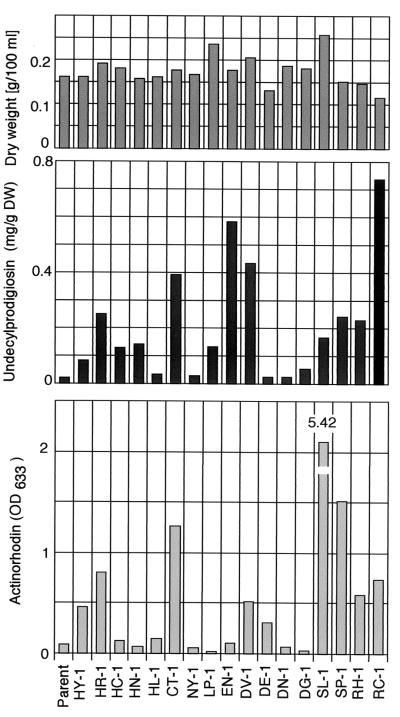
Act and Red production by S. lividans 66 (parental strain) and the rif mutants. Incubation was carried out in GYM liquid medium at 30°C for 6 days, and Act and Red levels were determined as described in Materials and Methods.
FIG. 4.
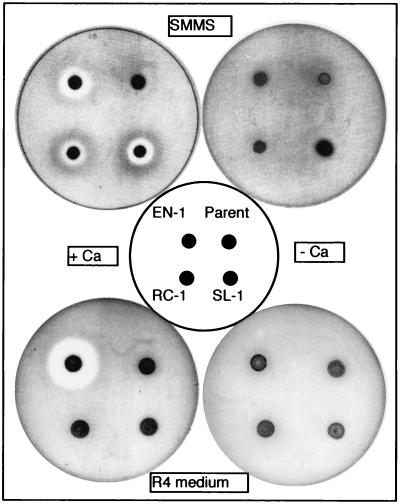
Determination of CDA produced by rif mutants (EN-1, RC-1, and SL-1) of S. lividans 66. The strains were grown on SMMS and R4 agar plates at 30°C for 48 h. Details of the CDA assay conditions are described in Materials and Methods.
Assays for antibiotics.
Act was assayed as previously described (22). When cultivation was carried out with GYM medium, 10 ml of 1 N KOH was added to every 100 ml of culture broth. After standing for 1 h at room temperature, the culture was filtered, and the optical density at 633 nm (OD633) of each filtrate was determined. For Red, 100 ml of culture broth was filtered, and the mycelial pellet was washed and dried under vacuum conditions. After being weighed, the dried mycelia were first extracted with 0.1 N KOH to dissolve the Act and then extracted with methanol (adjusted to pH 2) overnight at room temperature. The amount of Red was determined by measuring the OD530 (ɛ530 = 100,500) (26). The CDA was assayed according to the method of Kieser et al. (26). Then, 5 μl of spore suspension (containing ca. 105 spores) was spotted on R4 or SMMS agar plates, followed by incubation at 30°C for 48 h. Oxoid nutrient broth (10 ml) was inoculated with Staphylococcus aureus 209P, and the mixture was incubated at 30°C with shaking until the OD650 reached 0.7 to 0.8. For each culture plate, 0.5 ml of the S. aureus culture was added to 10 ml of soft nutrient agar (5 ml of Oxoid nutrient agar plus 5 ml of Oxoid nutrient broth) with or without 60 mM Ca(NO3)2. The mixture was used to overlay the S. lividans cultures. CDA production was detected as an inhibition zone of S. aureus after an overnight incubation at 30°C.
Mutation analysis of the rpoB gene.
The rpoB gene fragments in S. lividans 66 and the rif mutants were amplified by PCR by using the appropriate genomic DNA as templates, and the design of the synthetic oligonucleotide primers was based on the sequence for the S. coelicolor M145 rpoB gene (GenBank accession no. AL160431). Primers P1 (forward, 5′-CCGAGTTCACCAACAACGAGACC-3′) and P2 (reverse, 5′-CGATGACGAAGCGGTCCTCC-3′) were used to amplify a 1.2-kb fragment from nucleotides (nt) 374 to 1582. Primers P1, P2, and P3 (forward, 5′-GGCCGCTACAAGGTGAACAAGAAG-3′) were used as sequencing primers. Another fragment (nt 1453 to 2154) was amplified with primers P4 (forward, 5′-TCGCTCGCCTCGTACGGC-3′) and P5 (reverse, 5′-CTCGTAGTTGTGACCCTCCC-3′). PCR was performed with Taq polymerase (Takara LA-Taq) according to the manufacturer's instructions. A Perkin-Elmer-Cetus thermal cycler was used, and conditions were as follows: 5 min of preincubation at 96°C; followed by 30 cycles of 96°C for 0.3 min, 55°C for 0.2 min, and 72°C for 0.5 min; and final step at 72°C for 10 min. PCR products were directly sequenced by the dideoxy chain termination procedure by using the BigDye Terminator Cycle Sequencing kit (Perkin-Elmer/Applied Biosystems, Foster City, Calif.).
Western blot analysis.
Antibodies against S12, ActII-ORF4, ActIV-ORF1, RedZ, and RedD proteins were prepared by injecting purified recombinant proteins into rabbits intraperitoneally. After purification with conventional methods, these antibodies were used as primary antibodies at a dilution of 1:3,000, as described previously (24). Details of this antibody preparation will be reported elsewhere. The enhanced chemiluminescence Western blotting detection system for chemiluminescent detection was used as specified by the manufacturer (Amersham Pharmacia Biotech).
S1 nuclease protection assays.
RNA was extracted from cultures as described by Kieser et al. (26) by using Kirby method. For each S1 nuclease reaction, 50 μg of RNA was dried down with the appropriate 32P-labeled DNA probes (ca. 30,000 cpm) and hybridized overnight at 45°C in sodium-trichloroacetic acid buffer after denaturation at 65°C for 15 min. S1 nuclease digestions and analyses of RNA-protected fragments were performed as described previously (4). Uniquely end-labeled probes were generated by PCR as follows. For actII-ORF4, the primer 5′-GGTCCGCCCACAACTCCTC was labeled with [γ-32 P]ATP by using T4 polynucleotide kinase with the unlabeled primer 5′-GCCGTATCAGGAATGCCAGA and 1 μg of genomic DNA as a template. PCR conditions consisted of 30 cycles of 30 s at 95°C, 30 s at 58°C, and 50 s at 72°C in the presence of 5% glycerol. The resultant probe was 368 bp, which gave a protected fragment of 190 nt in S1 nuclease protection experiments. Probes for redZ and redD were made in a similar way but with the different set of primers. For redZ, labeled primer 5′-CCCAATATGTTGATTTCCACGC, unlabeled primer 5′-CTTCGTTTGCGTCGTTCAGTT and genomic DNA as a template produced a 367-bp product that yielded a protected fragment of 217 nt. For redD, labeled primer 5′-CACCAGTTCTTCGACCGACG and unlabeled primer 5′-AAGCCCCTCTCCAAGTGTGC produced a 480-bp product that yielded a protected fragment of 210 nt. Control experiments in which 50 μg of yeast tRNA replaced experimental total RNA samples were performed with each set of S1 nuclease protection assays to confirm that there were no signals. All of the S1 nuclease protection experiments were carried out twice with RNA isolated from independently cultured cells to ensure the reproducibility of the results.
Complementary experiment.
A 5.0-kb fragment from the NotI and BamHI digest of cosmid SCD82 (GenBank accession no. AL160431) that contains the intact rpoB gene of S. coelicolor M145 was cloned into the pV1 [a low-copy plasmid in Streptomyces spp. consisting of a pIJ941 sequence and a pBluescript SK(+) sequence (24)]. The replicative ligations were transformed into S. lividans rif mutants (EN-1 and SL-1). Streptomyces plasmids preparation and transformation were carried out as described by Kieser et al. (26).
RESULTS
Screening and mutation analysis of the rif mutants.
We first attempted to isolate a number of rif mutants, which developed spontaneously. When the spores of S. lividans 66 were spread and incubated on GYM agar containing 100 or 200 μg of rifampin/ml, spontaneous rif mutants developed after 4 to 7 days at a frequency of 10−7 to 10−9. Sixty mutants were randomly selected and examined for the production of Act and Red by using R4 and GYM media. Strikingly, >50% of the mutants tested exhibited a significantly elevated ability to produce Act and/or Red compared to the wild-type strain. In contrast, nearly 40% of the mutants showed the same or a reduced ability to produce Act and/or Red (data not shown). All of the mutants tested demonstrated a high level (20- to 40-fold) of resistance to rifampin (Table 1).
There is strong evidence that shows rifampin resistance frequently results from a mutation in the rpoB gene, which encodes the β-subunit of RNA polymerase (23, 39, 44). We therefore sequenced the rpoB gene from the 60 mutant isolates and compared them to the wild-type strain. Since all rif mutations in the rpoB gene found thus far are known to be located in three conserved regions, we focused on only three: region I covering nt 370 to 600, region II covering nt 700 to 1500, and region III covering nt 1600 to 2100. The sequencing data revealed that the vast majority (38 of 60) of the rif mutants possessed a point mutation in region II, 7 mutants were found with a point mutation in region III, and only 1 was found with a mutation in region I (Fig. 1). No mutation was detected in the rpoB gene of 10 rif mutant isolates, which were not listed in Table 1. In addition to point mutations, we found a deletion mutation (deletion of nt 1287 to 1289, resulting in the deletion of Asn-430) and a double substitution mutation (substitution of nt 1309 and 1310, resulting in an amino acid alteration at position 437). Thus, by analyzing 60 rif mutants we detected ultimately 15 kinds of point mutations at seven distinct positions, plus one deletion and one substitution mutation (Fig. 1). Among these mutations, changes of Leu-167 to Pro, His-437 to Cys, and the deletion of Asn-430 are novel rif mutations.
FIG. 1.
Nucleotide changes detected in rpoB gene and the corresponding amino acid alterations in the β-subunit of RNA polymerase in the S. lividans rif mutants. Numbering originates from the starting amino acid (Met) of the open reading frame. The mutation positions are indicated by arrows. Strain numbers, nucleotide changes, and amino acid changes are denoted in this order in boxes.
Activation of antibiotic biosynthesis by rif mutations. (i) Act production.
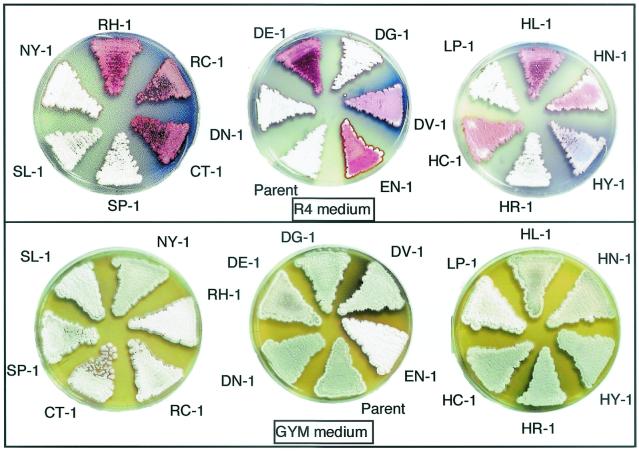
Although rif mutations occur in the rpoB gene and result in a high-level resistance to rifampin, only specific mutations could elicit S. lividans to produce Act as shown when examined in liquid culture by using R4 (Table 1) and GYM (Fig. 2) media. It is notable that the rif mutants which have the same mutation in rpoB (for example, all eight isolates in rif-1 type have the identical mutation; see Table 1) showed a similar phenotype with respect to antibiotic production. Further evidence for a causal relationship came from a complementary experiment (see Materials and Methods). By introducing the wild-type rpoB gene into mutant EN-1 (rif-9) or SL-1 (rif-14), the impaired sporulation and overproduction of Red or Act were both restored to the level of the parental (wild-type) strain (data not shown). These results indicate that the rif mutations detected are responsible for the observed changes in phenotype as presented in Table 1, where only the representative mutant strains are listed. Also, it is important to note that Act productivity depends not only on the mutational position but also on the amino acid species altered at that position. The mutants SL-1 (rif-14) and SP-1 (rif-15), which altered Ser-433, exhibited the highest Act productivity when cultured in GYM and R4 media, respectively. We then examined the mutants to see whether or not they could produce high levels of Act in solid culture. Our results show that, except for a couple of mutants (SP-1 and SL-1), almost all of the mutants that produced high levels of Act in liquid culture showed a high level of Act production in solid culture (Fig. 3).
FIG. 3.
Antibiotic production, aerial mycelia formation, and sporulation by S. lividans 66 and the rif mutants. Spores were inoculated on R4 or GYM agar plates and incubated at 30°C for 6 days. The blue color represents Act, and the red color represents Red.
(ii) Red production
S. lividans 66 produces a slight amount of the red antibiotic, Red. Like Act production, Red production was upregulated by the introduction of certain rif mutations, as represented by the mutants RC-1, EN-1, and DV-1 (Fig. 2 and 3 and Table 1). However, certain rif mutants (e.g., RC-1, CT-1, and SP-1) were shown to be able to upregulate Red only in certain growth media. Importantly, as examined with GYM liquid culture, the ability to upregulate Act correlated well with the ability to upregulate Red, except for the rif-9 mutation (strain EN-1), which activated Red but not Act (Fig. 2)—although the correlation was less apparent when examined with R4 plate cultures (Fig. 3).
Effects of rif mutations on growth, sporulation, and CDA biosynthesis.
We found that certain rif mutations markedly influence sporulation; this was especially pronounced in the white mutant EN-1, which lost almost completely the ability to sporulate on GYM plates, as determined by optical microscopy (Fig. 3).
To determine whether rif mutations influence production of CDA in S. lividans, three representative rif mutants (EN-1, SL-1, and RC-1) and the wild-type strain were monitored on R4 and SMMS agar plates. As shown in Fig. 4, all three mutants produced CDA on the SMMS plate. The productivity of CDA was especially pronounced when strain EN-1 was cultivated on the R4 plate. In contrast, strains RC-1 and SL-1 failed to produce CDA on the R4 plate, indicating medium dependence for CDA production. Thus, like Act and Red, production of CDA can be activated by certain rif mutations.
Expression of actII-ORF4, actIV-ORF1, redZ, and redD. (i) Western blotting analysis.
The ActII-ORF4 protein, which is encoded by the actII-ORF4 gene, has been characterized as a DNA-binding protein that positively regulates the transcription of the Act biosynthesis genes in S. coelicolor A3 (2). One of the Act biosynthesis genes, actIV-ORF1, encodes a dehydrogenase that catalyzes an early reductive step in the Act biosynthesis pathway (17). Red biosynthesis in S. coelicolor is known to depend on two pathway-specific regulatory genes: redD and redZ. Moreover, redD transcription is highly dependent on redZ, and transcription of redZ appears to be negatively autoregulated (41, 43). We analyzed the expression patterns of these key genes, comparing the mutants with the wild-type strain. We used three mutants (SL-1, RC-1, and EN-1) as representative rif mutants, since these three produced an abundant amount of Act and/or Red (Fig. 2). The profile of Act and Red production in GYM medium is shown in Fig. 5A. Western analysis clearly demonstrated that ActII-ORF4 is upregulated in the mutants SL-1 and RC-1 (Fig. 5B), but not in the mutant EN-1 which did not produce Act (Fig. 5A). The biosynthesis gene product ActIV-ORF1 was expressed just after the upregulation of ActII-ORF4 occurred in mutants SL-1 and RC-1 (Fig. 5B), followed by the onset of Act production (Fig. 5A). It was surprising that, although the wild-type strain accumulated a considerable amount of ActII-ORF4, only a slight amount of ActIV-ORF1 was detected (Fig. 5B).
FIG. 5.
Act and Red production and Western blotting analysis of the ActII-ORF4, ActIV-ORF1, RedZ, and RedD proteins. (A) Mycelial growth and antibiotic production by S. lividans 66 and the rif mutants in GYM liquid cultures. Symbols: ▪, S. lividans 66 (wild-type strain); □, EN-1 (rif-9); ▴, SL-1 (rif-14); ▵, RC-1 (rif-17). (B) Western analysis of the ActII-ORF4, ActIV-ORF1, and ribosomal S12 proteins. Cells grown in GYM liquid cultures at 30°C were harvested at the indicated time, followed by the preparation of cell extracts for Western analysis. The expression of the ribosomal S12 protein was analyzed in parallel as an internal control. Each lane contained 20 μg of total proteins. (C) Western analysis of the RedZ and RedD proteins. Cells used for this analysis were same as those used in panel B.
Results from Western analysis of RedZ and RedD also clearly indicate a relationship between the expression of these genes and Red production (Fig. 5C). All three mutants exhibited an upregulation of RedZ. It should be pointed out that RedD was expressed immediately after the upregulation of RedZ occurred (Fig. 5C), reflecting the onset of Red production (Fig. 5A). Thus, the rif-14 and rif-17 mutations (in strains SL-1 and RC-1) enable cells to activate two signal transduction pathways, the ActII-ORF4/ActIV-ORF1 and RedZ/RedD pathways, whereas the rif-9 mutation (in strain EN-1) can activate only the RedZ/RedD pathway.
(ii) S1 nuclease protection assay.
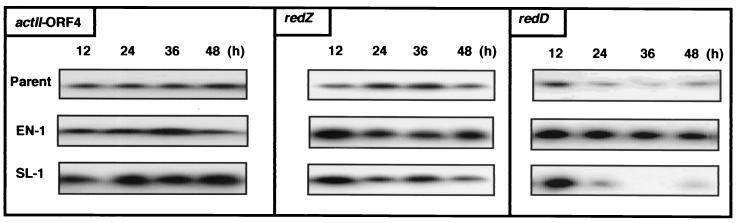
We conducted S1 nuclease protection assays to detect the level of transcripts of actII-ORF4, redZ, and redD (Fig. 6). The results obtained showed good correlation between the levels of transcripts of each gene (Fig. 6) and the actual amount of protein produced for ActII-ORF4, RedZ, and RedD (Fig. 5B and C), accounting for the elevated levels of these regulatory proteins in the rif mutants examined. It is also notable that the expression of actII-ORF4 and redD (and redZ) is characterized to be growth phase dependent, as has been shown in S. coelicolor A3(2) (17, 41). Importantly, unlike the wild-type strain, the mutant strains EN-1 and SL-1 revealed growth-phase-independent expression of redZ and actII-ORF4, respectively (Fig. 6).
FIG. 6.
Expression of actII-ORF4, redZ, and redD mRNA in parent (wild-type), EN-1 (rif-9), and SL-1 (rif-14) strains. RNAs for S1 nuclease protection assays were isolated from cells grown in the same GYM liquid medium for Western analysis. The probes for actII-ORF4, redZ, and redD were described in Materials and Methods.
DISCUSSION
The results described here establish that the biosyntheses of Red and Act in S. lividans can be activated by introducing mutations (rif) that confer resistance to rifampin and that this activation is dependent on the mutation's position and the species of amino acid altered in the β-subunit of RNA polymerase. Moreover, specific rif mutations can activate CDA biosynthesis, suggesting that regulating antibiotic biosynthesis by rif mutation is pleiotropic. Consistent with previous studies (7, 41-43), the activation of Red and Act biosynthesis in S. lividans rif mutants results from the activated expression of redD and actII-ORF4, which are pathway-specific regulatory genes. Despite the lack of detail for the regulatory cascade of these two pathways, our finding may be helpful in the elucidation of the regulatory mechanisms for antibiotic biosynthesis in Streptomyces.
The taxonomy of S. lividans 66 is closely related to S. coelicolor A3(2) (25). Nevertheless, it is well known that although it possesses complete Act and Red biosynthesis gene clusters, S. lividans produces no or only a slight amount of Act and Red. What is the mechanism that represses Act and Red biosynthesis in S. lividans? Previous studies addressed this point and partly uncovered this mechanism, focusing on the cutRS, afsR2, and orf10 genes of S. lividans. Although the cutRS signal transduction system and LysR-type transcriptional regulation mediated by orf10 both function to repress the biosynthesis of Act (7, 30), afsR2 positively regulates the biosynthesis of Act and Red (42). afsR2, when present at a high copy number, stimulates transcription of biosynthetic and regulatory genes in the Act gene cluster (act) and also stimulates the synthesis of Red (42). The cutRS operon is the second two-component system found in Streptomyces that negatively regulates antibiotic production; introduction of a mutation into cutR or cutS results in accerelated and increased production of Act (7). Likewise, disruption of the orf10 gene, encoding the LysR-type transcriptional regulator, causes Act overproduction (30). In addition to these previous findings, our present observations suggest that the RNA polymerase may also be involved in the repression of Act and Red biosynthesis in S. lividans. Recent studies dealing with S. coelicolor suggest that both redD and actII-ORF4 genes are recognized in vivo by the RNA polymerase holoenzyme containing σHrdB (3, 16, 18). Consequently, it is possible that the rif mutations, which are able to activate Act and/or Red biosynthesis, alter the three-dimensional structure or the conformation of the β-subunit of RNA polymerase, thereby stabilizing the σHrdB-RNA polymerase complex and resulting in the efficient transcription of actII-ORF4 and redZ (and redD). The accelerated expression of actII-ORF4, redZ, and redD in mutants SL-1 and RC-1 (Fig. 5) supports this hypothesis. Also, it is likely that the dependence of activation on the mutation position in the rpoB gene and the resulting type of amino acid substitution can be attributed to forming different conformation and/or three-dimensional structures of the β-subunit of RNA polymerase. It is worth mentioning that, in contrast to the rif-14 and rif-17 mutations (in SL-1 and RC-1), the rif-9 mutation (in EN-1) resulting in the deletion of Asn-430 failed to activate Act production, but rif-9 was quite effective in activating Red production (Fig. 2). It is conceivable that the altered conformational status of the RNA polymerase resulting from rif-9, rif-14, or rif-17 gave rise to different promoter selectivity (or affinity), leading to different expression levels of actII-ORF4 and redZ. Differential effects of rif mutations on the ability to sporulate (Fig. 3) may be explained in a similar way.
Previous studies of various bacteria, including S. coelicolor, demonstrated that mutations in the rpoB gene, which encodes the β-subunit of RNA polymerase, are responsible for the acquisition of resistance to rifampin (1, 36, 44). Almost all of the mutations are located in several conserved regions. In the present study, 17 kinds of mutations were detected in regions I, II, and III of the rpoB gene. Recent studies demonstrated that ppGpp binds to the β-subunit of E. coli RNA polymerase (11) and plays a causal role in activating actII-ORF4 transcription in S. coelicolor (18). These observations, taken together with our present and previously published observations (6, 33, 34, 40), show that the guanine nucleotide ppGpp is a pivotal signal molecule for initiating the onset of antibiotic production. ppGpp (and pppGpp) is believed to be responsible for the stringent response, which causes an immediate cessation of RNA synthesis and other cellular reactions (reviewed by Cashel et al. [5]). It is reasonable to consider that the enhanced expression of redZ (and redD) or actII-ORF4, which accompanies the activation of Red or Act biosynthesis, is based at least partly on the independence of cells from ppGpp for initiating the secondary metabolism. Thus, the mutated RNA polymerase may function by mimicking the ppGpp-bound form.
In S. coelicolor, transcription of the activator genes actII-ORF4 and redD has been characterized to be growth phase dependent, and the transcription of the antibiotic structural genes actIV-ORF1 (for Act) and redP (for Red) has been shown to occur after the accumulation of almost maximal levels of actII-ORF4 or redD transcripts (17, 41). This suggests that a threshold concentration of the activator is needed for transcription of the antibiotic genes. Consistent with this, our present work with S. lividans also demonstrated a growth phase dependence of the transcription of actII-ORF4, redZ, and redD in the wild-type strain (Fig. 6). However, the low level of ActII-ORF4 or RedZ protein expressed in wild-type S. lividans 66 (Fig. 5B and C) apparently was not enough to activate Act or Red biosynthesis. In contrast, in rif mutants expression of the actII-ORF4 or redZ was high during the transition or stationary phase, or even during the early growth phase (for redZ), resulting in the activation of Act or Red biosynthesis (Fig. 5 and 6).
It has been demonstrated that cluster I (region II in our study), which harbors most of the rif mutations, is the part of the “5′-face” of the active center and is the rifampin-binding site (37). Moreover, cluster I (which encodes amino acids 400 to 466) interacts with the +1 position of DNA-RNA hybrid in the transcription elongation complex (28). Consequently, it is possible that rif mutations in region II of the rpoB gene activate Act or Red biosynthesis by the efficient transcription of the activator genes such as actII-ORF4, redZ, and redD (or the inefficient transcription of the repressor genes). The dependence of activation on the mutation position and type of amino acid substitution implicates the existence of a relationship between the function and structure of the β-subunit of RNA polymerase in initiating bacterial secondary metabolism.
Acknowledgments
This work was supported by a grant from the Organized Research Combination System of the Science and Technology Agency of Japan.
We thank Y. Tozawa and S. Okamoto for preparation of the antibodies used here and Y. Ohnishi and S. Horinouchi for advice on the S1 mapping assay.
REFERENCES
- 1.Aboshkiwa, W., G. Rowland, and G. Coleman. 1995. Nucleotide sequence of the Staphylococcus aureus RNA polymerase rpoB gene and comparison of its predicated amino acid sequence with those of other bacteria. Biochem. Biophys. Acta 1262:73-78. [DOI] [PubMed] [Google Scholar]
- 2.Adamidis, T., P. Riggle, and W. Champness. 1990. Mutations in a new Streptomyces coelicolor locus which globally block antibiotic biosynthesis but not sporulation. J. Bacteriol. 172:2962-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aigle, B., A. Weitzorrek, E. Takano, and M. J. Bibb. 2000. A single amino acid substitution in region 1.2 of the principal sigma factor of Streptomyces coelicolor A3(2) results in pleiotropic loss of antibiotic production. Mol. Microbiol. 37:995-1004. [DOI] [PubMed] [Google Scholar]
- 4.Arias, P., M. A. Fernández-Moreno, and F. Malpartida. 1999. Characterization of the pathway-specific positive transcriptional regulator for actinorhodin biosynthesis in Streptomyces coelicolor A3(2) as a DNA-binding protein. J. Bacteriol. 181:6958-6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cashel, M., D. R. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 6.Chakraburtty, R., J. White, E. Takano, and M. Bibb. 1996. Cloning, characterization and disruption of a (p)ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2). Mol. Microbiol. 19:357-368. [DOI] [PubMed] [Google Scholar]
- 7.Chang, H. M., M. Y. Chen, Y. T. Shieh, M. J. Bibb, and C. W. Chen. 1996. The cutRS signal transduction system of Streptomyces lividans represses the biosynthesis of the polyketide antibiotic actinorhodin. Mol. Microbiol. 21:1075-1085. [PubMed] [Google Scholar]
- 8.Chater, K. F. 1989. Sporulation in Streptomyces, p. 277-299. In R. I. Smith, A. Slepecky, and P. Setlow (ed.), Regulation of procaryotic development. American Society for Microbiology, Washington, D.C.
- 9.Chater, K. F., and C. J. Bruton. 1985. Resistance, regulatory and production genes for the antibiotic methylenomycin are clustered. EMBO J. 4:1893-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chater, K. F., and M. J. Bibb. 1996. Regulation of bacterial antibiotic production. Bio/Technology 7:57-105. [Google Scholar]
- 11.Chatterji, D., N. Fujita, and A. Ishihama. 1998. The mediator for stringent control, ppGpp, binds to the β-subunit of Escherichia coli RNA polymerase. Genes Cells 3:279-287. [DOI] [PubMed] [Google Scholar]
- 12.Chong, P. P., S. M. Podmore, H. M. Kieser, M. Redenbach, K. Turgay, M. Marahiel, D. A. Hopwood, and C. P. Smith. 1998. Physical identification of a chromosomal locus encoding biosynthetic genes for the lipopeptide calcium-dependent antibiotic (CDA) of Streptomyces coelicolor A3(2). Microbiology 144(Pt. 1):193-199. [DOI] [PubMed] [Google Scholar]
- 13.Feitelson, J. S., F. Malpartida, and D. A. Hopwood. 1985. Genetic and biochemical characterization of the red gene cluster of Streptomyces coelicolor A3(2). J. Gen. Microbiol. 131:2431-2441. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Moreno, M. A., A. J. Martín-Triana, E. Martí nez, J. Niemi, H. M. Kieser, D. A. Hopwood, and F. Malpartida. 1992. abaA, a new pleiotropic regulatory locus for antibiotic production in Streptomyces coelicolor. J. Bacteriol. 174:2958-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Moreno, M. A., J. L. Caballrero, D. A. Hopwood, and F. Malpartida. 1991. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell 66:769-780. [DOI] [PubMed] [Google Scholar]
- 16.Fujii, T., H. C. Gramajo, E. Takano, and M. J. Bibb. 1996. redD and actII-ORF4, pathway-specific regulatory genes for antibiotic production in Streptomyces coelicolor A3(2), are transcribed in vitro by an RNA polymerase holoenzyme containing sigma HrdD. J. Bacteriol. 178:3402-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gramajo, H. C., E. Takano, and M. J. Bibb. 1993. Stationary-phase production of the antibiotic actinorhodin in Streptomyces coelicolor A3(2) is transcriptionally regulated. Mol. Microbiol. 7:837-845. [DOI] [PubMed] [Google Scholar]
- 18.Hesketh, A., J. Sun, and M. Bibb. 2001. Induction of ppGpp synthesis in Streptomyces coelicolor A3(2) grown under conditions of nutritional sufficiency elicits actII-ORF4 transcription and actinorhodin biosynthesis. Mol. Microbiol. 39:136-144. [DOI] [PubMed] [Google Scholar]
- 19.Hopwood, D. A., K. F. Chater, and M. J. Bibb. 1994. Antibiotic production in Streptomyces coelicolor A3(2), p. 71-108. In L. C. Vining and C. Stuttard (ed.), Regulation and biochemistry of antibiotic production. Butterworth-Heinemann, Newton, Mass.
- 20.Horinouchi, S., M. Kito, M. Nishiyama, K. Furuya, S. K. Hong, K. Miyake, and T. Beppu. 1990. Primary structure of AfsR, a global regulatory protein for secondary metabolite formation in Streptomyces coelicolor A3(2). Gene 95:49-56. [DOI] [PubMed] [Google Scholar]
- 21.Hosoya, Y., S. Okamoto, H. Muramatsu, and K. Ochi. 1998. Acquisition of certain streptomycin-resistant (str) mutations enhances antibiotic production in bacteria. Antimicrob. Agents Chemother. 42:2041-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, H., and K. Ochi. 2001. Novel approach for improving the productivity of antibiotic-producing strains by inducing combined resistant mutations. Appl. Environ. Microbiol. 67:1885-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin, D. J., and C. A. Gross. 1988. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J. Mol. Biol. 202:45-58. [DOI] [PubMed] [Google Scholar]
- 24.Kawamoto, S., D. Zhang, and K. Ochi. 1997. Molecular analysis of the ribosomal L11 protein gene (rplK=relC) of Streptomyces griseus and identification of a deletion allele. Mol. Gen. Genet. 255:549-560. [DOI] [PubMed] [Google Scholar]
- 25.Kawamoto, S., and K. Ochi. 1998. Comparative ribosomal protein (L11 and L30) sequence analyses of several Streptomyces spp. commonly used in genetic studies. Int. J. Syst. Bacteriol. 48:597-600. [DOI] [PubMed] [Google Scholar]
- 26.Kieser, T., M. J. Bibb, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, United Kingdom.
- 27.Kim, E. S., H. J. Hong, C. Y. Choi, and S. N. Cohen. 2001. Modulation of actinorhodin biosynthesis in Streptomyces lividans by glucose repression of afsR2 gene transcription. J. Bacteriol. 183:2198-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korzheva, N., A. Mustaev, M. Kozlov, A. Malhotra, V. Nikiforov, A. Goldfarb, and S. A. Darst. 2000. A structural model of transcription elongation. Science 289:619-625. [DOI] [PubMed] [Google Scholar]
- 29.Malpartida, F., J. Niemi, R. Navarrete, and D. A. Hopwood. 1990. Cloning and expression in a heterologous host of the complete set of genes for biosynthesis of the Streptomyces coelicolor antibiotic undecylprodigiosin. Gene 93:91-99. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-costa, O., A. J. Martin-Triana, E. Martinez, M. A. Fernandze-Moreno, and F. Malpartida. 1999. An additional regulatory gene for actinorhodin production in Streptomyces lividans involves a LysR-type transcriptional regulator. J. Bacteriol. 181:4353-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narva, K. E., and J. S. Feitelson. 1990. Nucleotide sequence and transcriptional analysis of the redD locus of Streptomyces coelicolor A3(2). J. Bacteriol. 172:326-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochi, K. 1987. Metabolic initiation of differentiation and secondary metabolism by Streptomyces griseus: significance of the stringent response (ppGpp) and GTP content in relation to A-factor. J. Bacteriol. 169:3608-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochi, K. 1990. A relaxed (rel) mutant of Streptomyces coelicolor A3(2) with a missing ribosomal protein lacks the ability to accumulate ppGpp, A-factor and prodigiosin. J. Gen. Microbiol. 136:2405-2412. [DOI] [PubMed] [Google Scholar]
- 34.Ochi, K., D. Zhang, S. Kawamoto, and A. Hesketh. 1997. Molecular and functional analysis of the ribosomal L11 and S12 protein genes (rplK and rpsL) of Streptomyces coelicolor A3(2). Mol. Gen. Genet. 256:488-498. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto, S., and K. Ochi. 1998. An essential GTP-binding protein functions as a regulator for differentiation in Streptomyces coelicolor. Mol. Microbiol. 30:107-119. [DOI] [PubMed] [Google Scholar]
- 36.Serverinov, K., M. Soushko, A. Goldfarb, and V. Nikiforrov. 1994. Rif mutations in the beginning of the Escherichia coli rpoB gene. Mol. Gen. Genet. 244:120-126. [DOI] [PubMed] [Google Scholar]
- 37.Severinov, K., A. Mustaev, E. Severinova, M. Kozlov, S. A. Darst, and A. Goldfarb. 1995. The β subunit Rif-cluster I is only angstroms away from the active center of Escherichia coli RNA polymerase. J. Biol. Chem. 270:29428-29432. [DOI] [PubMed] [Google Scholar]
- 38.Shima, J., A. Hesketh, S. Okamoto, S. Kawamoto, and K. Ochi. 1996. Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2). J. Bacteriol. 178:7276-7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer, M., D. J. Jin, W. A. Walter, and C. A. Cross. 1993. Genetic evidence for the interaction between cluster I and cluster III rifampicin resistant mutations. J. Mol. Biol. 231:1-5. [DOI] [PubMed] [Google Scholar]
- 40.Sun, J., A. Hesketh, and M. Bibb. 2001. Functional analysis of relA and rshA, two relA/spoT homologues of Streptomyces coelicolor A3(2). J. Bacteriol. 183:3488-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takano, E., H. C. Gramajo, E. Strauch, N. Andres, J. White, and M. J. Bibb. 1992. Transcriptional regulation of the redD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2). Mol. Microbiol. 6:2797-2804. [DOI] [PubMed] [Google Scholar]
- 42.Vogtli, M., P. C. Chang, and S. N. Cohen. 1994. afsR2: a previously undetected gene encoding a 63-amino-acid protein that stimulates antibiotic production in Streptomyces lividans. Mol. Microbiol. 14:643-653. [DOI] [PubMed] [Google Scholar]
- 43.White, J., and M. Bibb. 1997. bldA dependence of undecylprodigiosin production in Streptomyces coelicolor A3(2) involves a pathway-specific regulatory cascade. J. Bacteriol. 179:627-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wichelhaus, T. A., V. Schäfer, V. Brade, and B. Böddinghaus. 1999. Molecular characterization of rpoB mutations conferring cross-resistance to rifamycins on methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2813-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]