Abstract
1. The time course of the washout of the extracellular markers, inulin, raffinose, sucrose and sorbitol, has been determined for the isolated rat heart perfused for 20 min with Krebs bicarbonate medium containing the marker, and washed out with marker-free perfusate.
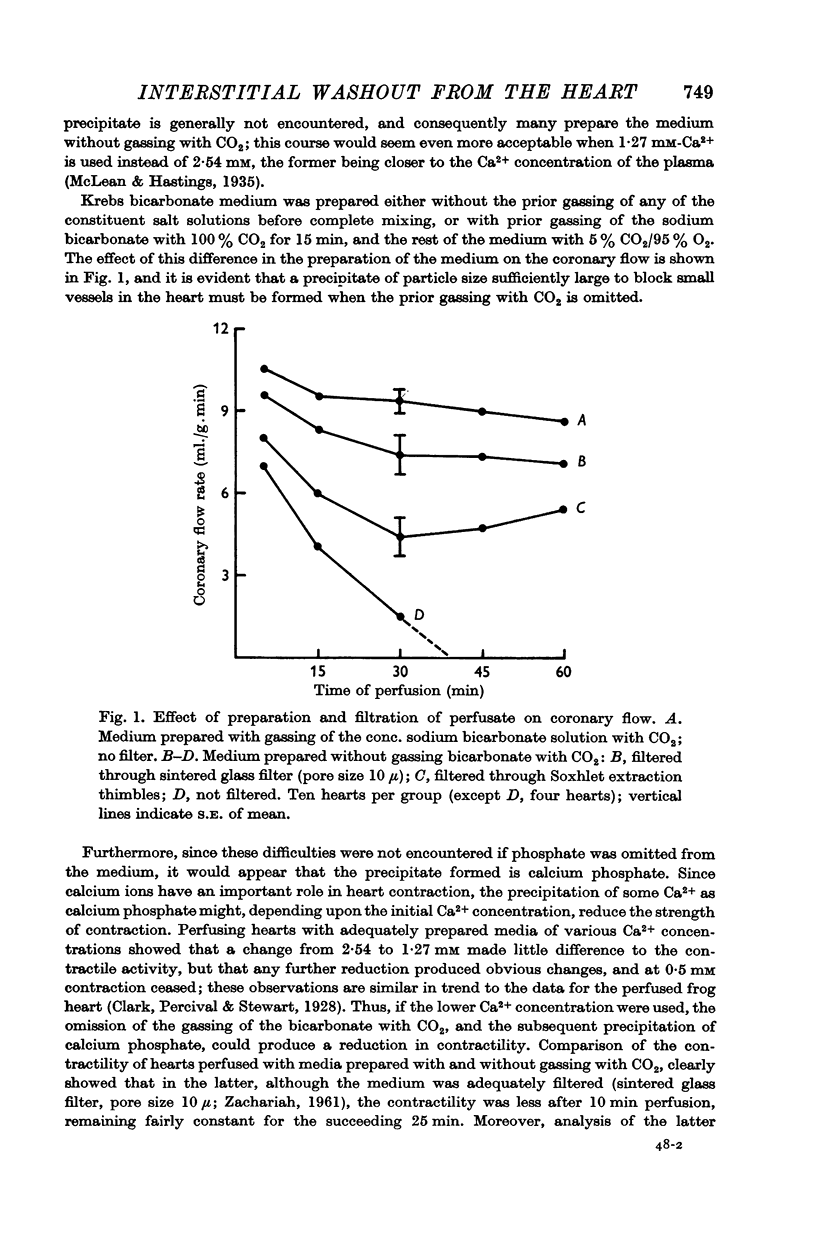
2. The filtration problem for the perfused rat heart was found to be due to omission of the gassing of the concentrated sodium bicarbonate solution with CO2 in the preparation of the perfusate. This probably caused calcium phosphate to be precipitated. The reduced contractility of the heart was reflected in the washout of the extracellular markers.
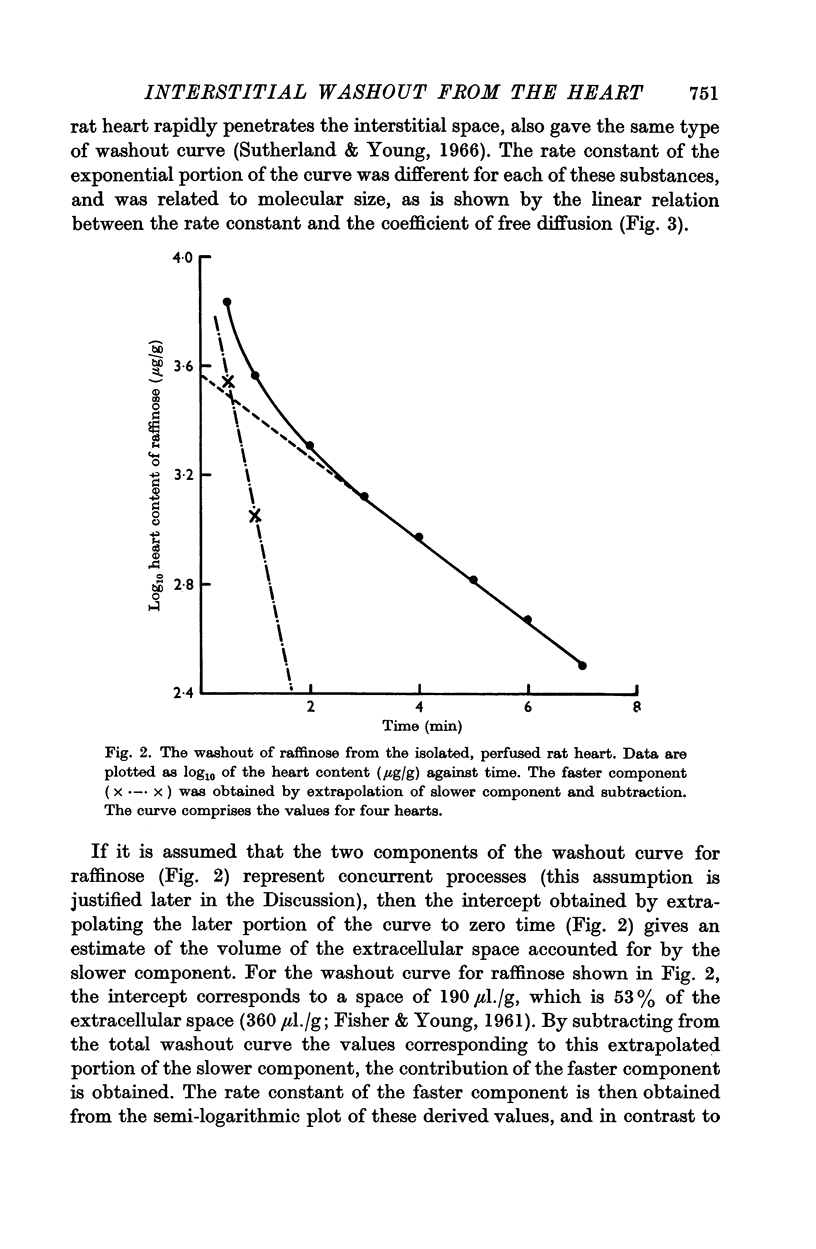
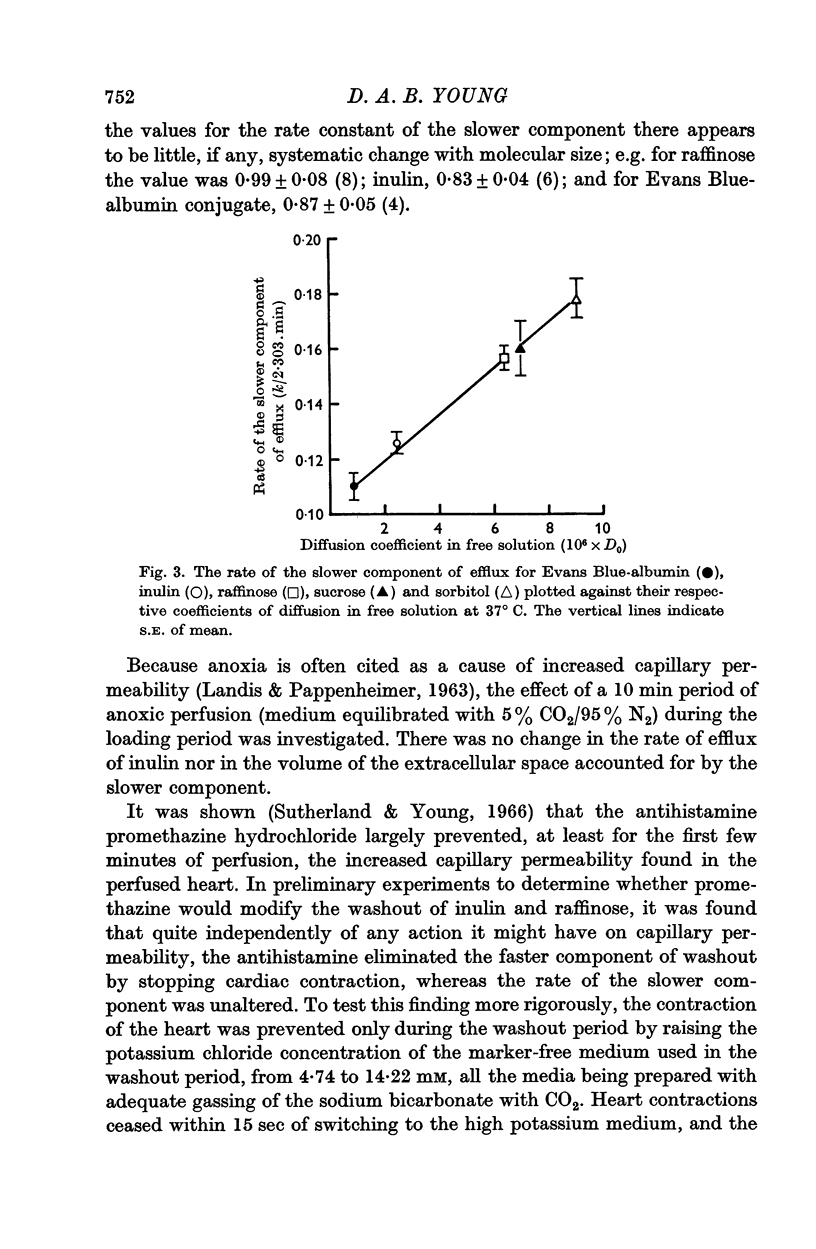
3. The logarithmic plot of the heart content of the marker against time showed two components. The slower was an exponential process, the rate of which was linearly related to the diffusion coefficient for the various markers.
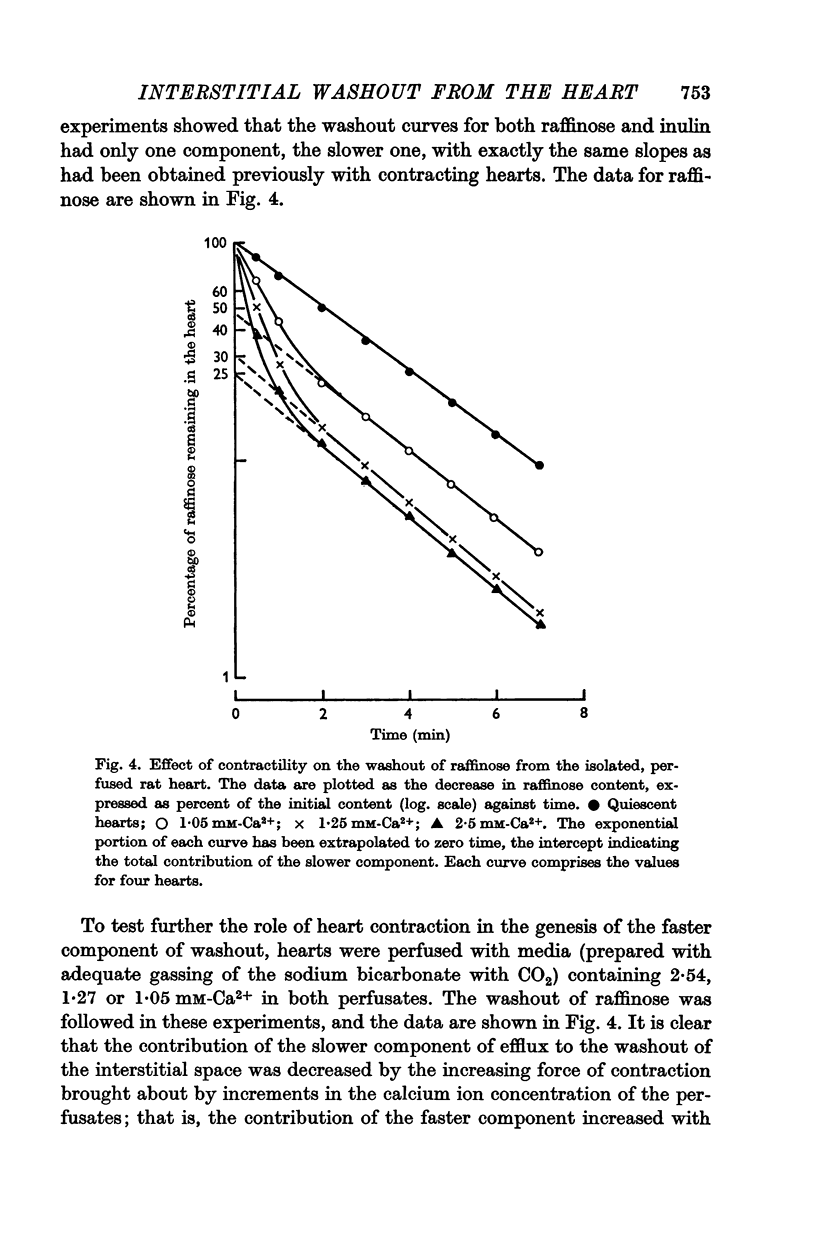
4. The faster component was absent in the quiescent heart, and in the beating heart its contribution to the washout of the interstitial space was related to the strength of contraction.
5. Similar washout curves were obtained for Evans Blue-albumin conjugate, which readily penetrates the interstitial space of the isolated rat heart.
6. It is suggested that the two components of efflux are a diffusion process limited by perfusate flow, and bulk movements of fluid.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASSIR O. Molecular inhomogeneity as a source of error in inulin clearance studies. J Physiol. 1956 Mar 28;131(3):586–591. doi: 10.1113/jphysiol.1956.sp005484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLEEHEN N. M., FISHER R. B. The action of insulin in the isolated rat heart. J Physiol. 1954 Feb 26;123(2):260–276. doi: 10.1113/jphysiol.1954.sp005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon J. S., Bell D. J. Fructose and glucose in the blood of the foetal sheep. Biochem J. 1948;42(3):397–405. doi: 10.1042/bj0420397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo R., Marks H. P. The action of insulin on the aseptically perfused heart. J Physiol. 1927 Aug 8;63(3):242–248. doi: 10.1113/jphysiol.1927.sp002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COTRAN R. S., MAJNO G. THE DELAYED AND PROLONGED VASCULAR LEAKAGE IN INFLAMMATION. I. TOPOGRAPHY OF THE LEAKING VESSELS AFTER THERMAL INJURY. Am J Pathol. 1964 Aug;45:261–281. [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Percival G. H., Stewart C. P. Action of calcium ions on the frog's heart. J Physiol. 1928 Dec 20;66(4):346–355. doi: 10.1113/jphysiol.1928.sp002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., YOUNG D. A. Direct determination of extracellular fluid in the rat heart. J Physiol. 1961 Sep;158:50–58. doi: 10.1113/jphysiol.1961.sp006753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HLAD C. J., Jr, ELRICK H. Further studies of the kinetics of glucose utilization. I. A new method of data analysis. J Clin Endocrinol Metab. 1959 Oct;19:1258–1273. doi: 10.1210/jcem-19-10-1258. [DOI] [PubMed] [Google Scholar]
- HURLEY J. V., SPECTOR W. G. A TOPOGRAPHICAL STUDY OF INCREASED VASCULAR PERMEABILITY IN ACUTE TURPENTINE-INDUCED PLEURISY. J Pathol Bacteriol. 1965 Jan;89:245–254. [PubMed] [Google Scholar]
- Locke F. S., Rosenheim O. Contributions to the physiology of the isolated heart: The consumption of dextrose by mammalian cardiac muscle. J Physiol. 1907 Dec 31;36(4-5):205–220. doi: 10.1113/jphysiol.1907.sp001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
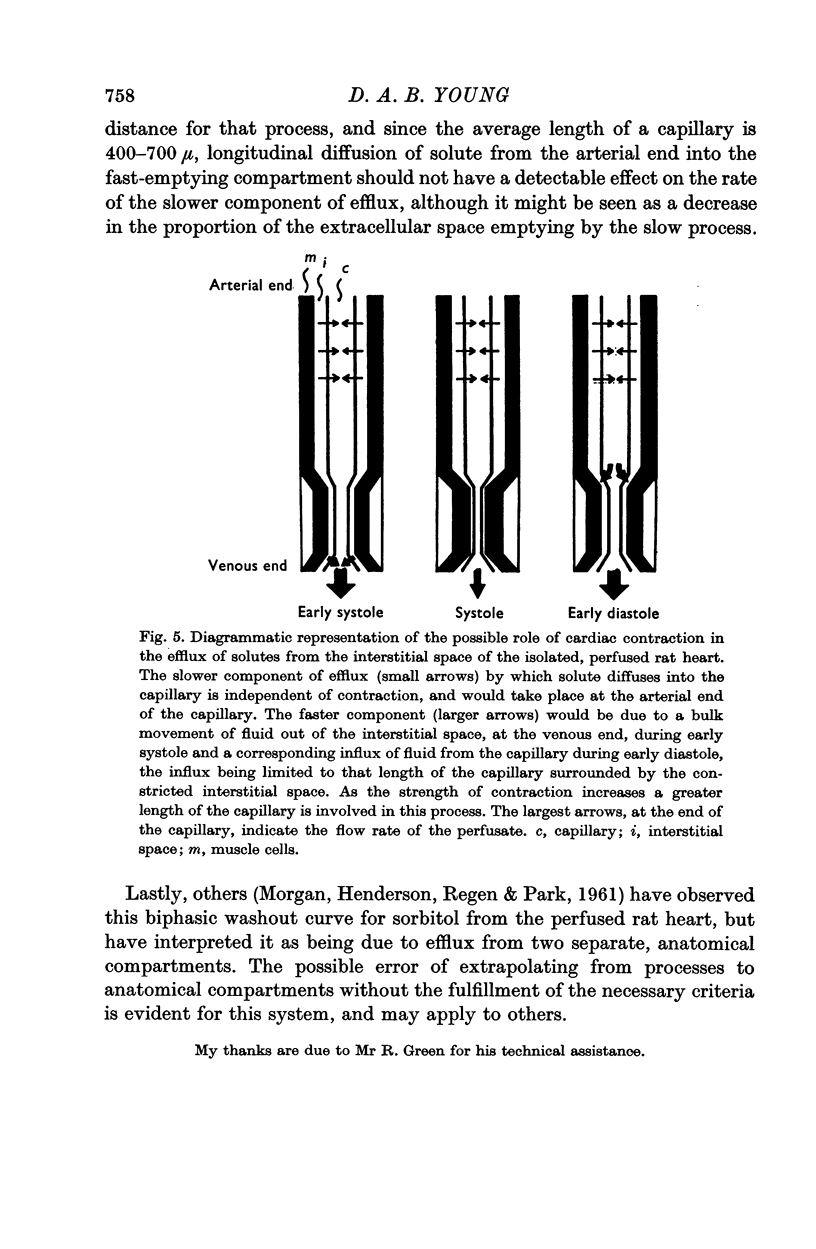
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- SCHAFER D. E., JOHNSON J. A. PERMEABILITY OF MAMMALIAN HEART CAPILLARIES TO SUCROSE AND INULIN. Am J Physiol. 1964 May;206:985–991. doi: 10.1152/ajplegacy.1964.206.5.985. [DOI] [PubMed] [Google Scholar]
- STUBBS J., WIDDAS W. F. Extravascular fluid losses in the perfused isolated rabbit heart. J Physiol. 1959 Oct;148:393–402. doi: 10.1113/jphysiol.1959.sp006295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland T. M., Young D. A. Increased permeability of the capillaries of the rat heart to plasma albumin with asphyxiation and with perfusion. J Physiol. 1966 Mar;183(1):112–122. doi: 10.1113/jphysiol.1966.sp007854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZACHARIAH P. Contractility and sugar permeability in the perfused rat heart. J Physiol. 1961 Sep;158:59–72. doi: 10.1113/jphysiol.1961.sp006754. [DOI] [PMC free article] [PubMed] [Google Scholar]


