Abstract
1. Indirect evidence suggests that the concentration of arginine vasopressin in the plasma of normally hydrated man is about 1 μ-u./ml., but this is usually considered to be below the limit of sensitivity of the standard assay preparation, the water-loaded Wistar rat under ethanol anaesthesia.
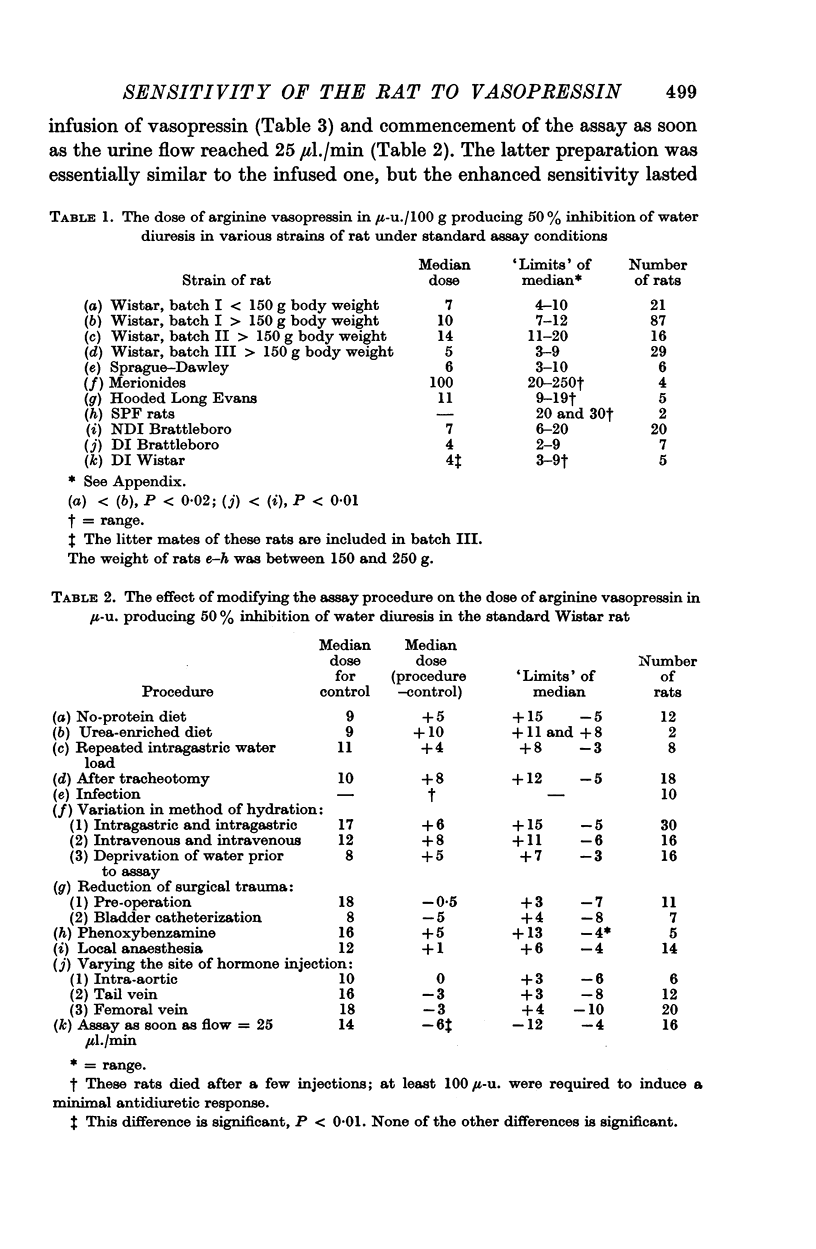
2. It was found that there was a surprising variation in sensitivity to vasopressin between batches of Wistar rats, and that other varieties of rat (including those with diabetes insipidus) were no more sensitive.
3. Three modifications of the standard assay procedure produced an increase in sensitivity:
(a) using Wistar rats weighing 100-150 g, rather than larger animals;
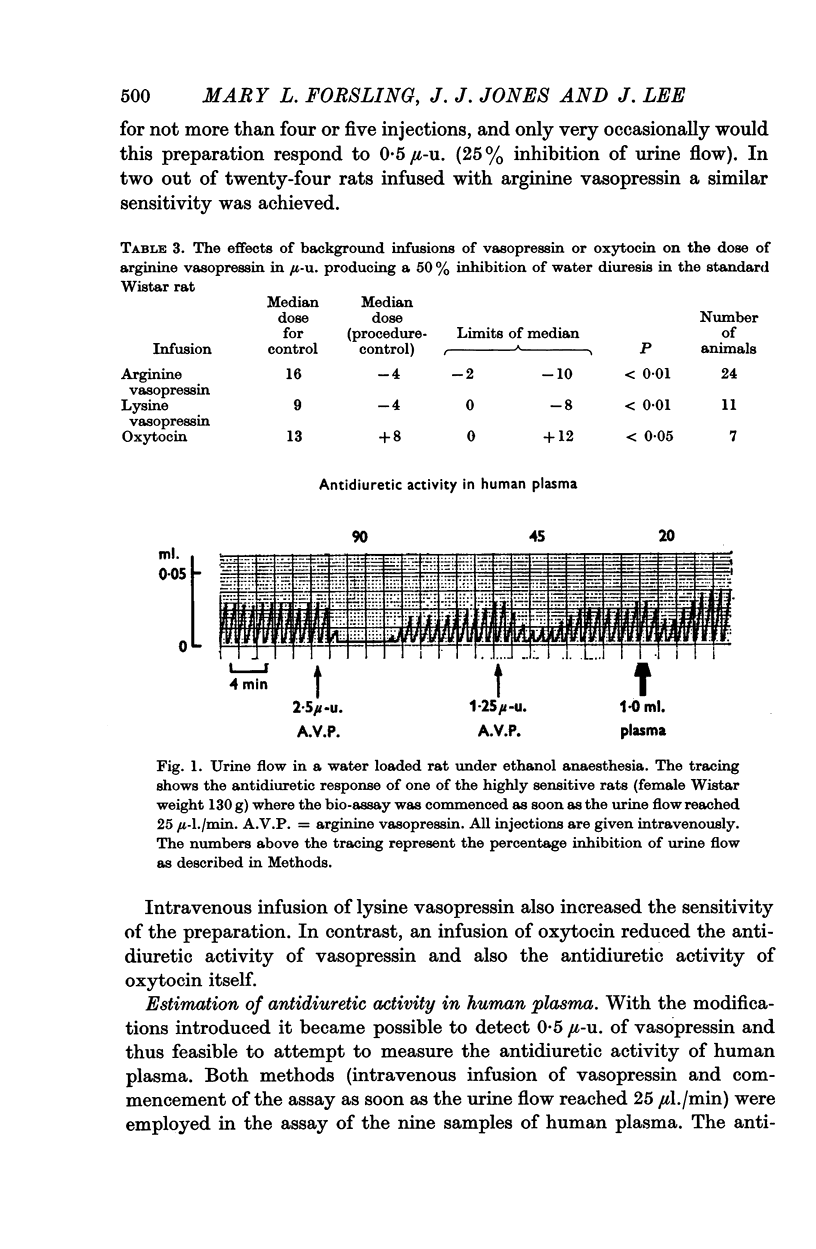
(b) commencing the assay shortly after surgery, i.e. as soon as the urine flow reached 25 μl./min;
(c) infusing vasopressin intravenously (0·5-3 μ-u./min). By using modification (a) with either (b) or (c) it was possible to detect as little as 0·5 μ-u.
4. With these modifications antidiuretic activity equivalent to 0·5-2·0 μ-u./ml. of arginine vasopressin was measured in nine samples of plasma from a normally hydrated subject.
5. It is suggested that the frequent reports of enhanced sensitivity may have been due to the fortuitous use of a particularly sensitive batch of rats, or to a high endogenous secretion of vasopressin due to operative trauma.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barraclough M. A., Jones J. J., Lee J. Production of vasopressin by anaplastic oat cell carcinoma of the bronchus. Clin Sci. 1966 Aug;31(1):135–144. [PubMed] [Google Scholar]
- Beránková-Ksandrová Z., Bisset G. W., Jost K., Krejci I., Pliska V., Rudinger J., Rychlík I., Sorm F. Synthetic analogues of oxytocin acting as hormonogens. Br J Pharmacol Chemother. 1966 Mar;26(3):615–632. doi: 10.1111/j.1476-5381.1966.tb01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CZACZKES J. W., KLEEMAN C. R., KOENIG M. PHYSIOLOGIC STUDIES OF ANTIDIURETIC HORMONE BY ITS DIRECT MEASUREMENT IN HUMAN PLASMA. J Clin Invest. 1964 Aug;43:1625–1640. doi: 10.1172/JCI105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E. A method for the assay of very small amounts of antidiuretic activity with a note on the antidiuretic titre of rat's blood. J Physiol. 1953 Oct;122(1):149–157. doi: 10.1113/jphysiol.1953.sp004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsling M. L., Jones J. J., Lee J. A change of sensitivity to neurohypophysial hormones induced by their intravenous infusion in the rat. J Physiol. 1967 Jul;191(2):127P–128P. [PubMed] [Google Scholar]
- Forsling M. L., Jones J. J., Lee J. Intravenous infusion of vasopressin to increase the sensitivity of its assay in the water-ethanol loaded rat. Nature. 1967 Jul 22;215(5099):433–434. doi: 10.1038/215433a0. [DOI] [PubMed] [Google Scholar]
- Gupta K. K., Chaudhury R. R., Chhuttani P. N. Plasma antidiuretic hormone concentrations in normal subjects and in persons with oedema of cardiac and renal origin, and in normal pregnancy. Indian J Med Res. 1967 Jun;55(6):643–651. [PubMed] [Google Scholar]
- HOLLIDAY M. A., BURSTIN C., HARRAH J. EVIDENCE THAT THE ANTIDIURETIC SUBSTANCE IN THE PLASMA OF CHILDREN WITH NEPHROGENIC DIABETES INSIPIDUS IS ANTIDIURETIC HORMONE. Pediatrics. 1963 Sep;32:384–388. [PubMed] [Google Scholar]
- Jones J. J., Lee J. The value of rats with hereditary hypothalamic diabetes insipidus for the bioassay of vasopressin. J Endocrinol. 1967 Mar;37(3):335–344. doi: 10.1677/joe.0.0370335. [DOI] [PubMed] [Google Scholar]
- Jones J. J., Lee J. The value of the pre-operated rat in the bioassay of vasopressin. J Endocrinol. 1965 Oct;33(2):329–330. doi: 10.1677/joe.0.0330329. [DOI] [PubMed] [Google Scholar]
- KENNEDY G. C., LIPSCOMB H. S., HAGUE P. PLASMA CORTICOSTERONE IN RATS WITH EXPERIMENTAL DIABETES INSIPIDUS. J Endocrinol. 1963 Nov;27:345–353. doi: 10.1677/joe.0.0270345. [DOI] [PubMed] [Google Scholar]
- Lauson H. D. Metabolism of antidiuretic hormones. Am J Med. 1967 May;42(5):713–744. doi: 10.1016/0002-9343(67)90091-5. [DOI] [PubMed] [Google Scholar]
- Sawyer W. H., Valtin H. Antidiuretic responses of rats with hereditary hypothalamic diabetes insipidus to vasopressin, oxytocin and nicotine. Endocrinology. 1967 Jan;80(1):207–210. doi: 10.1210/endo-80-1-207. [DOI] [PubMed] [Google Scholar]
- Tata P. S., Buzalkov R. Vasopressin studies in the rat. 3. Inability of ethanol anesthesia to prevent ADH secretion due to pain and hemorrhage. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;290(4):294–297. [PubMed] [Google Scholar]
- Tata P. S., Gauer O. H. Vasopressin studies in the rat. I. A sensitive bioassay for exogenous vasopressin. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;290(4):279–285. doi: 10.1007/BF00363305. [DOI] [PubMed] [Google Scholar]
- Vierling A. F., Little J. B., Radford E. P., Jr Antidiuretic hormone bio-assay in rats with hereditary hypothalamic diabetes insipidus (Brattleboro strain). Endocrinology. 1967 Jan;80(1):211–214. doi: 10.1210/endo-80-1-211. [DOI] [PubMed] [Google Scholar]


