Abstract
1. Protein fractions were prepared from serum and various organs of pigs following the methods used to extract and purify neurophysin from posterior pituitaries.
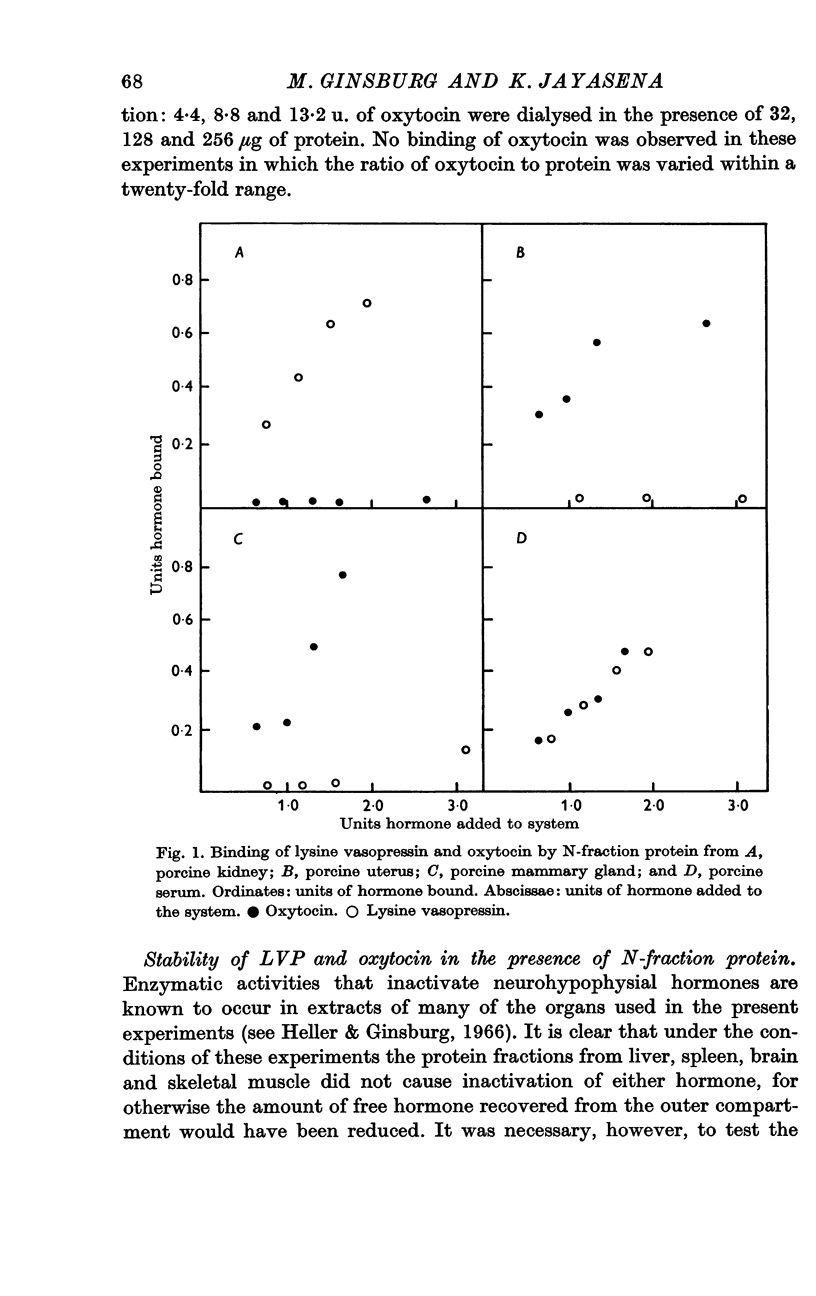
2. Protein fractions extracted from porcine kidney, uterus, mammary gland or serum, which contain antigen reacting with anti-neurophysin serum, form non-dialysable complexes with oxytocin and/or lysine vasopressin.
3. Protein from uterus or mammary gland bound oxytocin but not lysine vasopressin, while protein extracted from kidney bound lysine vasopressin but not oxytocin: protein from serum bound both hormones.
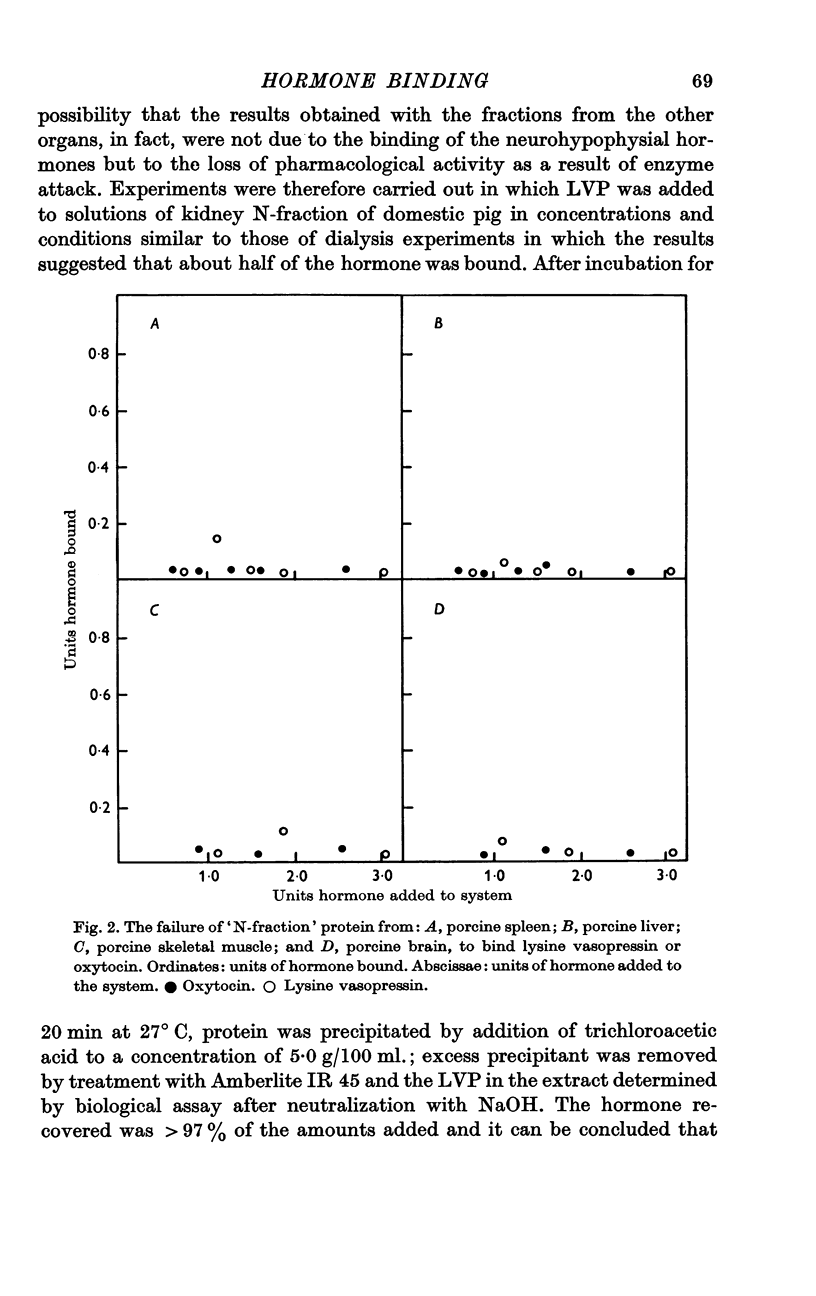
4. Protein fractions prepared in the same way from porcine liver, spleen, skeletal muscle and brain, which do not contain antigen reacting with anti-neurophysin serum, did not form complexes with neurohypophysial hormone.
5. The formation of complexes between the renal or uterine protein fractions and lysine vasopressin or oxytocin is inhibited by the addition of 1·0 × 10-6 M-CaCl2.
6. 1-Desamino 8-arginine vasopressin is not bound by neurophysin prepared from porcine posterior pituitaries or the protein from porcine kidneys, while 8-arginine vasopressin does form non-dialysable complexes with proteins from both sources.
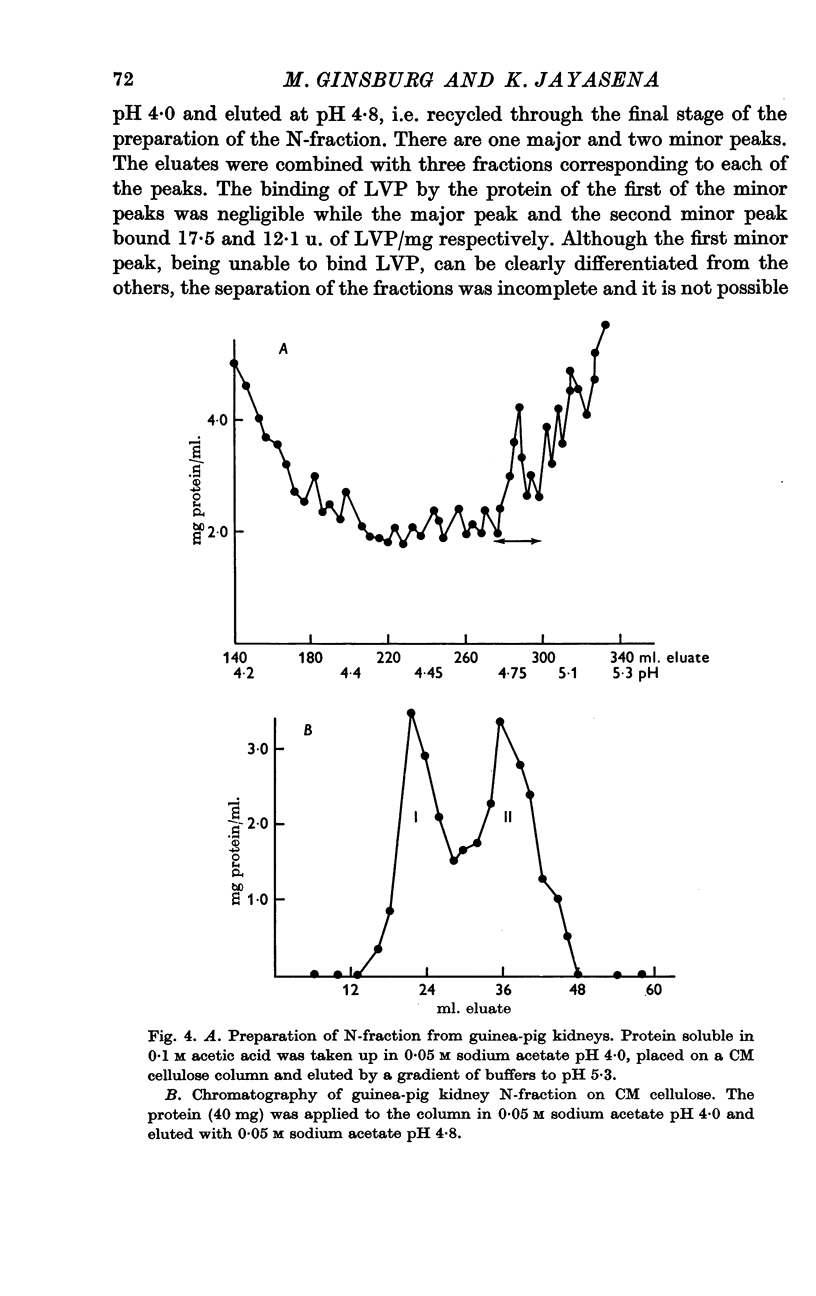
7. A protein fraction extracted from guinea-pig kidney by similar preparative methods also bound lysine vasopressin and the binding was inhibited by addition of CaCl2.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUDRAIN L., CLAUSER H. [Mechanism of the inactivation of oxytocin by uterine tissue]. Biochim Biophys Acta. 1960 Mar 11;38:494–501. doi: 10.1016/0006-3002(60)91284-1. [DOI] [PubMed] [Google Scholar]
- BIRNIE J. H. The inactivation of posterior pituitary antidiuretic hormone by liver extracts. Endocrinology. 1953 Jan;52(1):33–38. doi: 10.1210/endo-52-1-33. [DOI] [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKER S. E., GREENBAUM A. L. Inactivation of the antidiuretic activity of vasopressin by tissue homogenates. J Physiol. 1956 Apr 27;132(1):199–212. doi: 10.1113/jphysiol.1956.sp005514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG M., IRELAND M. BINDING OF VASOPRESSIN AND OXYTOCIN TO PROTEIN IN EXTRACTS OF BOVINE AND RABBIT NEUROHYPOPHYSES. J Endocrinol. 1964 Aug;30:131–145. doi: 10.1677/joe.0.0300131. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Jayasena K. The occurrence of antigen reacting with antibody to porcine neurophysin. J Physiol. 1968 Jul;197(1):53–63. doi: 10.1113/jphysiol.1968.sp008545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg M., Jayasena K., Thomas P. J. The preparation and properties of porcine neurophysin and the influence of calcium on the hormone-neurophysin complex. J Physiol. 1966 May;184(2):387–401. doi: 10.1113/jphysiol.1966.sp007921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLER H., ZAIDI S. M. The metabolism of exogenous and endogenous antidiuretic hormone in the kidney and liver in vivo. Br J Pharmacol Chemother. 1957 Sep;12(3):284–292. doi: 10.1111/j.1476-5381.1957.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller H. The state in the blood and the excretion by the kidney of the antidiuretic principle of posterior pituitary extracts. J Physiol. 1937 Feb 19;89(1):81–95. doi: 10.1113/jphysiol.1937.sp003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller H., Urban F. F. The fate of the antidiuretic principle of postpituitary extracts in vivo and in vitro. J Physiol. 1935 Dec 16;85(4):502–518. doi: 10.1113/jphysiol.1935.sp003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER G. E., TOWNSEND C. E. The in vitro inactivation of pitressin by normal and cirrhotic human liver. J Clin Invest. 1954 Apr;33(4):549–554. doi: 10.1172/JCI102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNSICK R. A. Effect of magnesium ion on the response of the rat uterus to neurohypophysial hormones and analogues. Endocrinology. 1960 Mar;6:451–457. doi: 10.1210/endo-66-3-451. [DOI] [PubMed] [Google Scholar]
- SAWYER W. H. Inactivation of oxytocin by homogenates of uteri and other tissues from normal and pregnant rats. Proc Soc Exp Biol Med. 1954 Nov;87(2):463–465. doi: 10.3181/00379727-87-21413. [DOI] [PubMed] [Google Scholar]
- THORN N. A., SILVER L. Chemical form of circulating antidiuretic hormone in rats. J Exp Med. 1957 Jun 1;105(6):575–583. doi: 10.1084/jem.105.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]


