Abstract
1. The spino-olivocerebellar path ascending through the ventral funiculus (VF-SOCP) was investigated in decerebrate cats with the cord transected in the third cervical segment except for the left ventral funiculus. The climbing fibre responses evoked in Purkinje cells were studied by recording from single cells and by recording the mass activity at the cerebellar surface or in the molecular layer.
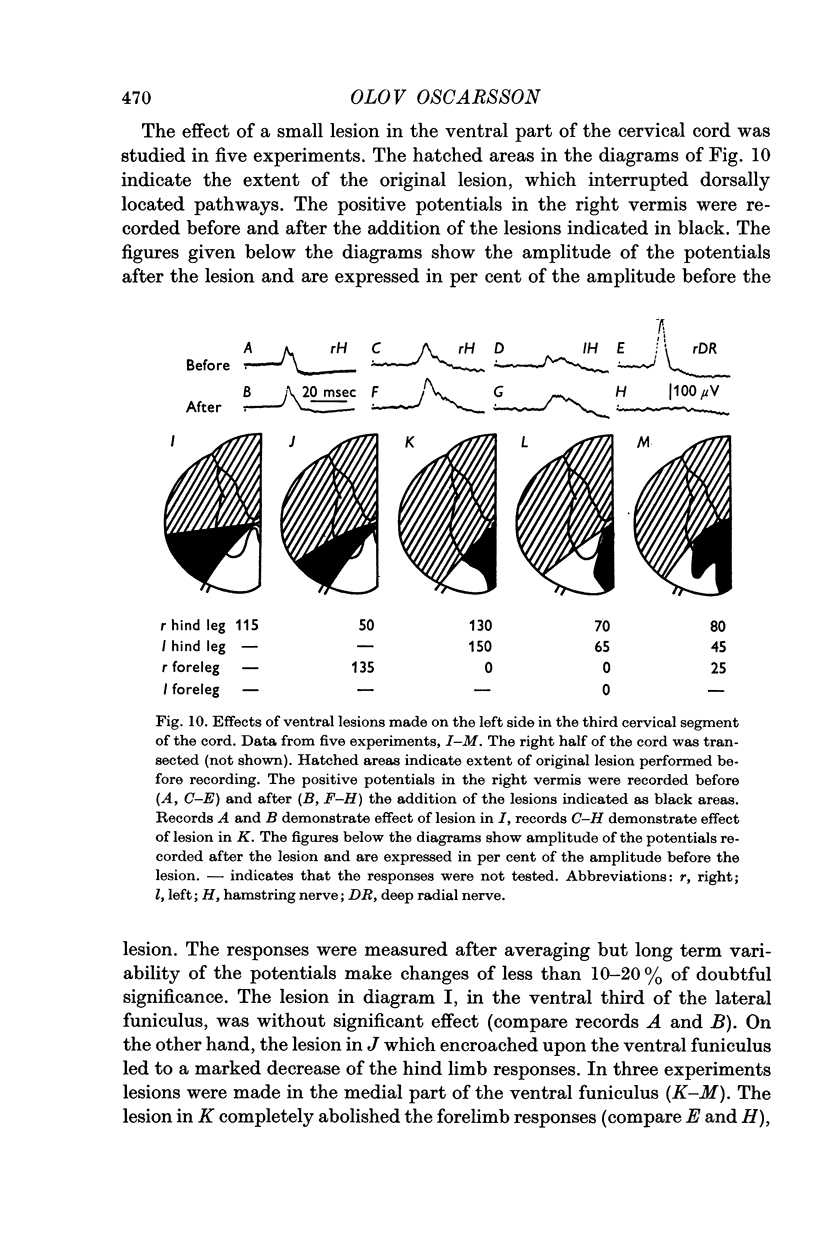
2. In the spinal cord the forelimb component of the VF-SOCP occupies a medial part and the hind limb component a lateral part of the ventral funiculus.
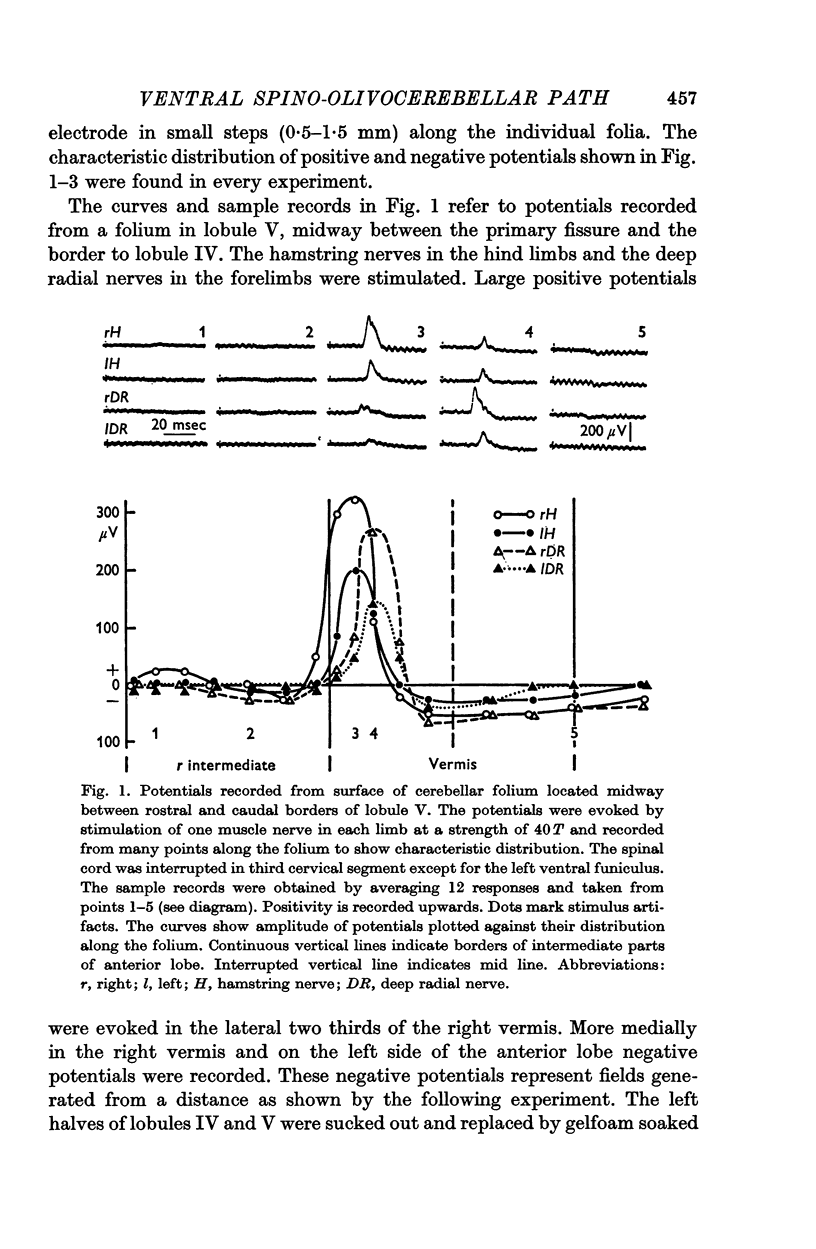
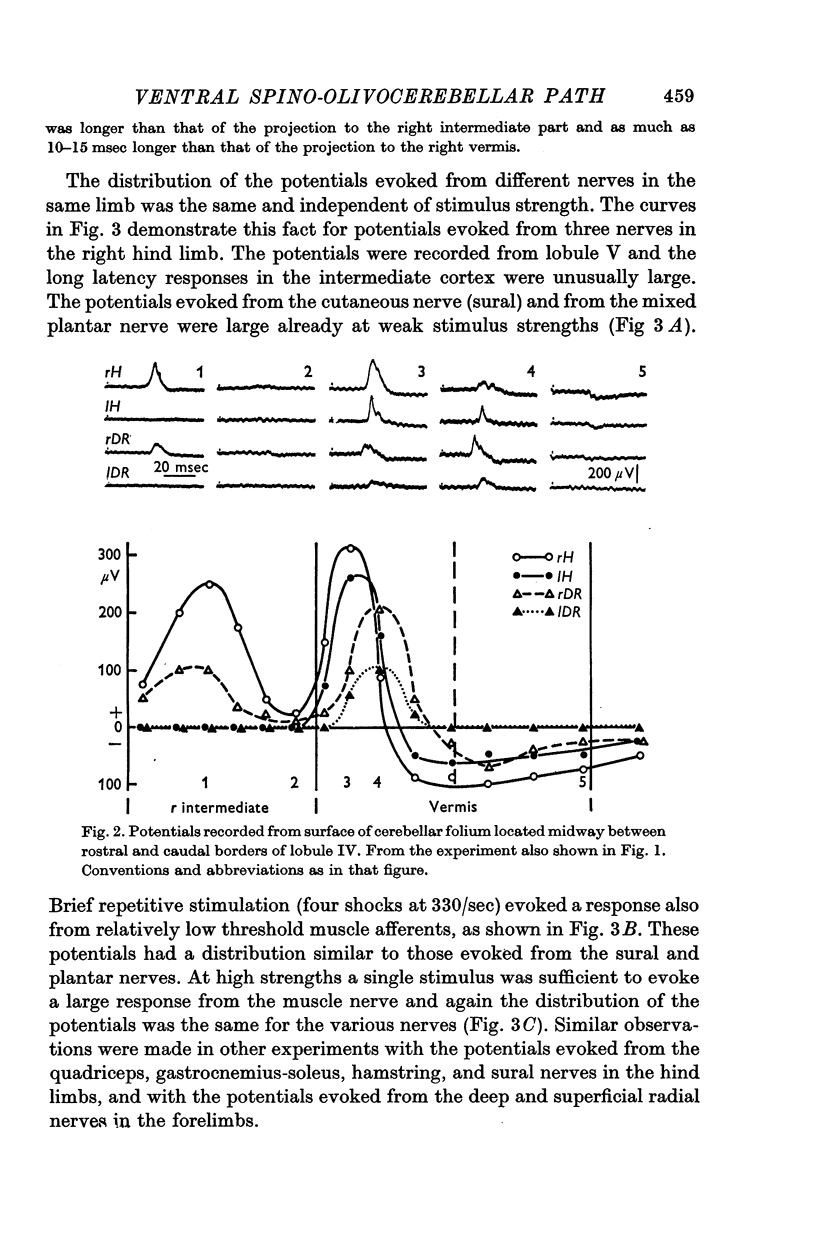
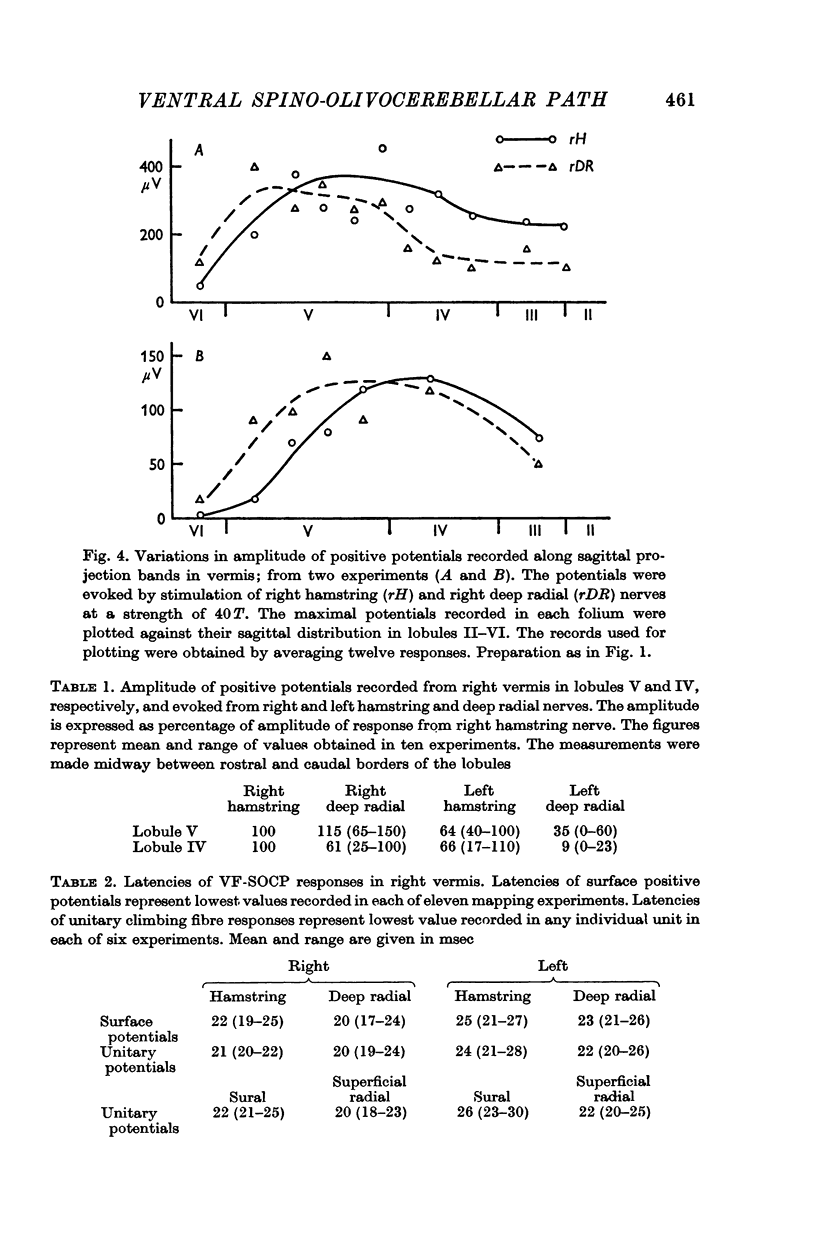
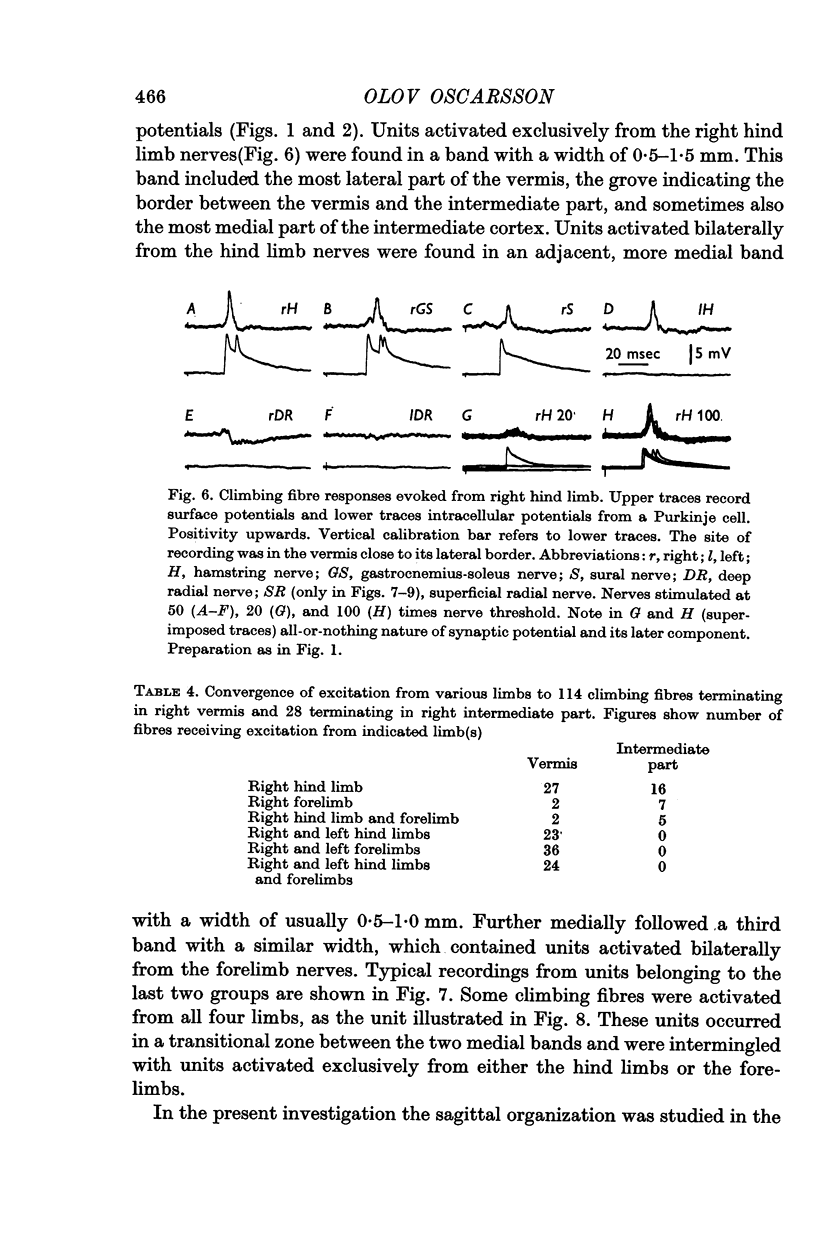
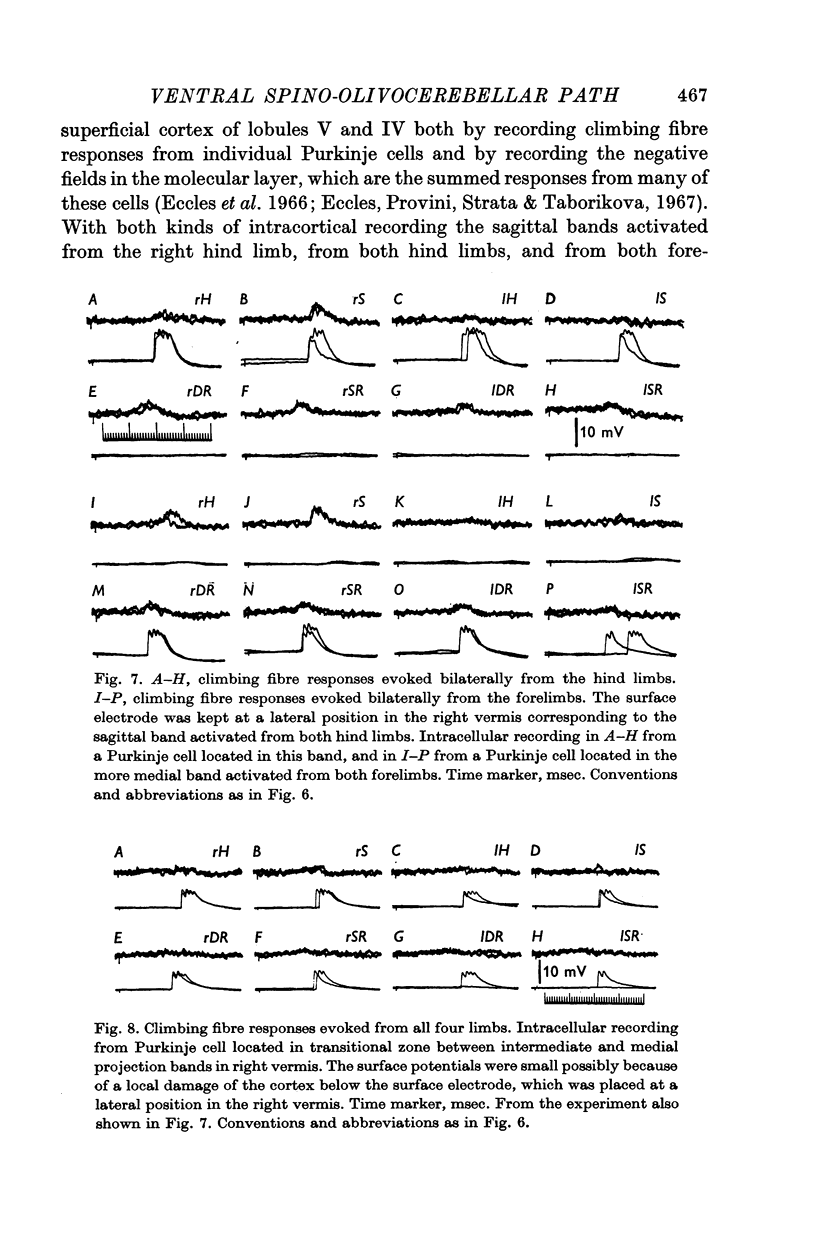
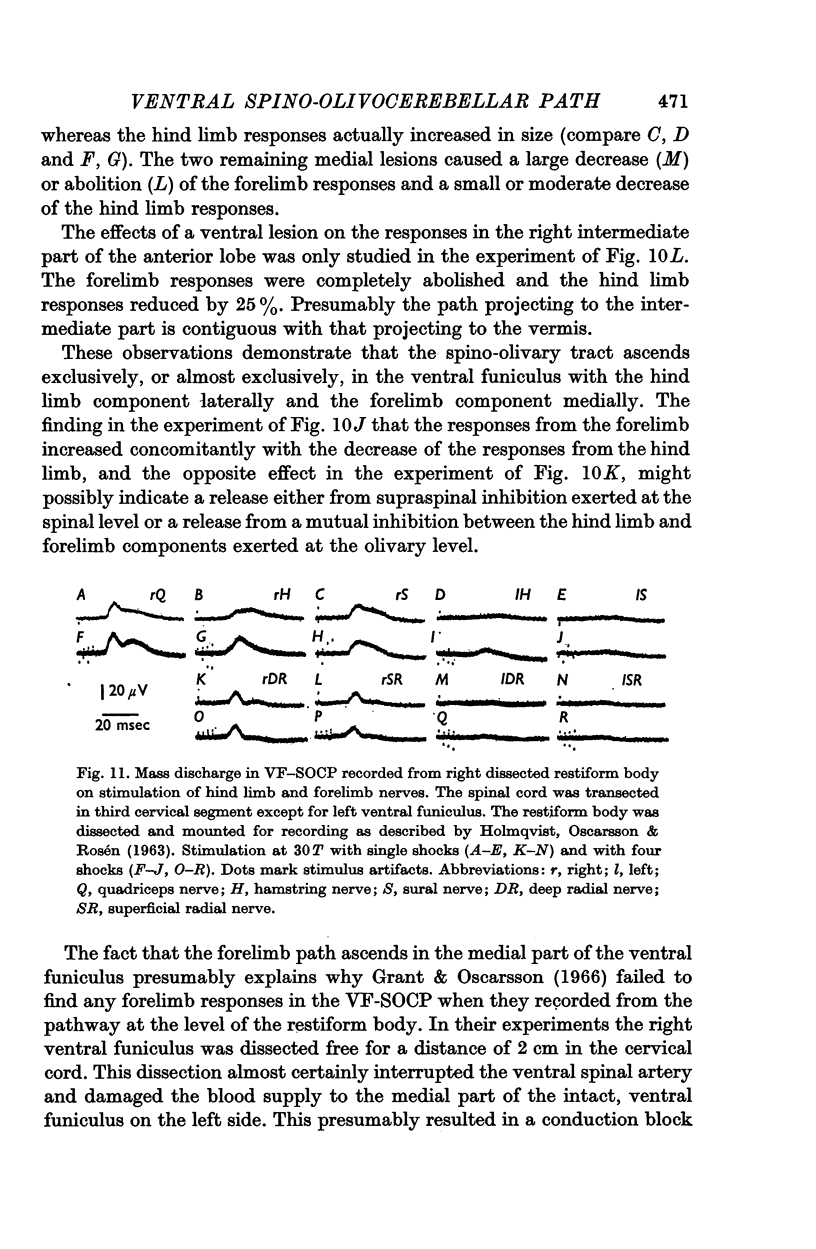
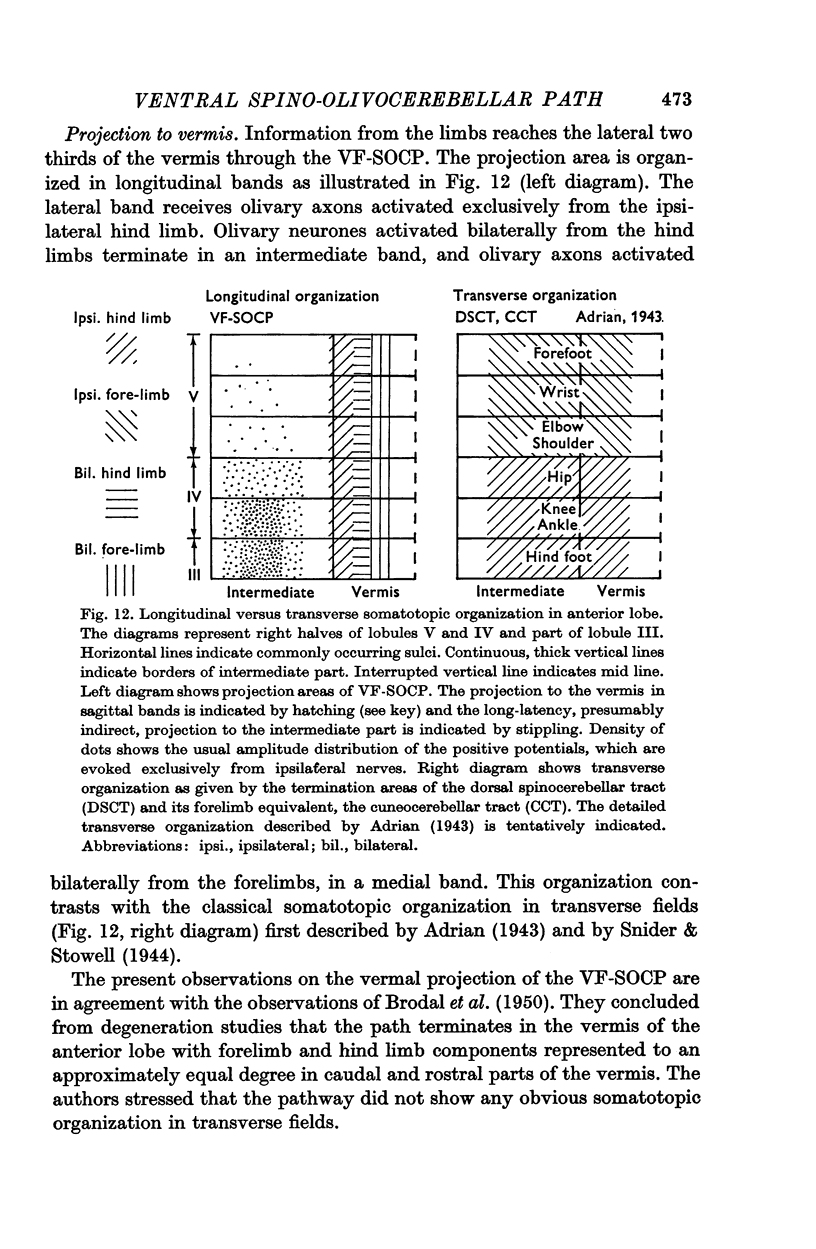
3. The main projection of the limb nerves through the VF-SOCP is to the lateral two thirds of the vermis of the anterior lobe. The projection area consists of three sagittally arranged bands: a lateral band receiving olivary axons activated exclusively from the ipsilateral hind limb, an intermediate band receiving olivary axons activated bilaterally from the hind limbs, and a medial band receiving olivary axons activated bilaterally from the forelimbs. Some olivary neurones are activated from all four limbs.
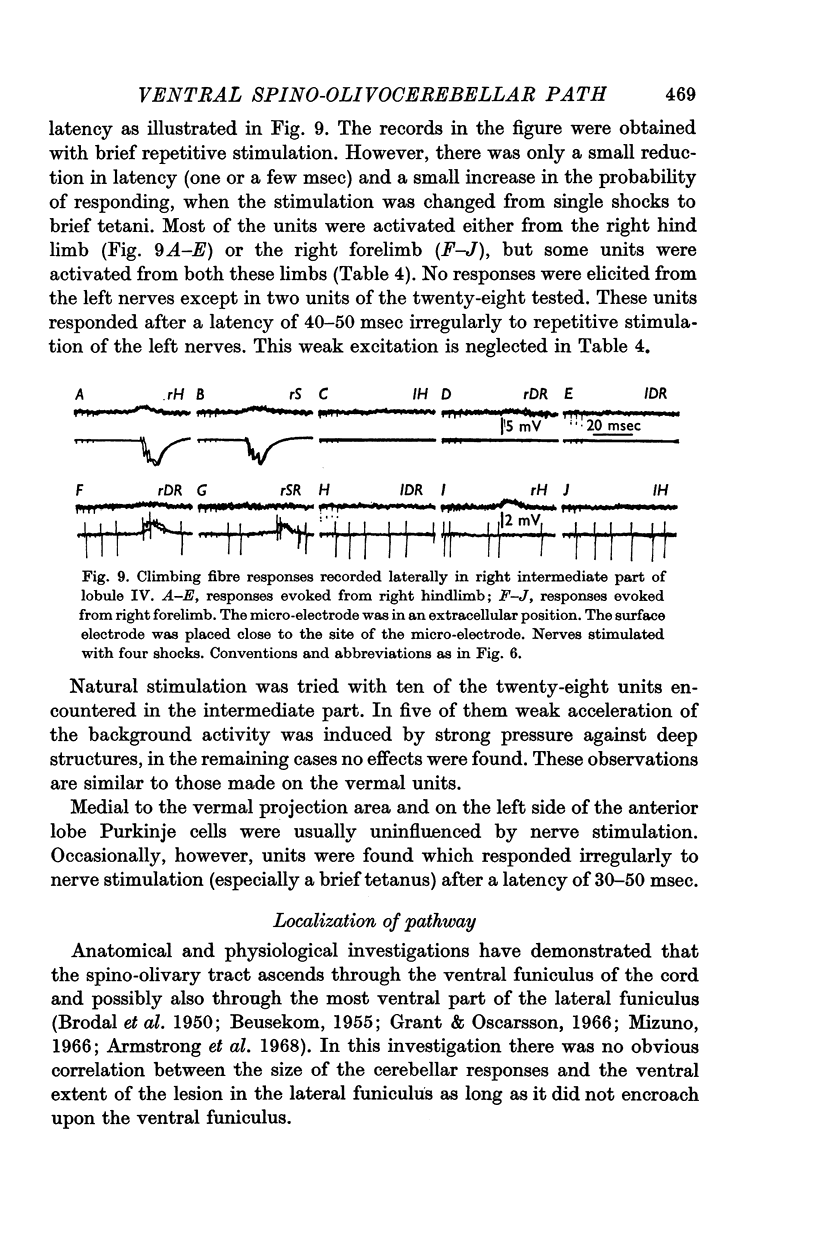
4. The latency of the climbing fibre responses was about 22 msec on stimulation of ipsilateral hind limb nerves and about 20 msec on stimulation of ipsilateral forelimb nerves. The responses evoked from contralateral nerves had a latency which was about 3 msec longer than the latency of the corresponding ipsilateral responses.
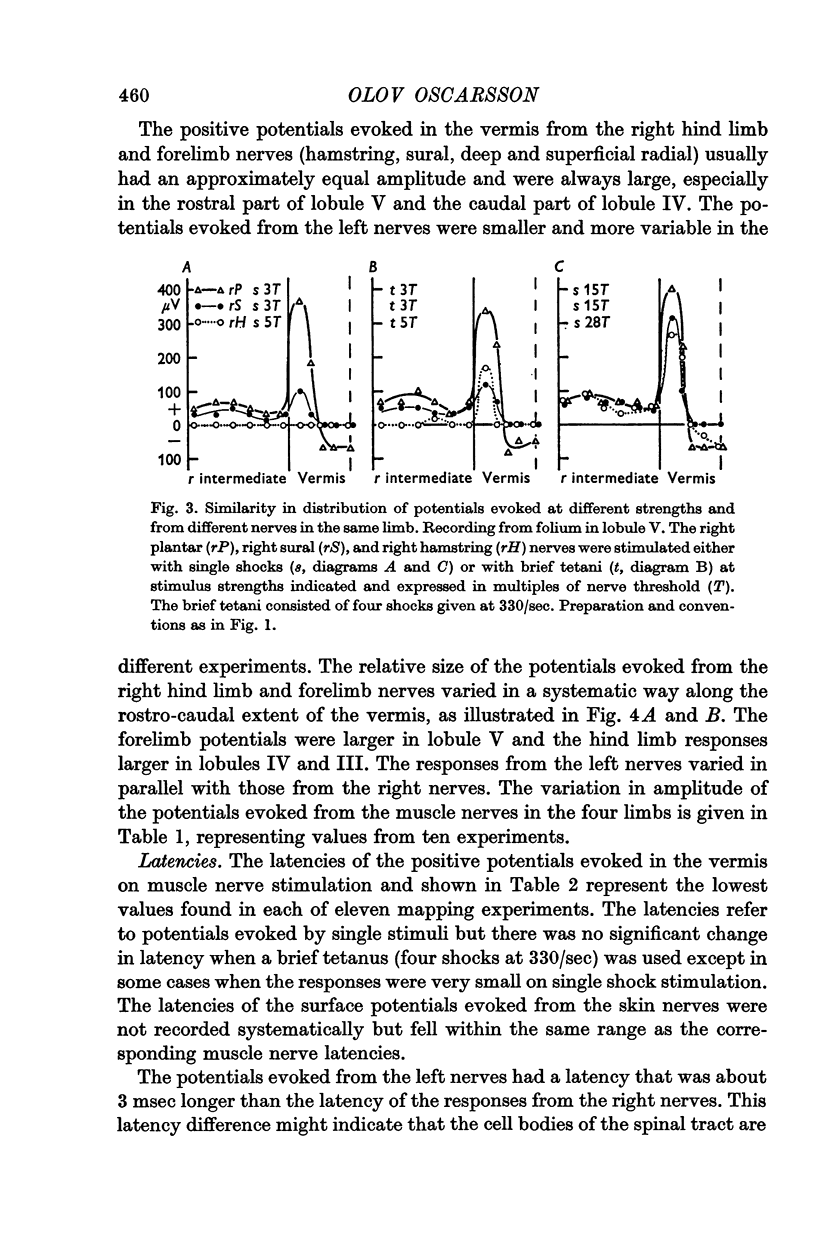
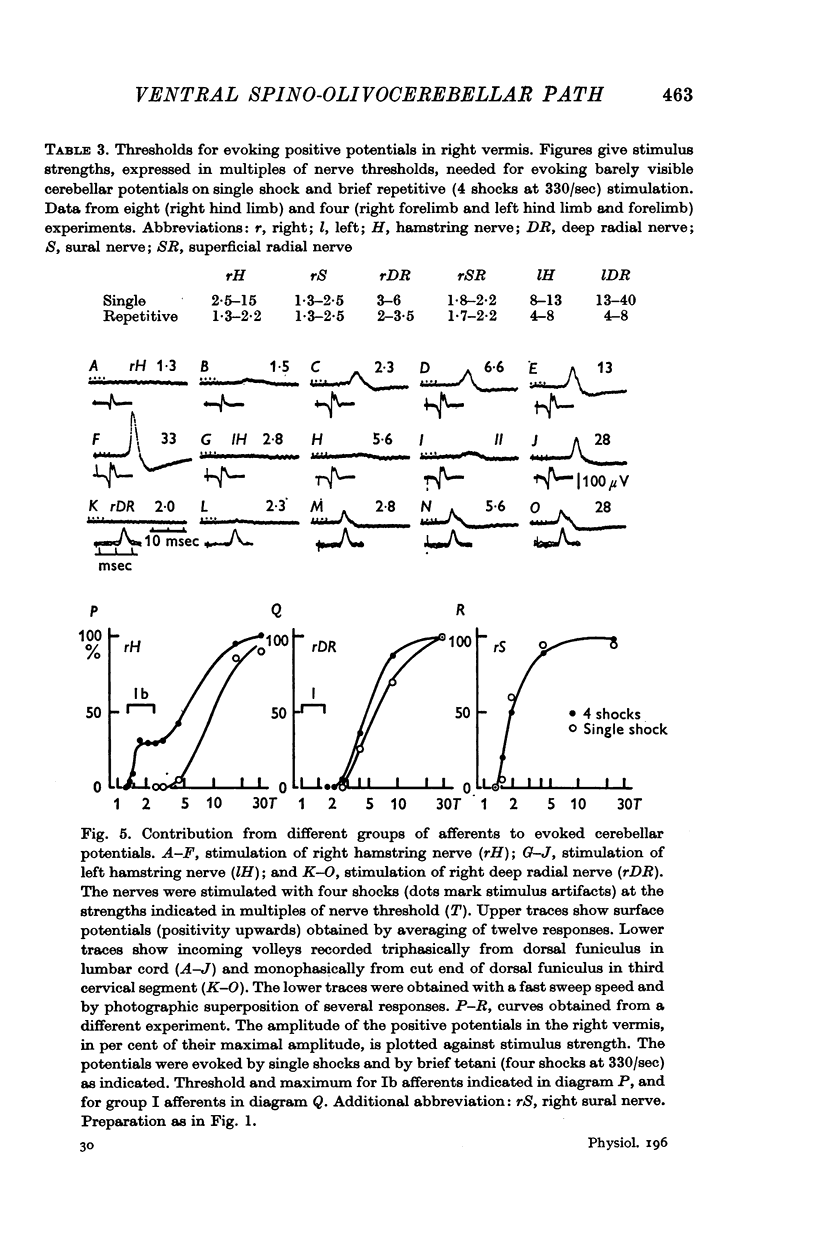
5. The olivary neurones were usually activated from all tested muscle and skin nerves in the limb(s) constituting the receptive field. Cutaneous afferents and groups II and III muscle afferents were responsible for the excitation elicited by single shock stimulation of the nerves. Brief repetitive stimulation revealed additional activation from mainly Ib, but also Ia, afferents in ipsilateral hind limb nerves.
6. Natural stimulation of receptors evoked responses in about half of the olivary neurones tested. The responses were elicited by strong pressure against deep structures. Inhibitory effects were seldom observed.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Eccles J. C., Harvey R. J., Matthews P. B. Responses in the dorsal accessory olive of the cat to stimulation of hind limb afferents. J Physiol. 1968 Jan;194(1):125–145. doi: 10.1113/jphysiol.1968.sp008398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Harvey R. J. Responses to a spino-olivo-cerebellar pathway in the cat. J Physiol. 1968 Jan;194(1):147–168. doi: 10.1113/jphysiol.1968.sp008399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODAL A., WALBERG F., BLACKSTAD T. Termination of spinal afferents to inferior olive in cat. J Neurophysiol. 1950 Nov;13(6):431–454. doi: 10.1152/jn.1950.13.6.431. [DOI] [PubMed] [Google Scholar]
- CARREA R. M., GRUNDFEST H. Electrophysiological studies of cerebellar inflow. I. Origin, conduction and termination of ventral spino-cerebellar tract in monkey and cat. J Neurophysiol. 1954 May;17(3):208–238. doi: 10.1152/jn.1954.17.3.208. [DOI] [PubMed] [Google Scholar]
- COMBS C. M. Electro-anatomical study of cerebellar localization; stimulation of various afferents. J Neurophysiol. 1954 Mar;17(2):123–143. doi: 10.1152/jn.1954.17.2.123. [DOI] [PubMed] [Google Scholar]
- Crichlow E. C., Kennedy T. T. Functional characteristics of neurons in the lateral reticular nucleus with reference to localized cerebellar potentials. Exp Neurol. 1967 Jun;18(2):141–153. doi: 10.1016/0014-4886(67)90036-2. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., HUBBARD J. I., OSCARSSON O. Intracellular recording from cells of the ventral spinocerebellar tract. J Physiol. 1961 Oct;158:486–516. doi: 10.1113/jphysiol.1961.sp006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C., Llinás R., Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966 Jan;182(2):268–296. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANT G. Projection of the external cuneate nucleus onto the cerebellum in the cat: an experimental study using silver methods. Exp Neurol. 1962 Mar;5:179–195. doi: 10.1016/0014-4886(62)90032-8. [DOI] [PubMed] [Google Scholar]
- Grant G., Oscarsson O. Mass discharges evoked in the olivocerebellar tract on stimulation of muscle and skin nerves. Exp Brain Res. 1966;1(4):329–337. doi: 10.1007/BF00237705. [DOI] [PubMed] [Google Scholar]
- Grant G., Oscarsson O., Rosén I. Functional organization of the spinoreticulocerebellar path with identification of its spinal component. Exp Brain Res. 1966;1(4):306–319. doi: 10.1007/BF00237703. [DOI] [PubMed] [Google Scholar]
- HOLMQVIST B., OSCARSSON O., ROSEN I. Functional organization of the cuneocrebellar tract in the cat. Acta Physiol Scand. 1963 Jun-Jul;58:216–235. doi: 10.1111/j.1748-1716.1963.tb02643.x. [DOI] [PubMed] [Google Scholar]
- LARSELL O. The cerebellum of the cat and the monkey. J Comp Neurol. 1953 Aug;99(1):135–199. doi: 10.1002/cne.900990110. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., NORRSELL U., VOORHOEVE P. EFFECTS FROM THE SENSORIMOTOR CORTEX ON ASCENDING SPINAL PATHWAYS. Acta Physiol Scand. 1963 Dec;59:462–473. doi: 10.1111/j.1748-1716.1963.tb02762.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Two ascending spinal pathways in the ventral part of the cord. Acta Physiol Scand. 1962 Mar-Apr;54:270–286. doi: 10.1111/j.1748-1716.1962.tb02351.x. [DOI] [PubMed] [Google Scholar]
- MORIN F., LAMARCHE G., OSTROWSKI A. Z. Responses of the inferior olive to peripheral stimuli and the spinal pathways involved. Am J Physiol. 1957 May;189(2):401–406. doi: 10.1152/ajplegacy.1957.189.2.401. [DOI] [PubMed] [Google Scholar]
- Mizuno N. An experimental study of the spino-olivary fibers in the rabbit and the cat. J Comp Neurol. 1966 Jun;127(2):267–292. doi: 10.1002/cne.901270209. [DOI] [PubMed] [Google Scholar]
- OSCARSSON O. DIFFERENTIAL COURSE AND ORGANIZATION OF UNCROSSED AND CROSSED LONG ASCENDING SPINAL TRACTS. Prog Brain Res. 1964;12:164–178. doi: 10.1016/s0079-6123(08)60622-6. [DOI] [PubMed] [Google Scholar]
- OSCARSSON O. FUNCTIONAL ORGANIZATION OF THE SPINO- AND CUNEOCEREBELLAR TRACTS. Physiol Rev. 1965 Jul;45:495–522. doi: 10.1152/physrev.1965.45.3.495. [DOI] [PubMed] [Google Scholar]
- Oscarsson O., Rosén I. Response characteristics of reticulocerebellar neurones activated from spinal afferents. Exp Brain Res. 1966;1(4):320–328. doi: 10.1007/BF00237704. [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Termination and functional organization of a dorsal spino-olivocerebellar path. Brain Res. 1967 Aug;5(4):531–534. doi: 10.1016/0006-8993(67)90029-7. [DOI] [PubMed] [Google Scholar]
- Oscarsson O., Uddenberg N. Somatotopic termination of spino-olivocerebellar path. Brain Res. 1966 Dec;3(2):204–207. doi: 10.1016/0006-8993(66)90080-1. [DOI] [PubMed] [Google Scholar]
- Provini L., Redman S., Strata P. Somatotopic organization of mossy and climbing fibres to the anterior lobe of cerebellum activated by the sensorimotor cortex. Brain Res. 1967 Oct;6(2):378–381. doi: 10.1016/0006-8993(67)90205-3. [DOI] [PubMed] [Google Scholar]
- Thach W. T., Jr Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967 Jul;30(4):675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]


