Abstract
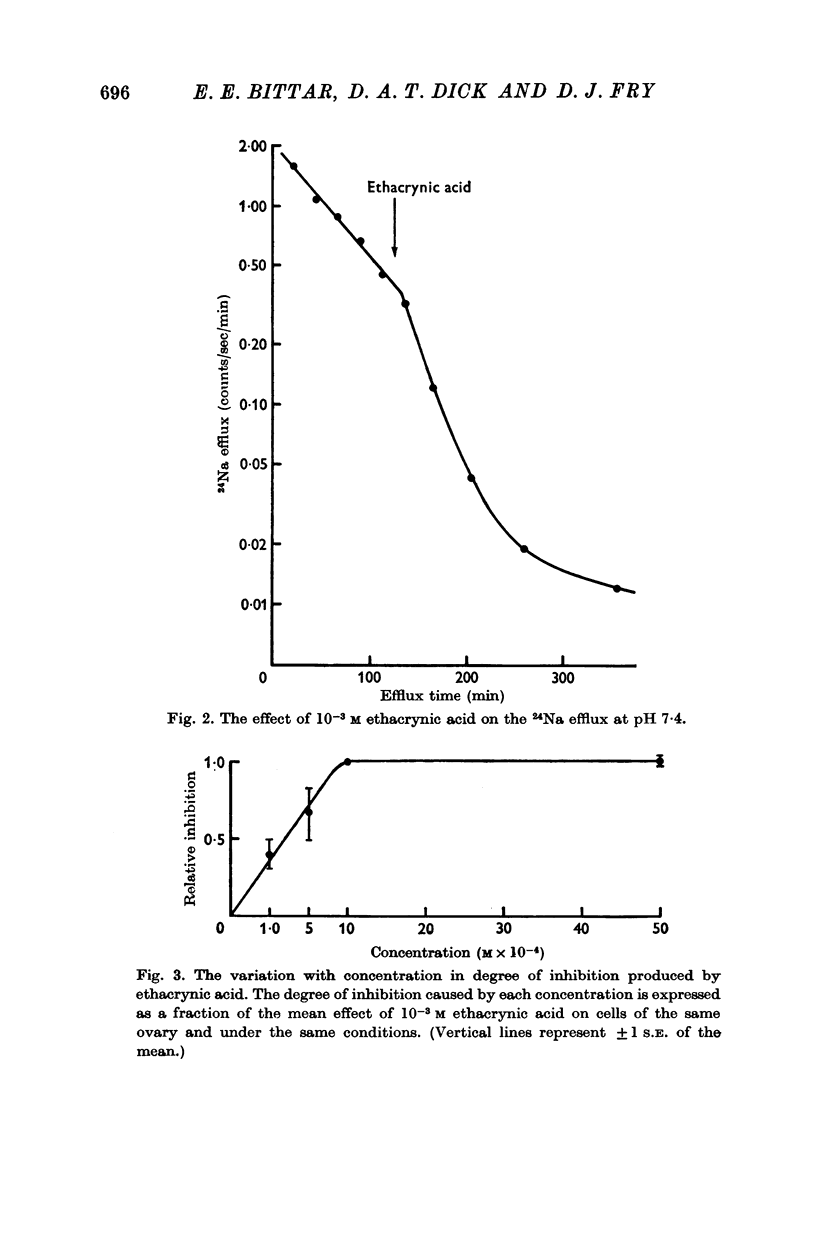
1. Ethacrynic acid (10-3 M) at pH 7·4 caused approximately 83% inhibition of Na efflux from single dissected toad oocytes.
2. Less inhibition was found at concentrations below 10-3 M but higher concentrations did not cause greater inhibition.
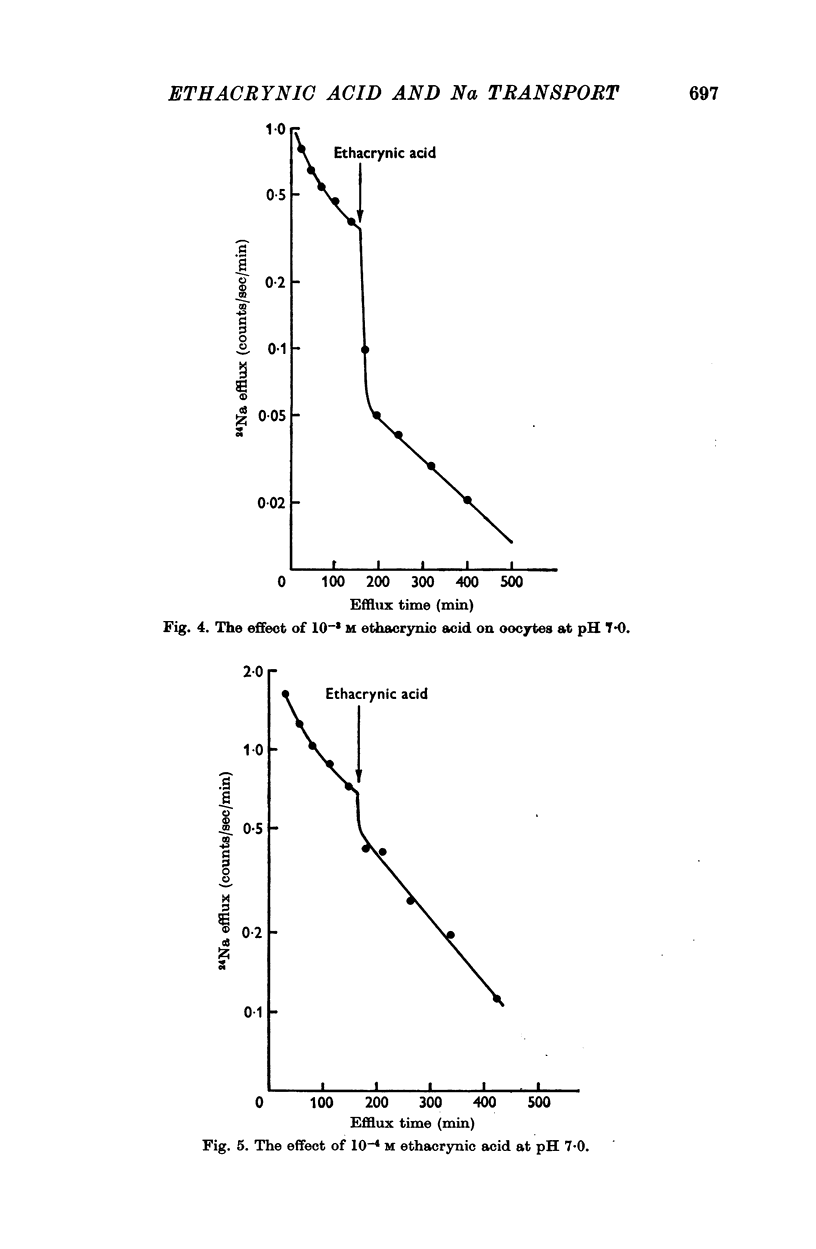
3. At an external pH of 7·0 the onset of inhibition was more rapid than at pH 7·4.
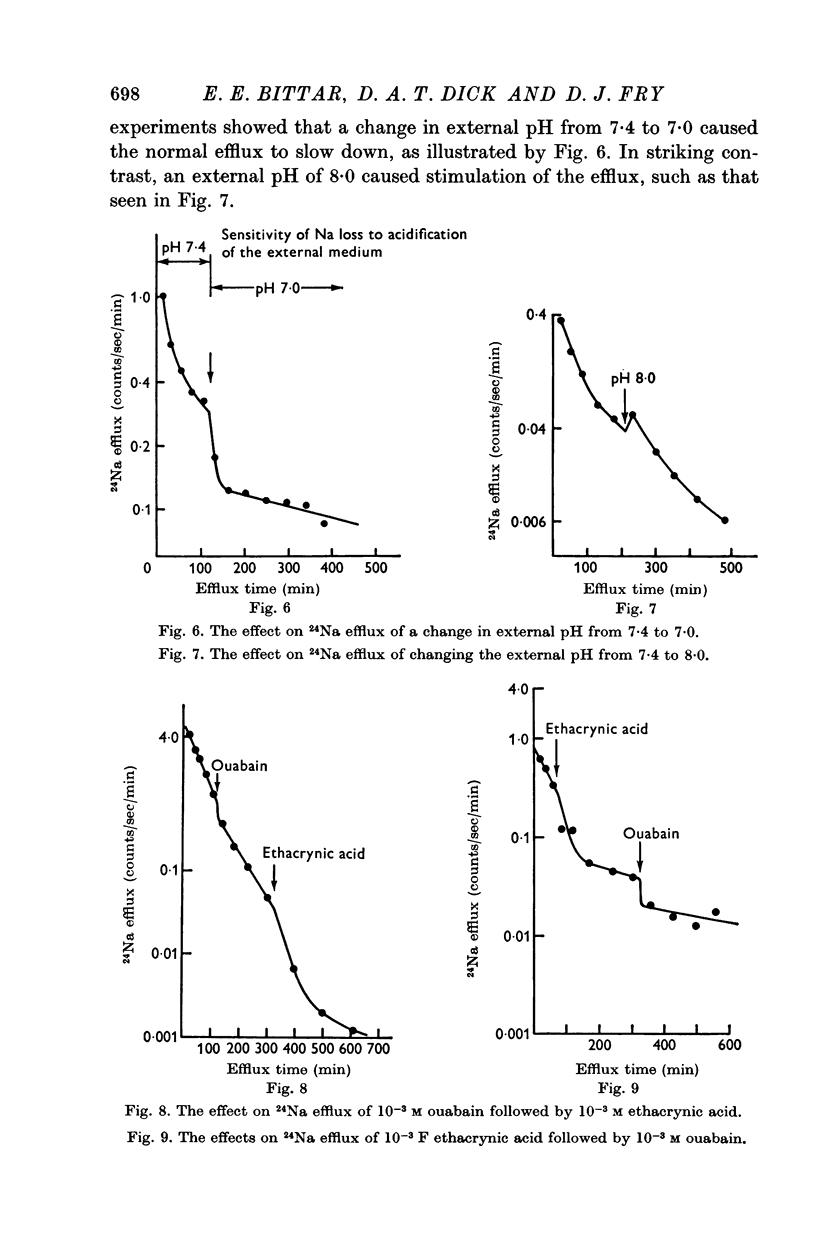
4. Ouabain (10-3 M) produced only 30% inhibition of the Na efflux, and the subsequent application of ethacrynic acid largely inhibited the remaining efflux.
5. Application of ouabain, after ethacrynic acid had produced some 80% inhibition, caused only a further 9% drop in efflux.
6. In uninhibited cells, Na efflux was found to be greater at pH 8·0, and less at pH 7·0 than at 7·4.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DICK D. A., LEA E. J. NA FLUXES IN SINGLE TOAD OOCYTES WITH SPECIAL REFERENCE TO THE EFFECT OF EXTERNAL AND INTERNAL NA CONCENTRATION ON NA EFFLUX. J Physiol. 1964 Oct;174:55–90. doi: 10.1113/jphysiol.1964.sp007474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. E. The effects of new diuretics on net ion movements in sodium-rich smooth muscles. Can J Physiol Pharmacol. 1967 Jan;45(1):149–159. doi: 10.1139/y67-015. [DOI] [PubMed] [Google Scholar]
- EDWARDS C., HARRIS E. J. Factors influencing the sodium movement in frog muscle with a discussion of the mechanism of sodium movement. J Physiol. 1957 Mar 11;135(3):567–580. doi: 10.1113/jphysiol.1957.sp005731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWICZ P., GERBER C. J. EFFECTS OF EXTERNAL POTASSIUM AND STROPHANTHIDIN ON SODIUM FLUXES IN FROG STRIATED MUSCLE. J Gen Physiol. 1965 Jan;48:489–514. doi: 10.1085/jgp.48.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- Hook J. B., Williamson H. E. Lack of correlation between natriuretic activity and inhibition of renal Nak-activated ATPase. Proc Soc Exp Biol Med. 1965 Nov;120(2):358–360. doi: 10.3181/00379727-120-30536. [DOI] [PubMed] [Google Scholar]
- MAIZELS M., REMINGTON M., TRUSCOE R. The effects of certain physical factors and of the cardiac glycosides on sodium transfer by mouse ascites tumour cells. J Physiol. 1958 Jan 23;140(1):61–79. doi: 10.1113/jphysiol.1958.sp005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNE M. D., SCRIBNER B. H., CRAWFORD M. A. Non-ionic diffusion and the excretion of weak acids and bases. Am J Med. 1958 May;24(5):709–729. doi: 10.1016/0002-9343(58)90376-0. [DOI] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- WHITTAM R. The asymmetrical stimulation of a membrane adenosine triphosphatase in relation to active cation transport. Biochem J. 1962 Jul;84:110–118. doi: 10.1042/bj0840110. [DOI] [PMC free article] [PubMed] [Google Scholar]


