Abstract
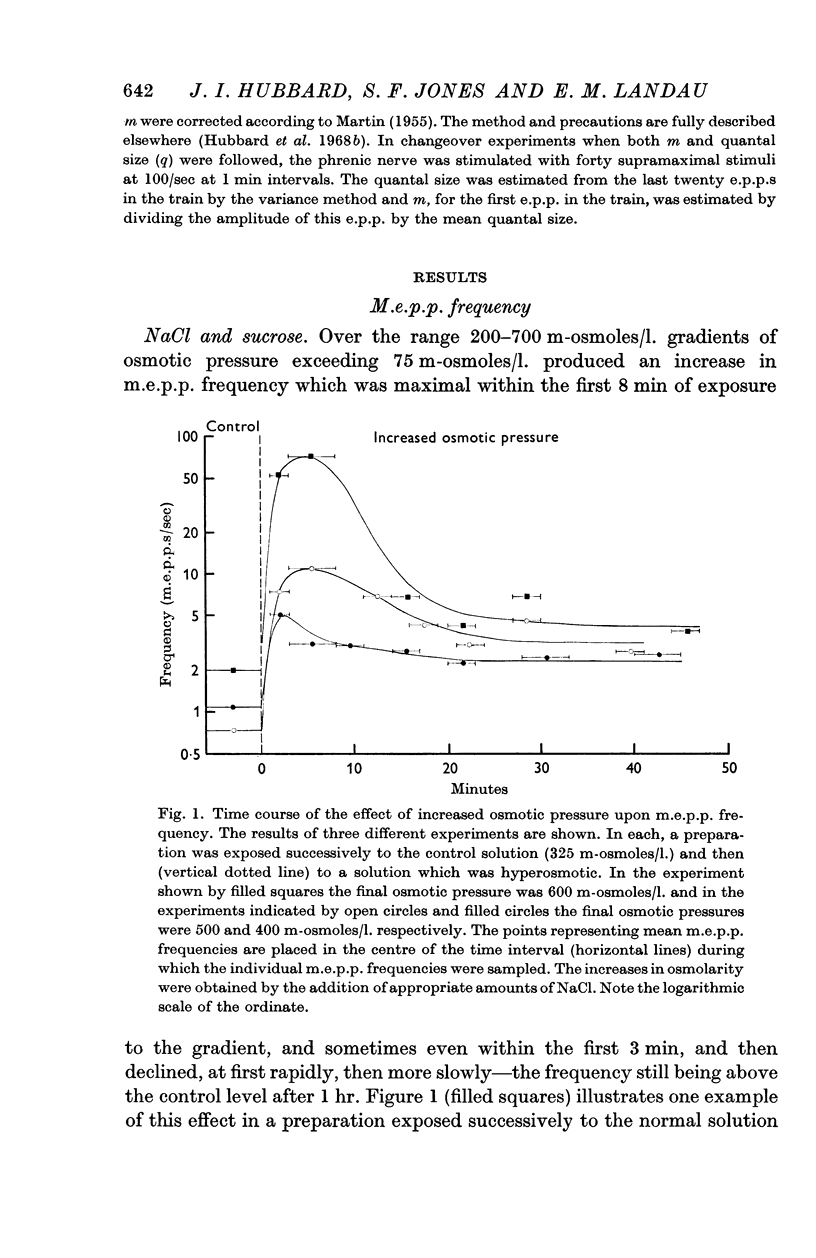
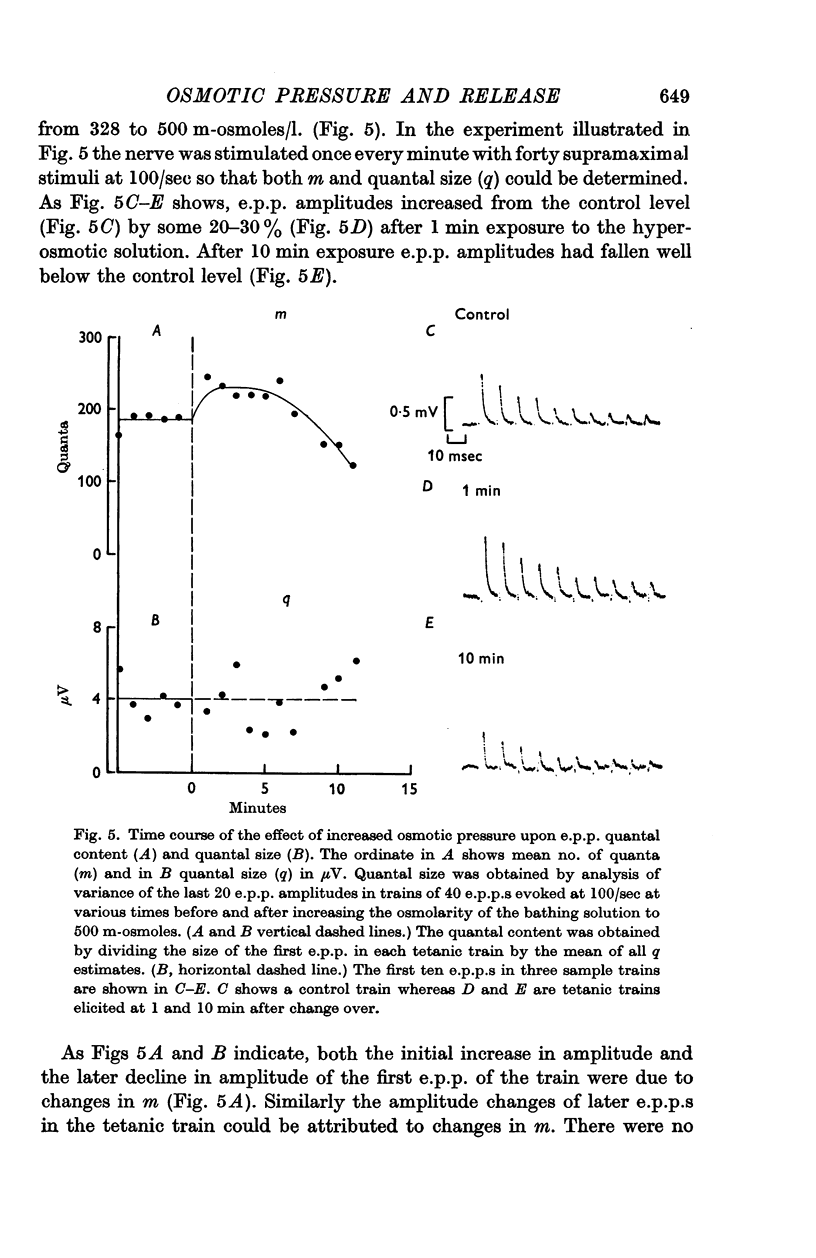
1. When the frequency of miniature end-plate potentials (m.e.p.p.s) was measured at neuromuscular junctions in rat diaphragm nerve preparations in vitro bathed in solutions having osmolarities between 200 and 700 m-osmoles/l. it was found that m.e.p.p. frequency was transiently increased by exposure to osmotic gradients exceeding 75 m-osmoles/l., and then declined, within 1 hr, to a steady level slightly higher than the control level of frequency. Smaller osmotic gradients caused a maintained increase in m.e.p.p. frequency. E.p.p. quantal content was initially increased and later profoundly decreased upon exposure of preparations to solutions with an osmotic pressure of 500 or 600 m-osmoles/l. but was unaffected by less hypertonic solutions.
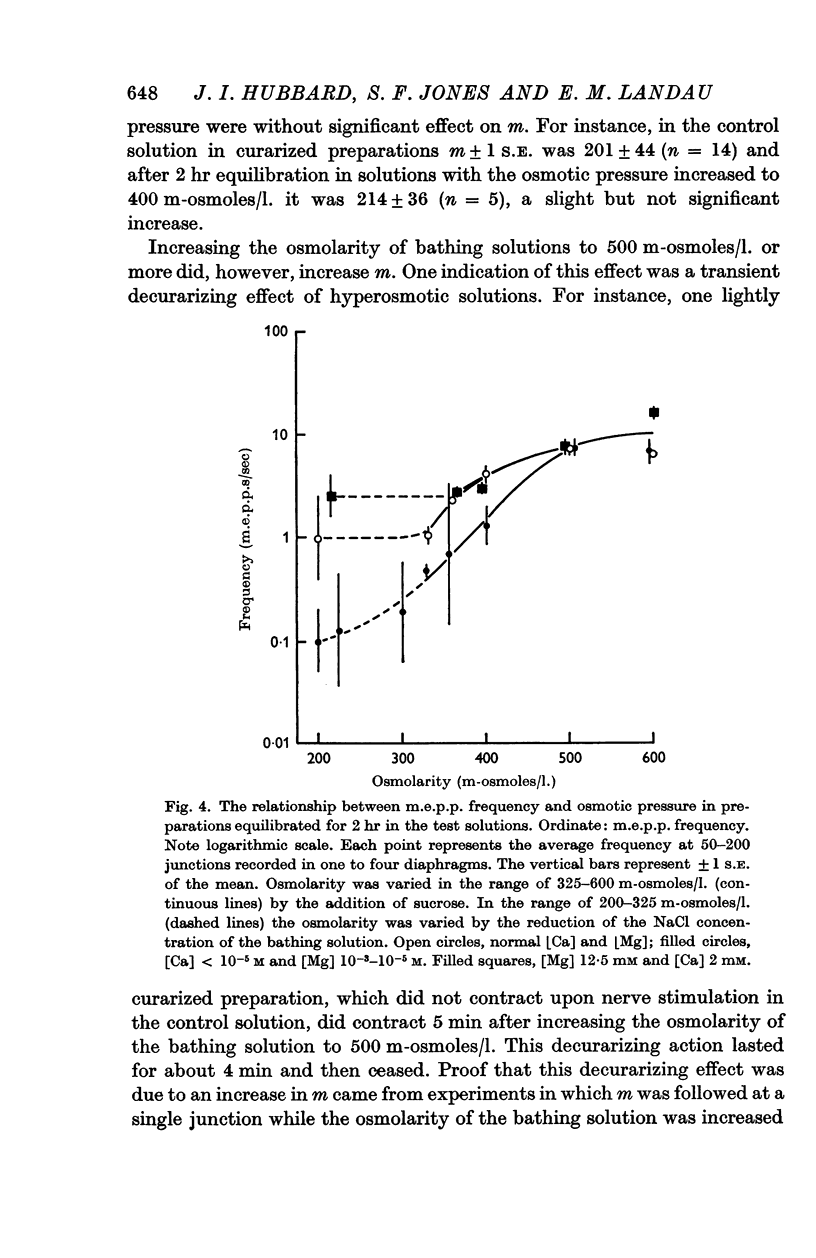
2. Variation of the Ca or Mg content of the bathing solutions did not alter these effects of osmotic pressure on the early transient increase in m.e.p.p. frequency or e.p.p. quantal content but affected the late steady increase in m.e.p.p. frequency.
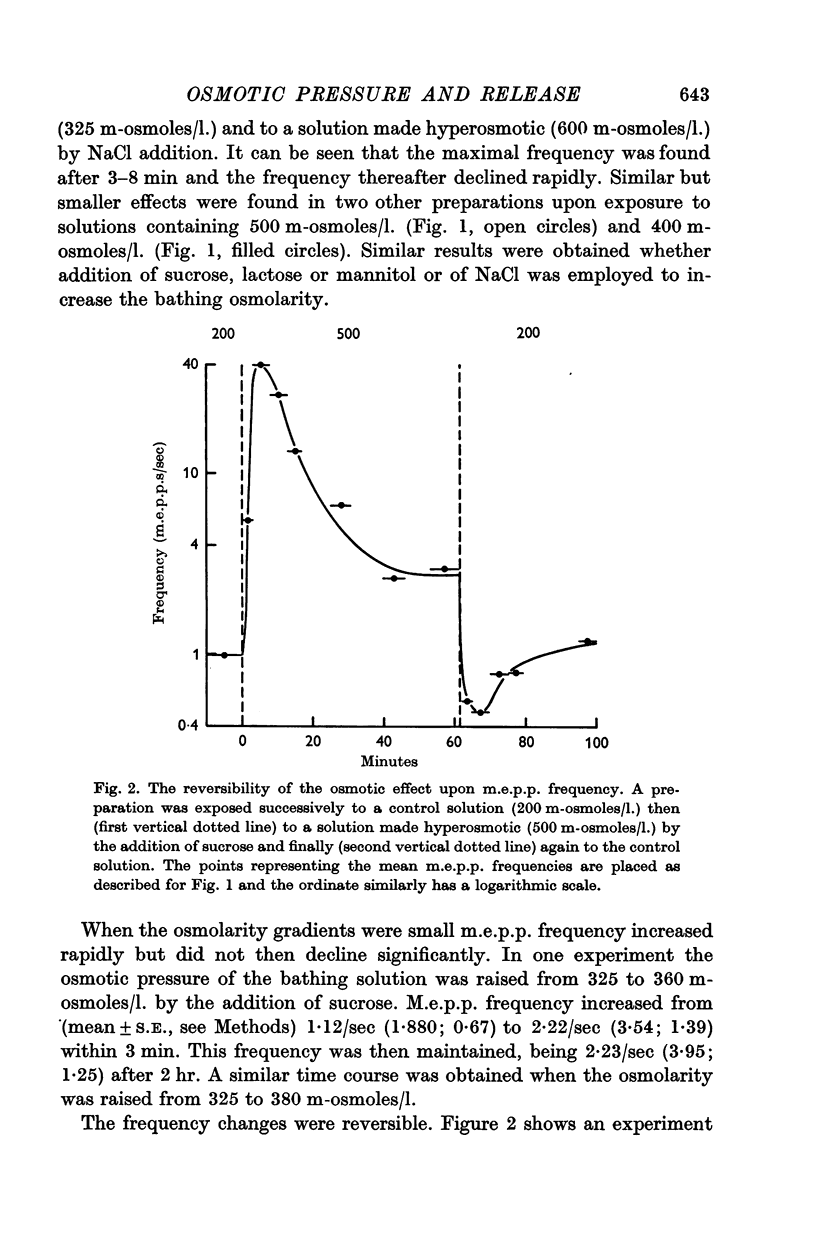
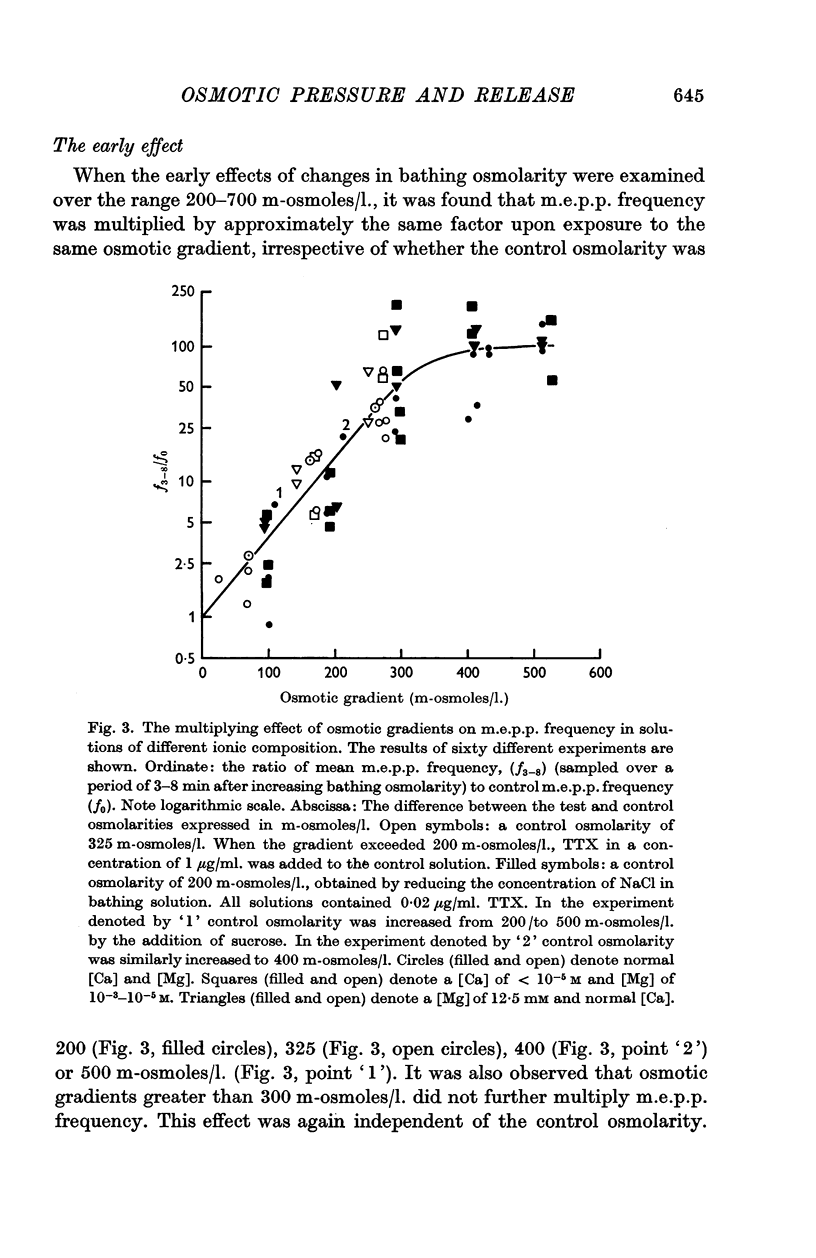
3. The value of the transient increase in m.e.p.p. frequency was exponentially related to the osmotic gradient in the range 0-300 m-osmoles/l. with a Q10 of 1·95 (range 11-34° C). Greater osmotic gradients did not further increase m.e.p.p. frequency. Variation of the ionic strength of the bathing medium did not influence osmotic effects upon frequency.
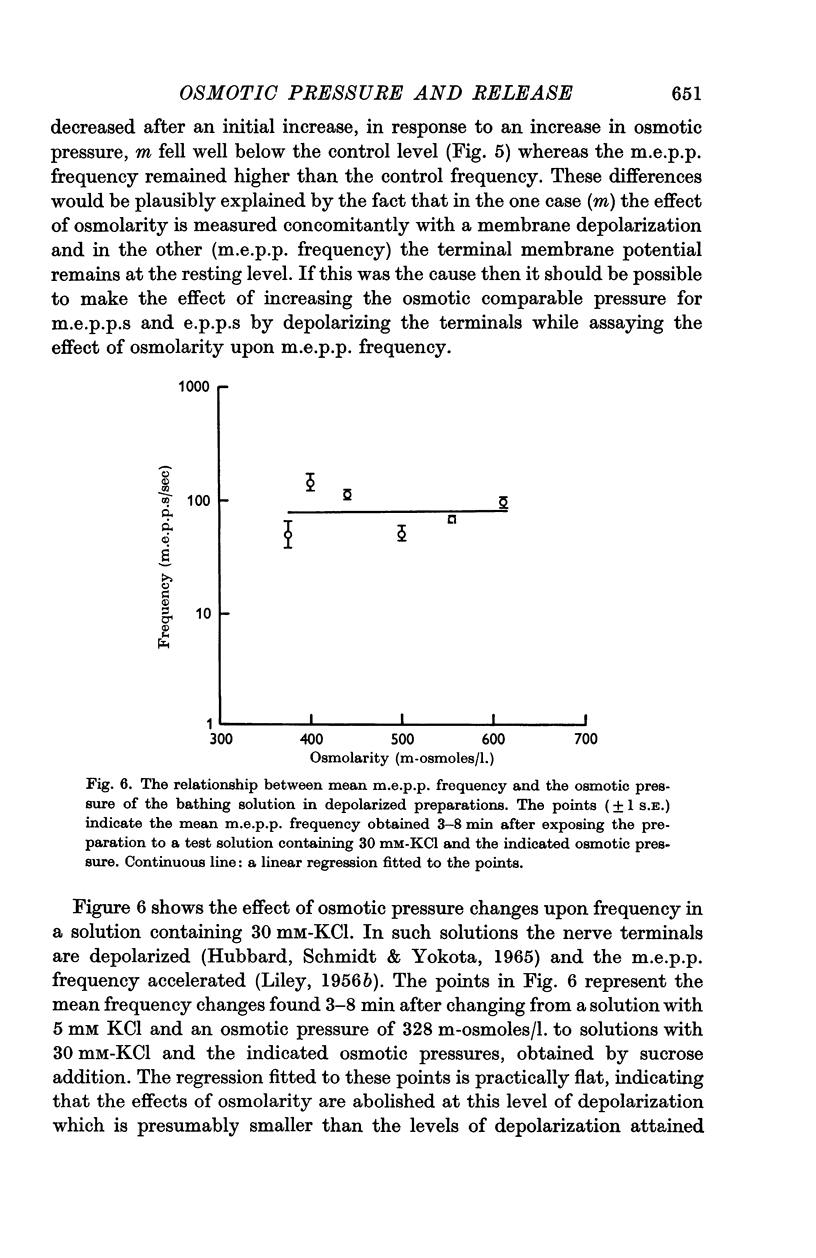
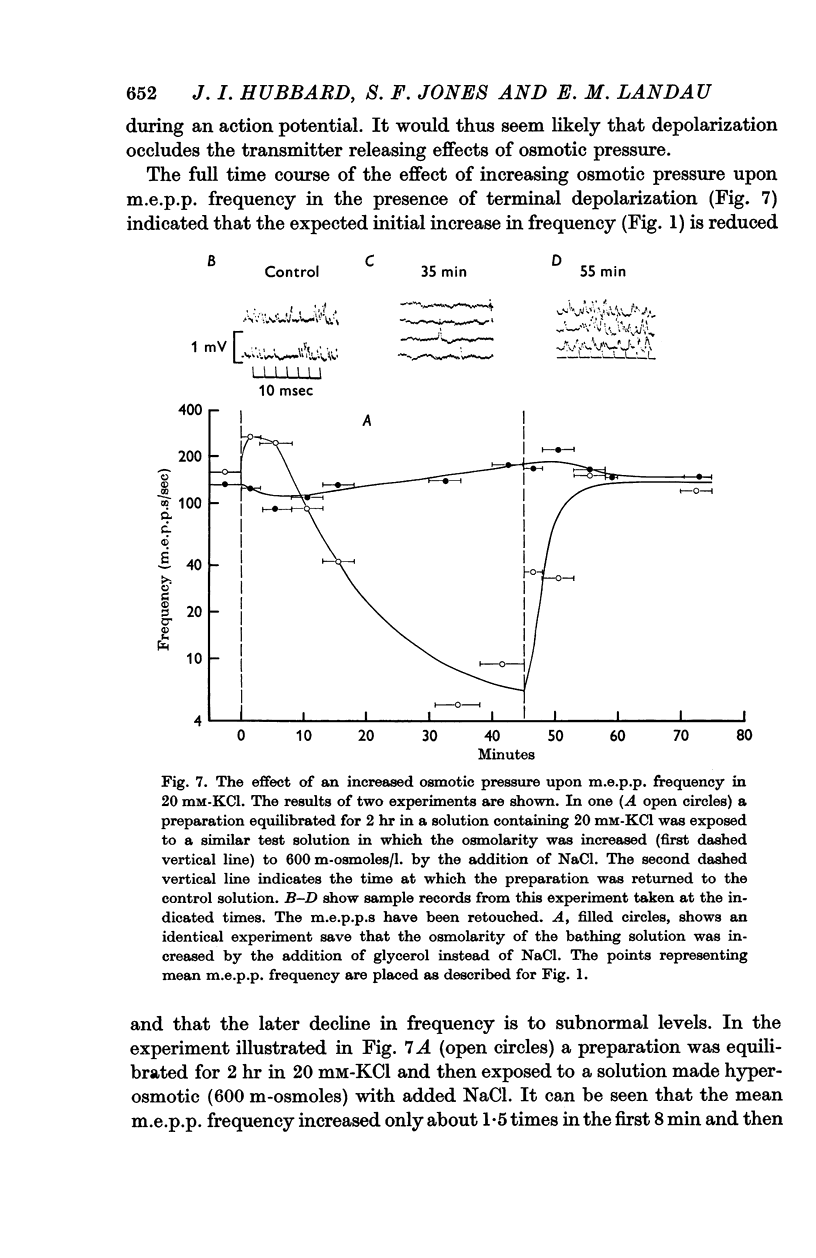
4. The discrepancy between the effects of osmotic gradients upon spontaneous and nerve-impulse induced transmitter release was explained by an occlusion of the osmotic effects by depolarization of nerve terminals. Time-course studies showed that in the presence of 20 mM-KCl the m.e.p.p. frequency increase in response to an increase in osmotic pressure was small and was followed by a reduction in frequency to below control levels while osmotic pressure changes had no immediate effect upon m.e.p.p. frequency in solutions containing 30 mM-KCl.
5. It was concluded that increased osmotic gradients could release transmitter by a mechanism independent of Ca and of nerve terminal depolarization.
6. It is suggested that the initial transient effects of changes of osmotic gradient upon transmitter release are related to flow of water through the nerve terminal membrane, while the later effects are related to nerve terminal volume changes.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass L., Moore W. J. Electrokinetic mechanism of miniature postsynaptic potentials. Proc Natl Acad Sci U S A. 1966 May;55(5):1214–1217. doi: 10.1073/pnas.55.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Changes in end-plate activity produced by presynaptic polarization. J Physiol. 1954 Jun 28;124(3):586–604. doi: 10.1113/jphysiol.1954.sp005131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Spontaneous activity at a mammalian neuromuscular junction in tetrodotoxin. Acta Physiol Scand. 1965 Aug;64(4):475–476. doi: 10.1111/j.1748-1716.1965.tb04206.x. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman A. R., Reuben J. P., Brandt P. W., Grundfest H. Osmometrically determined characteristics of the cell membrane of squid and lobster giant axons. J Gen Physiol. 1966 Nov;50(2):423–445. doi: 10.1085/jgp.50.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. An investigation of the post-tetanic potentiation of end-plate potentials at a mammalian neuromuscular junction. J Physiol. 1966 May;184(2):353–375. doi: 10.1113/jphysiol.1966.sp007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. The origin of the post-tetanic hyperpolarization of mammalian motor nerve terminals. J Physiol. 1966 May;184(2):335–352. doi: 10.1113/jphysiol.1966.sp007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Quastel D. M. Competition between sodium and calcium ions in transmitter release at mammalian neuromuscular junctions. J Physiol. 1966 Jul;185(1):95–123. doi: 10.1113/jphysiol.1966.sp007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL D. K. The volume change resulting from stimulation of a giant nerve fibre. J Physiol. 1950 Oct 16;111(3-4):304–327. doi: 10.1113/jphysiol.1950.sp004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARTH J. V. The behaviour of frog muscle in hypertonic solutions. J Physiol. 1958 Nov 10;144(1):167–175. doi: 10.1113/jphysiol.1958.sp006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I. The effect of calcium and magnesium on the spontaneous release of transmitter from mammalian motor nerve endings. J Physiol. 1961 Dec;159:507–517. doi: 10.1113/jphysiol.1961.sp006824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD J. I., WILLIS W. D. Hyperpolarization of mammalian motor nerve terminals. J Physiol. 1962 Aug;163:115–137. doi: 10.1113/jphysiol.1962.sp006961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the release of transmitter by nerve impulses. J Physiol. 1968 May;196(1):75–86. doi: 10.1113/jphysiol.1968.sp008495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. On the mechanism by which calcium and magnesium affect the spontaneous release of transmitter from mammalian motor nerve terminals. J Physiol. 1968 Feb;194(2):355–380. doi: 10.1113/jphysiol.1968.sp008413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. The relationship between the state of nerve-terminal polarization and liberation of acetylcholine. Ann N Y Acad Sci. 1967 Oct 31;144(2):459–470. doi: 10.1111/j.1749-6632.1967.tb53787.x. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Schmidt R. F., Yokota T. The effect of acetylcholine upon mammalian motor nerve terminals. J Physiol. 1965 Dec;181(4):810–829. doi: 10.1113/jphysiol.1965.sp007799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin and neuromuscular transmission. Proc R Soc Lond B Biol Sci. 1967 Jan 31;167(1006):8–22. doi: 10.1098/rspb.1967.0010. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The effects of presynaptic polarization on the spontaneous activity at the mammalian neuromuscular junction. J Physiol. 1956 Nov 28;134(2):427–443. doi: 10.1113/jphysiol.1956.sp005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEHEIL G., NAESS K. Note on the effect of increased NaCl-concentration on the neuromuscular transmission. Does desensitization to acetylcholine take place during tetanus? Acta Physiol Scand. 1961 May;52:23–31. doi: 10.1111/j.1748-1716.1961.tb02195.x. [DOI] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. Structural alterations in nerve fibers produced by hypotonic and hypertonic solutions. J Biophys Biochem Cytol. 1958 Jul 25;4(4):349–364. doi: 10.1083/jcb.4.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H. Osmotic properties of frog nerve. Comp Biochem Physiol. 1966 Sep;19(1):225–239. doi: 10.1016/0010-406x(66)90561-5. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Motor end-plate 'desensitization' by repetitive nerve stimuli. J Physiol. 1959 Oct;148:659–664. doi: 10.1113/jphysiol.1959.sp006314. [DOI] [PMC free article] [PubMed] [Google Scholar]


