Abstract
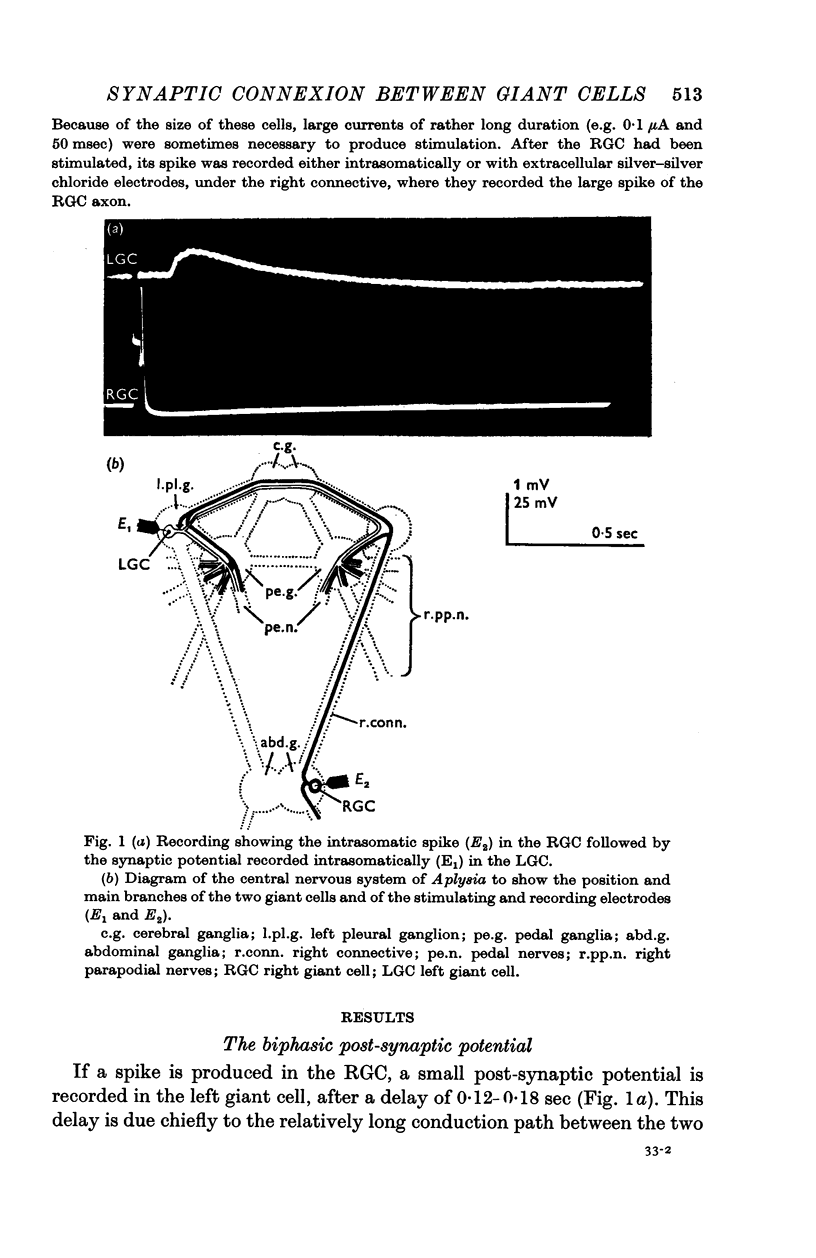
1. In Aplysia fasciata, intrasomatic stimulation of the giant cell (RGC) of the right upper quadrant of the abdominal ganglia is followed after a constant delay by the appearance of a synaptic potential recorded in the giant cell (LGC) of the left pleural ganglion.
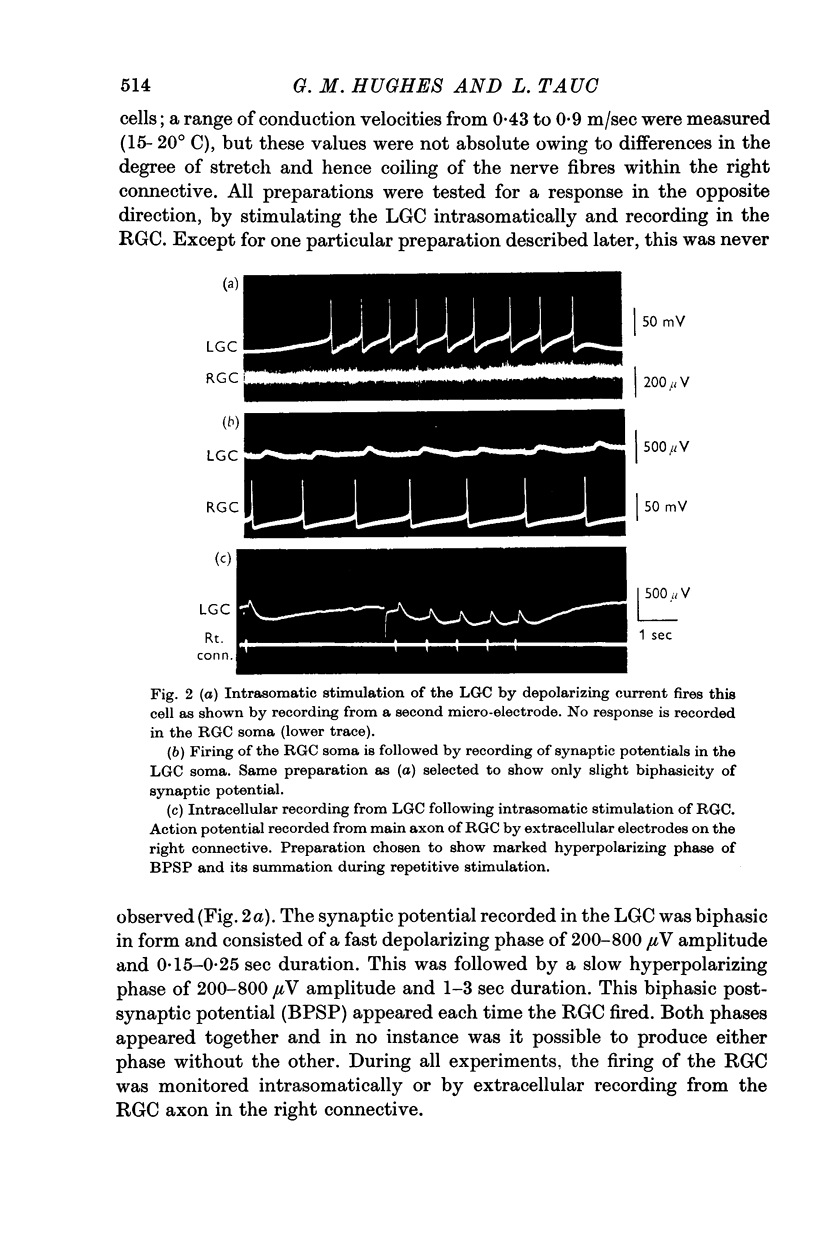
2. The synaptic potential recorded in the LGC soma has a biphasic form (hence biphasic post-synaptic potential or BPSP) consisting of a fast depolarizing phase of about 200-800 μV amplitude and 0·15-0·25 sec duration, followed by a slow hyperpolarizing phase of about 200-800 μV amplitude and 1-3 sec duration.
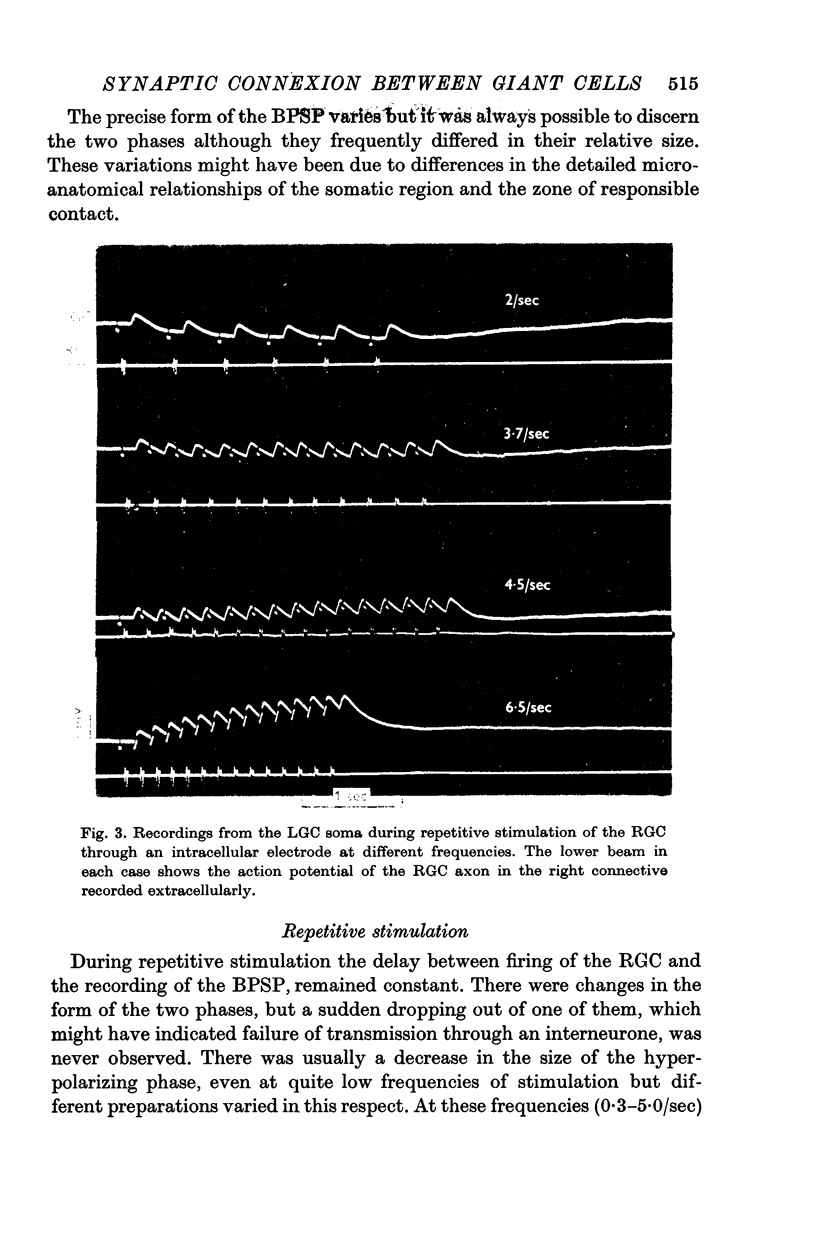
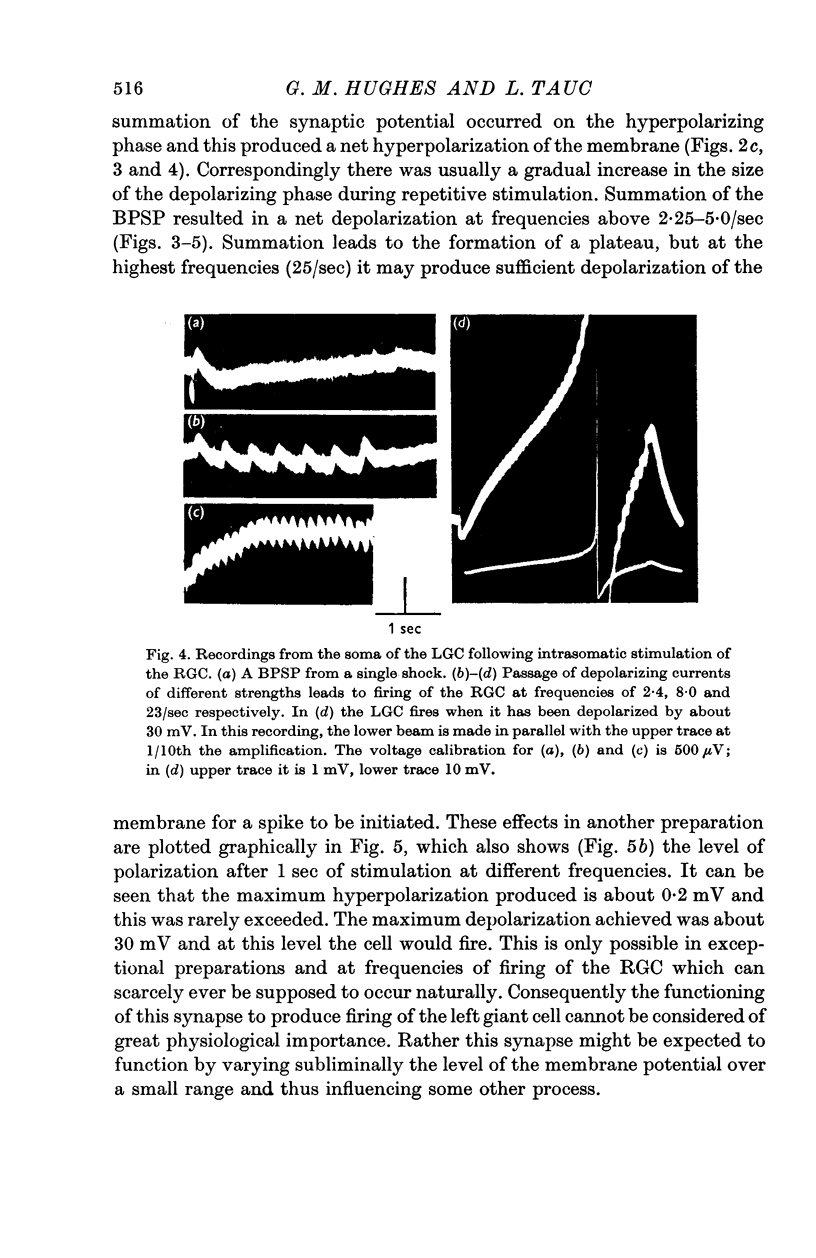
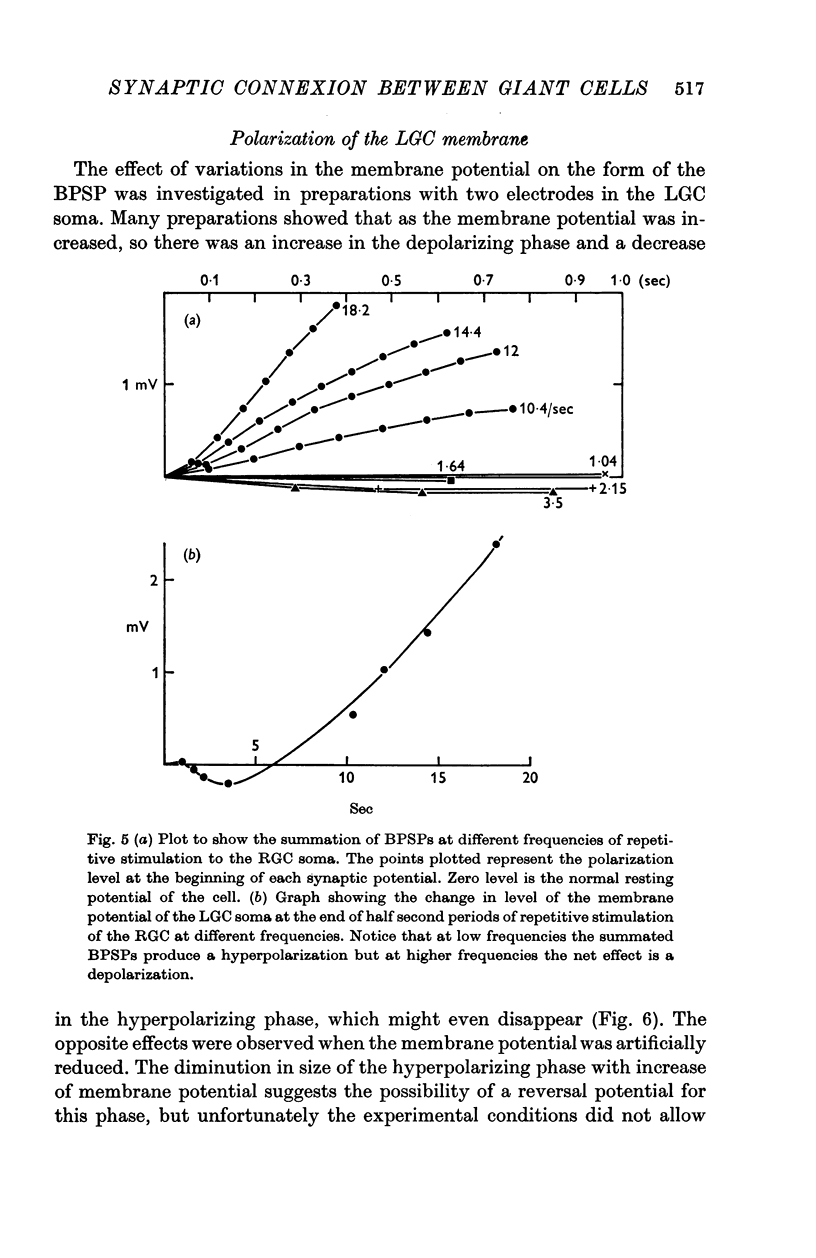
3. During repetitive stimulation summation results in an over-all hyperpolarization at low frequencies (less than 5/sec) and an over-all depolarization for higher frequencies. Very high frequencies (25/sec) of stimulation of the RGC may elicit a spike in the LGC.
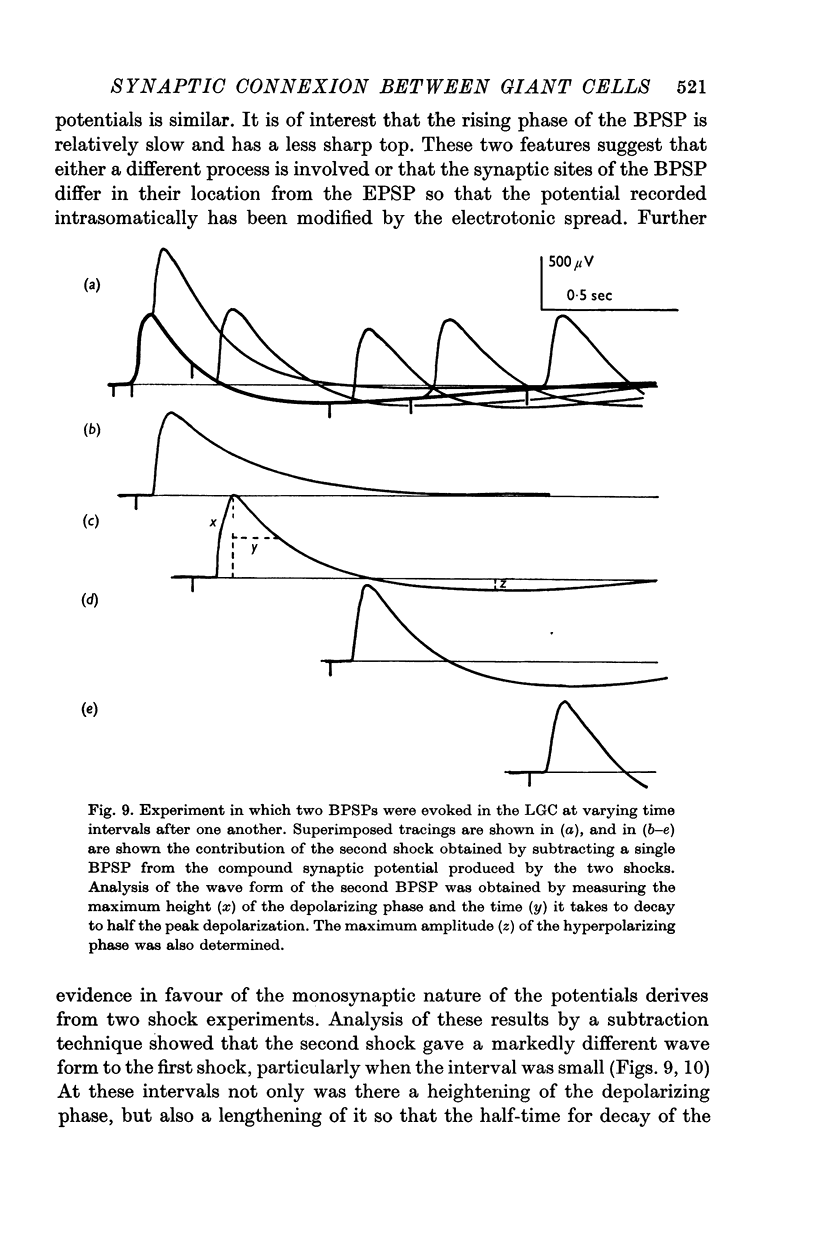
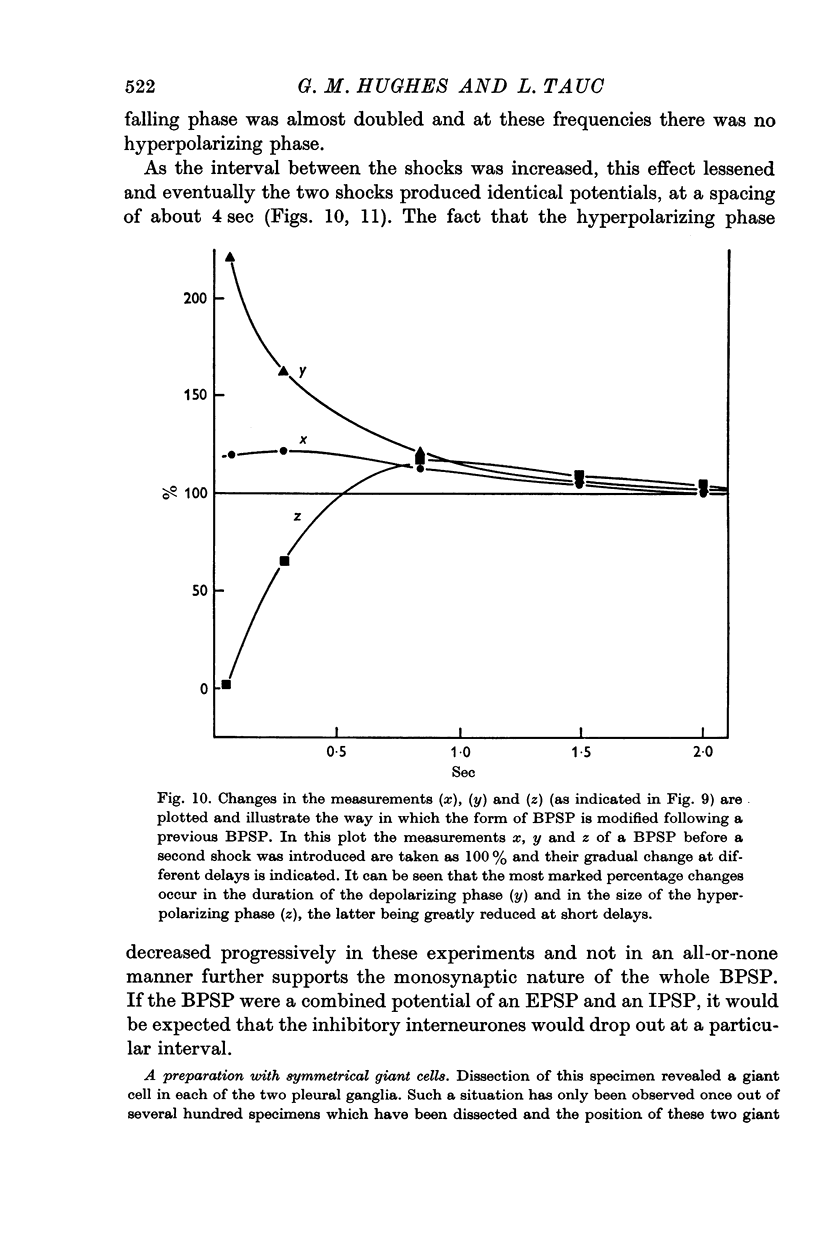
4. Stimulation with two shocks showed increasing effects with shorter intervals on both the depolarizing and hyperpolarizing phase. These effects were progressive and there was no falling out of one of the two phases as might be expected if the BPSP was a composite of an inhibitory post-synaptic potential (IPSP) and an excitatory post-synaptic potential (EPSP).
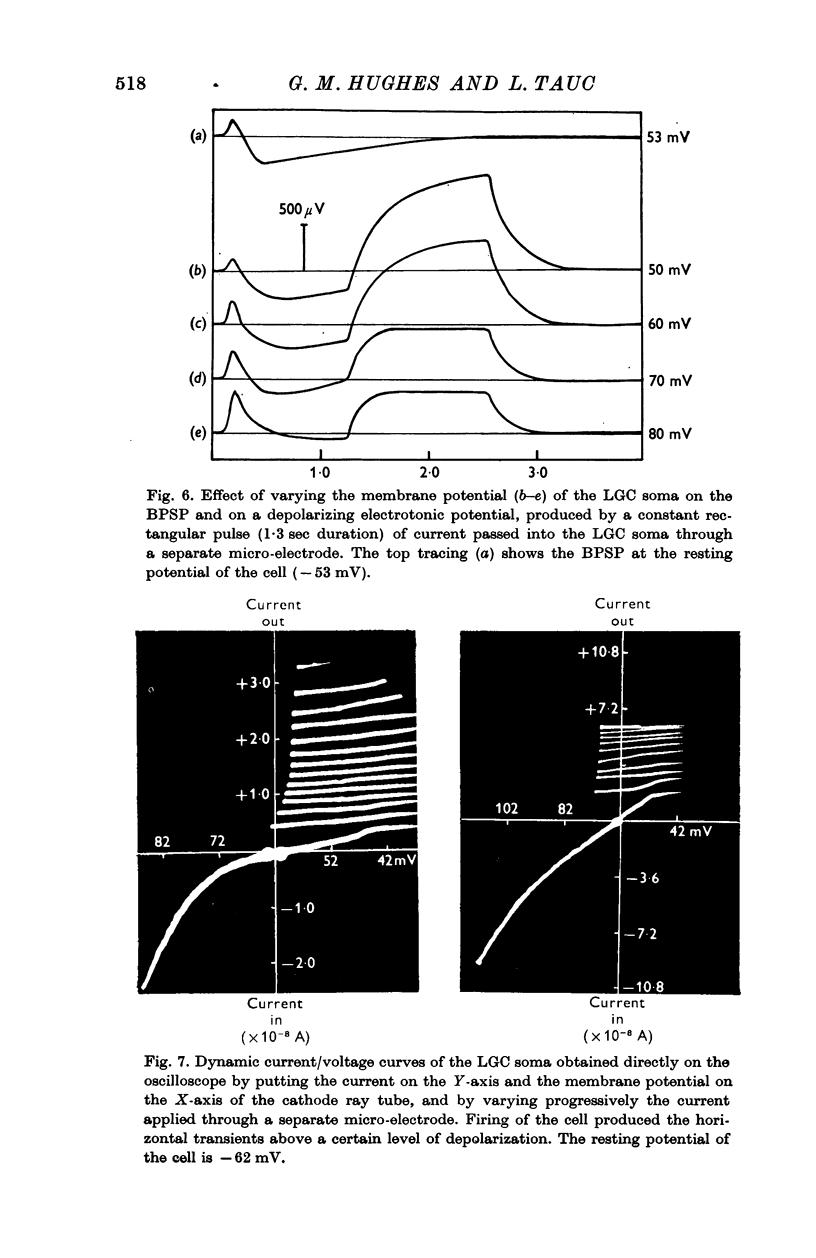
5. The effects of artificially imposed polarization of the LGC through a second micro-electrode suggest that the BPSP results from a chemical transmission mechanism for both its depolarizing and hyperpolarizing phases but electrical transmission cannot be excluded.
6. Curare has no effect on the BPSP and thus excludes a cholinergic transmission mechanism. Chloride ions injected into the LGC soma do not appear to modify the BPSP and hence it is concluded that the hyperpolarizing phase is different from IPSPs of the same cell.
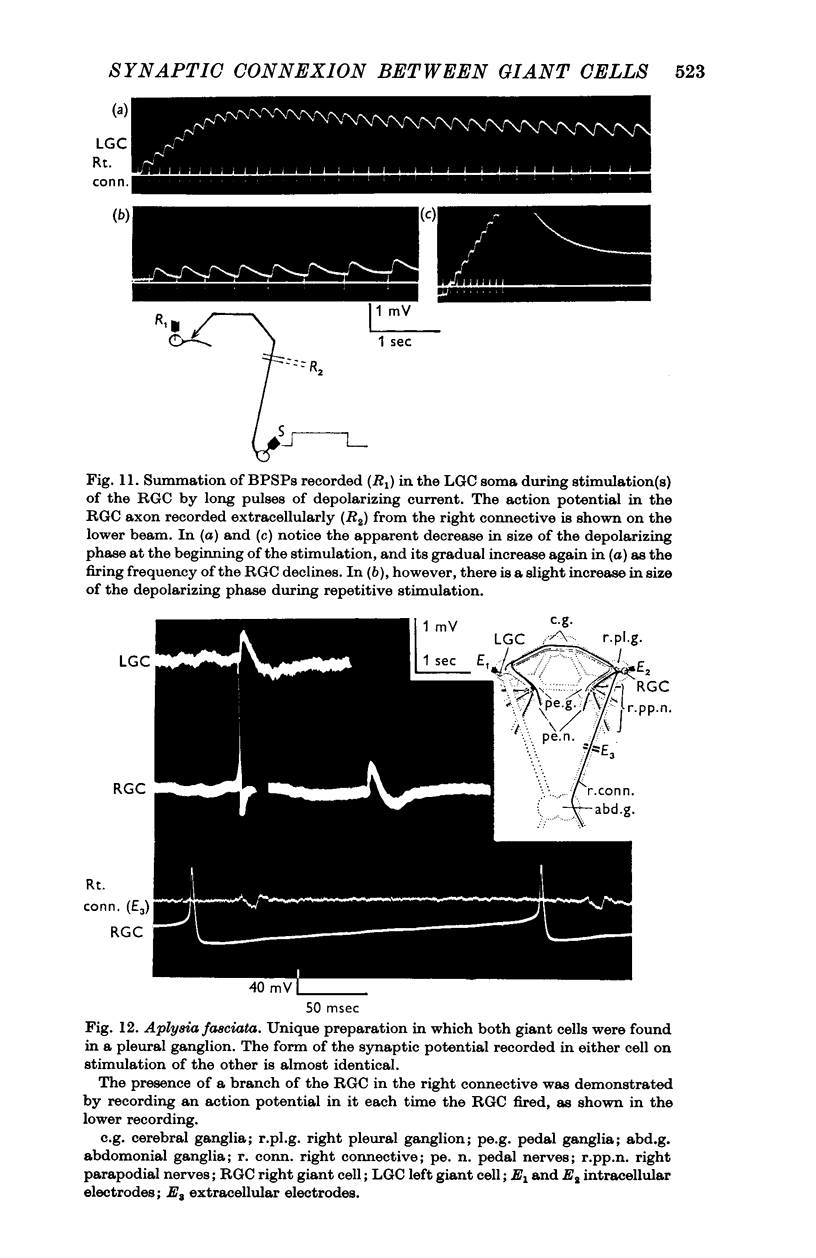
7. No synaptic potential is recorded in the RGC following stimulation of the LGC, except in a single preparation in which the RGC soma was situated in the right pleural ganglion. In this case the synaptic potential recorded in both giant cells following stimulation of the other, was biphasic in form.
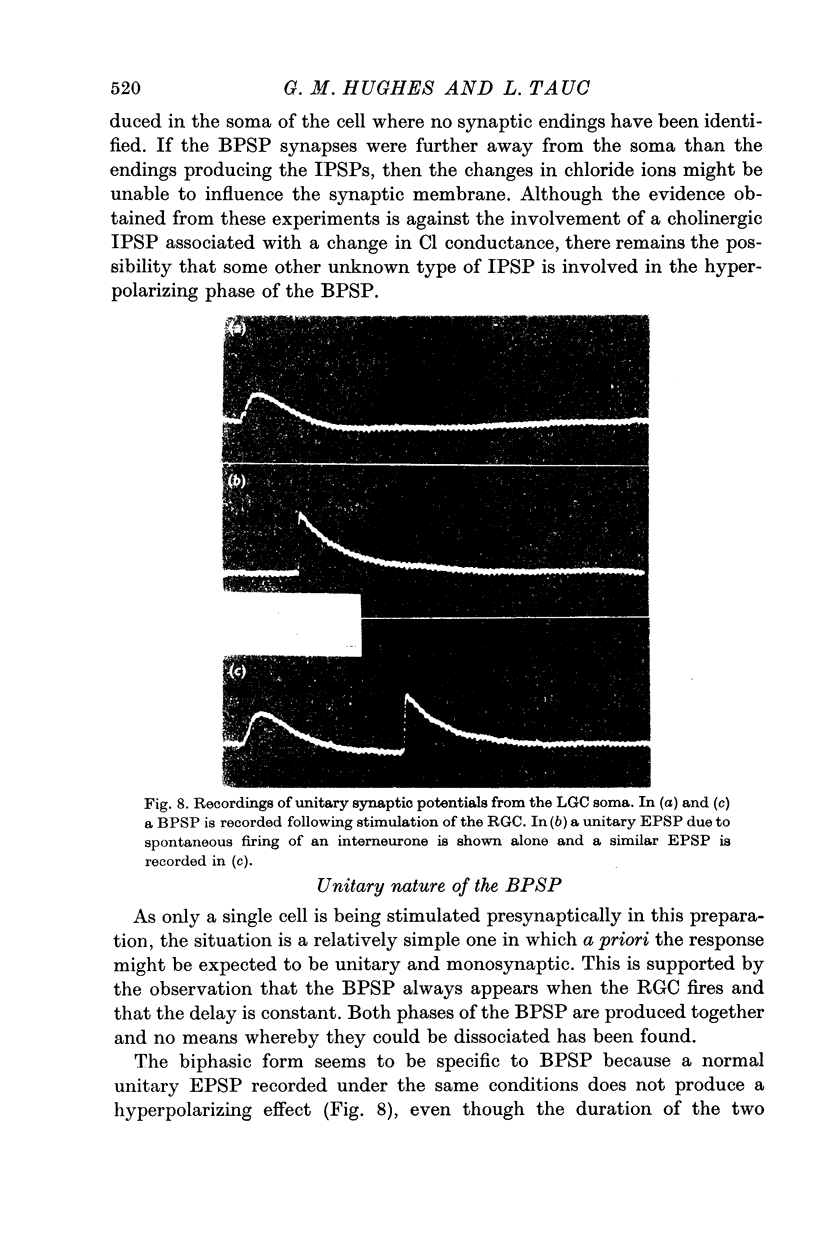
8. It is concluded that the BPSP is a unitary monosynaptic potential which is a characteristic feature of the organization of these two giant cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERKOWITZ E. C. Functional properties of spinal pathways in the carp, Cyprinus carpio L. J Comp Neurol. 1956 Dec;106(2):269–289. doi: 10.1002/cne.901060202. [DOI] [PubMed] [Google Scholar]
- Dale H. Pharmacology and Nerve-endings (Walter Ernest Dixon Memorial Lecture): (Section of Therapeutics and Pharmacology). Proc R Soc Med. 1935 Jan;28(3):319–332. [PMC free article] [PubMed] [Google Scholar]
- Eckert R. Electrical Interaction of Paired Ganglion Cells in the Leech. J Gen Physiol. 1963 Jan 1;46(3):573–587. doi: 10.1085/jgp.46.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J., FURUKAWA T. Intracellular and extracellular responses of the several regions of the Mauthner cell of the goldfish. J Neurophysiol. 1962 Nov;25:732–771. doi: 10.1152/jn.1962.25.6.732. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K. Synaptic inhibition in giant nerve cell of Onchidium verruculatum. J Neurophysiol. 1961 Mar;24:167–175. doi: 10.1152/jn.1961.24.2.167. [DOI] [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. AN ELECTROPHYSIOLOGICAL STUDY OF THE ANATOMICAL RELATIONS OF TWO GIANT NERVE CELLS IN APLYSIA DEPILANS. J Exp Biol. 1963 Sep;40:469–486. doi: 10.1242/jeb.40.3.469. [DOI] [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. Aspects of the organization of central nervous pathways in Aplysia depilans. J Exp Biol. 1962 Mar;39:45–69. doi: 10.1242/jeb.39.1.45. [DOI] [PubMed] [Google Scholar]
- HUGHES G. M., TAUC L. The path of the giant cell axons in Aplysia depilans. Nature. 1961 Jul 22;191:404–405. doi: 10.1038/191404a0. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W., VAUGHAN WILLIAMS E. M. Small-nerve junctional potentials; the distribution of small motor nerves to frog skeletal muscle, and the membrane characteristics of the fibres they innervate. J Physiol. 1953 Aug;121(2):289–317. doi: 10.1113/jphysiol.1953.sp004948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Coggeshall R. E. Opposite synaptic actions mediated by different branches of an identifiable interneuron in Aplysia. Science. 1967 Jan 20;155(3760):346–349. doi: 10.1126/science.155.3760.346. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Input organization of two symmetrical giant cells in the snail brain. J Physiol. 1966 Mar;183(2):269–286. doi: 10.1113/jphysiol.1966.sp007866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon D., Jr Analysis of compound postsynaptic potentials in the central nervous system of the surf clam. J Gen Physiol. 1967 Jan;50(3):759–778. doi: 10.1085/jgp.50.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA K. I. ELECTROPHYSIOLOGY OF THE FETAL SPINAL CORD. I. ACTION POTENTIALS OF THE MOTONEURON. J Gen Physiol. 1964 May;47:1003–1022. doi: 10.1085/jgp.47.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. A cholinergic mechanism of inhibitory synaptic transmission in a molluscan nervous system. J Neurophysiol. 1962 Mar;25:236–262. doi: 10.1152/jn.1962.25.2.236. [DOI] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. Cholinergic transmission mechanisms for both excitation and inhibition in molluscan central synapses. Nature. 1961 Oct 28;192:366–367. doi: 10.1038/192366a0. [DOI] [PubMed] [Google Scholar]


