Abstract
1. The effect of adrenaline on contracture and twitch tension in frog's ventricle has been examined, using the superfused preparation.
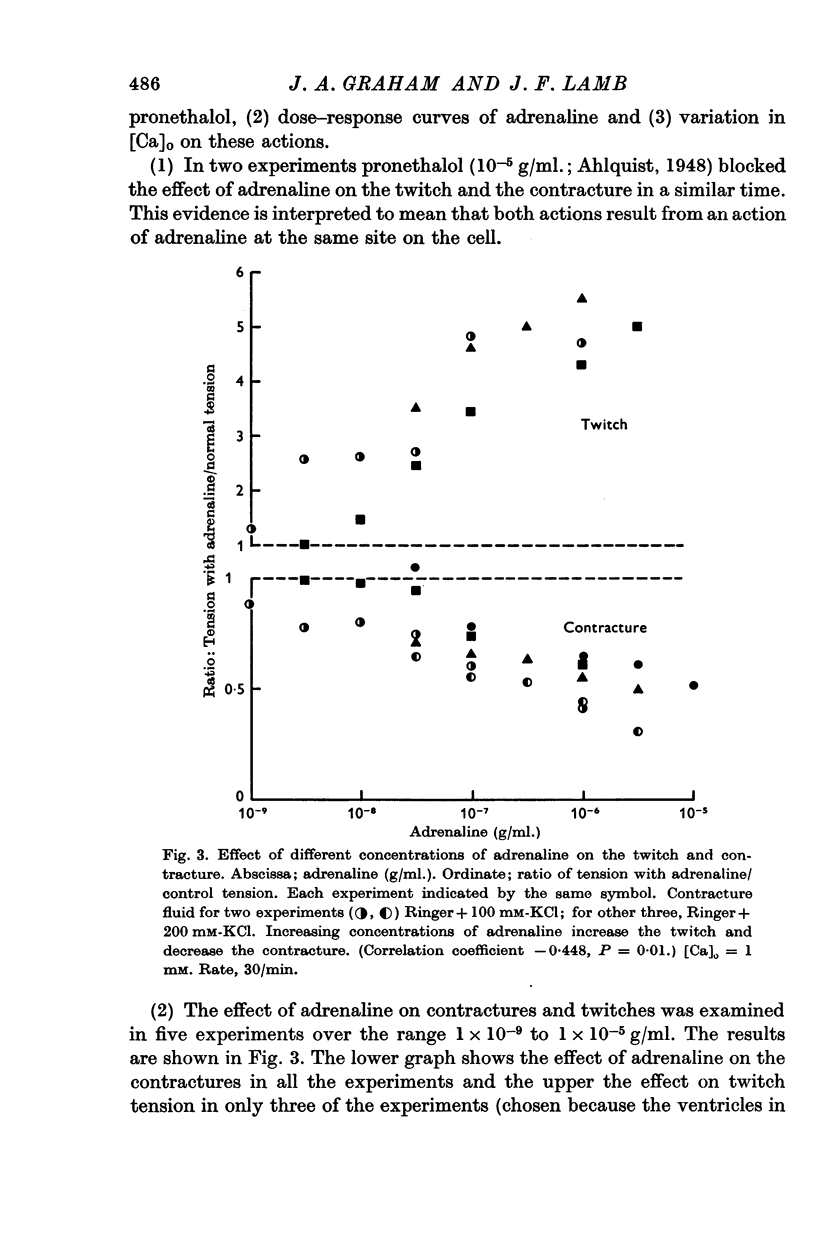
2. In 1 mM-Ca Ringer, contractures induced with excess KCl concentrations from 50 to 200 mM, are reduced by 1 × 10-6 g/ml. adrenaline to an average of 0·62 of control values, in marked contrast to the well known positive inotropic effect of adrenaline on the heart twitch. This effect of adrenaline is directly dose dependent. Increasing [Ca]o diminishes the effect of adrenaline on contracture tension, and on the twitch tension.
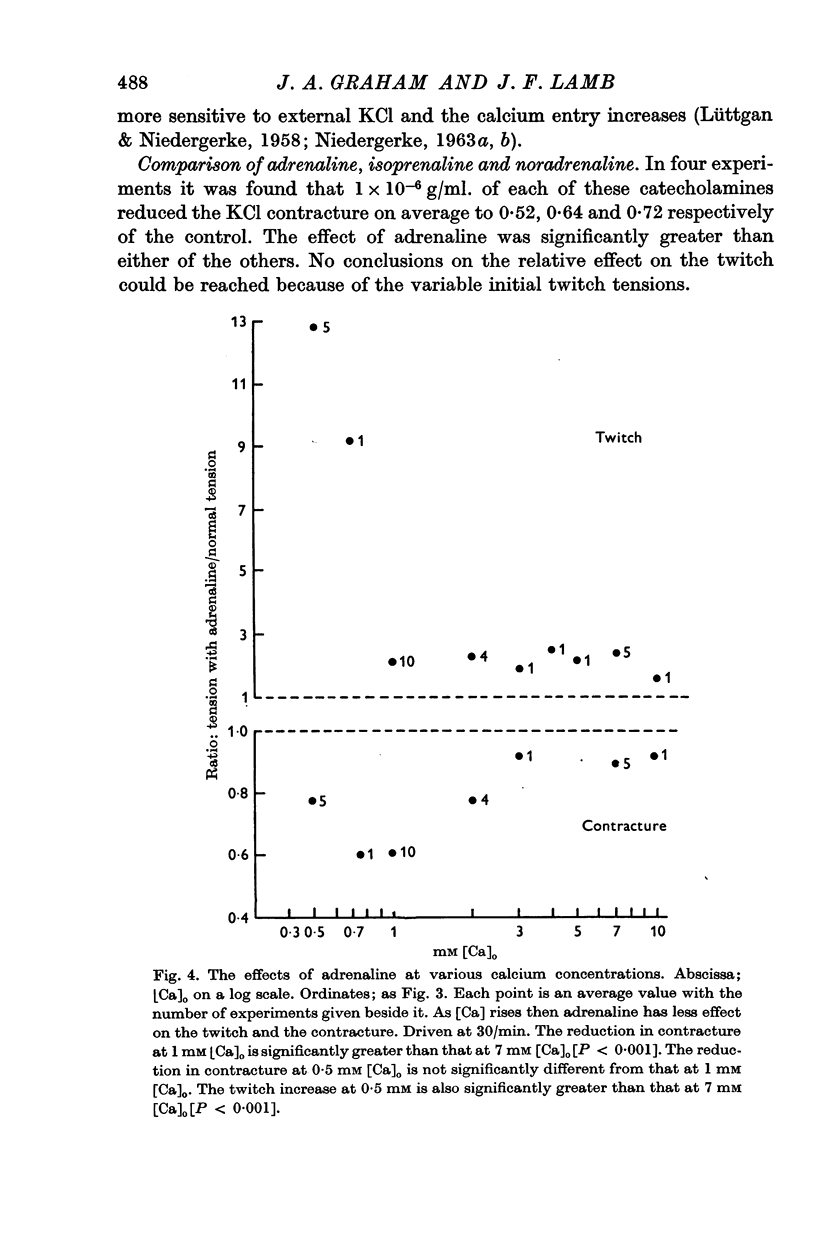
3. Adrenaline has a significantly greater effect on the KCl contracture tension than noradrenaline or isoprenaline.
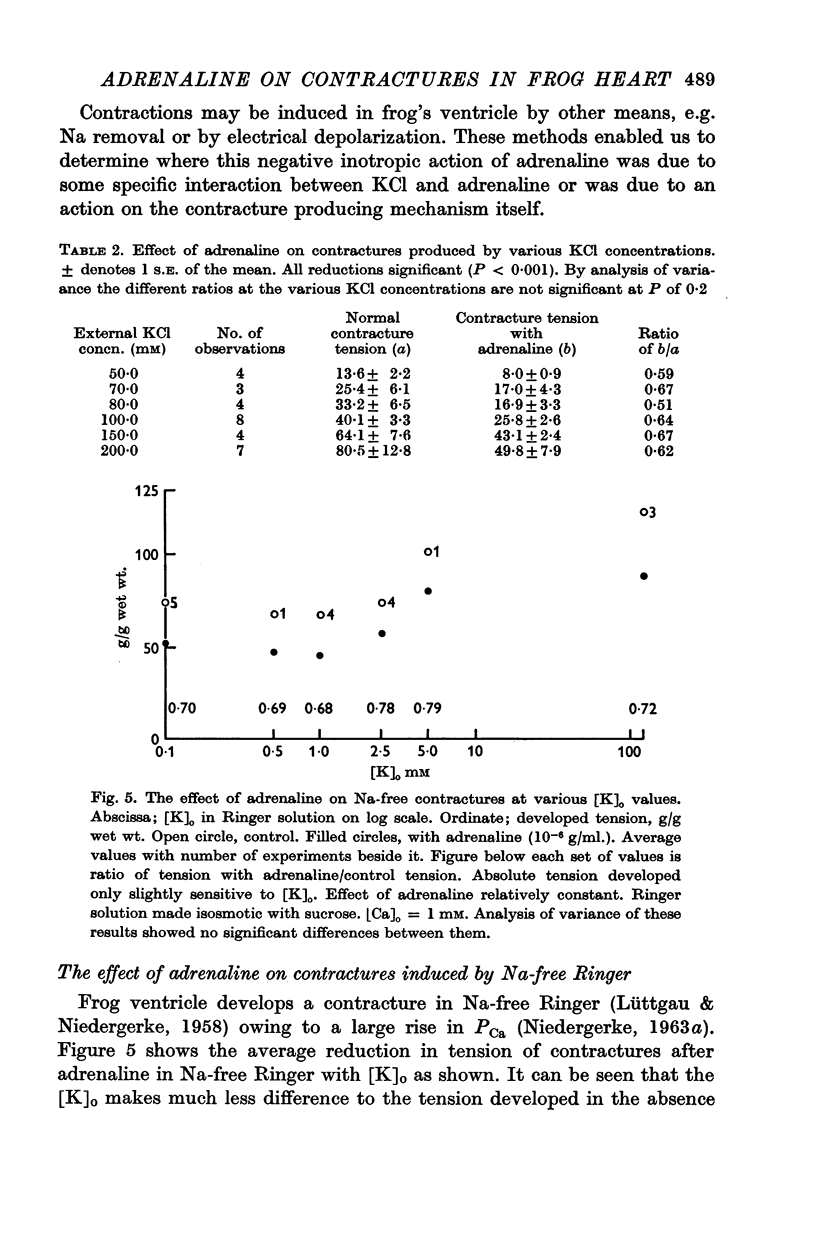
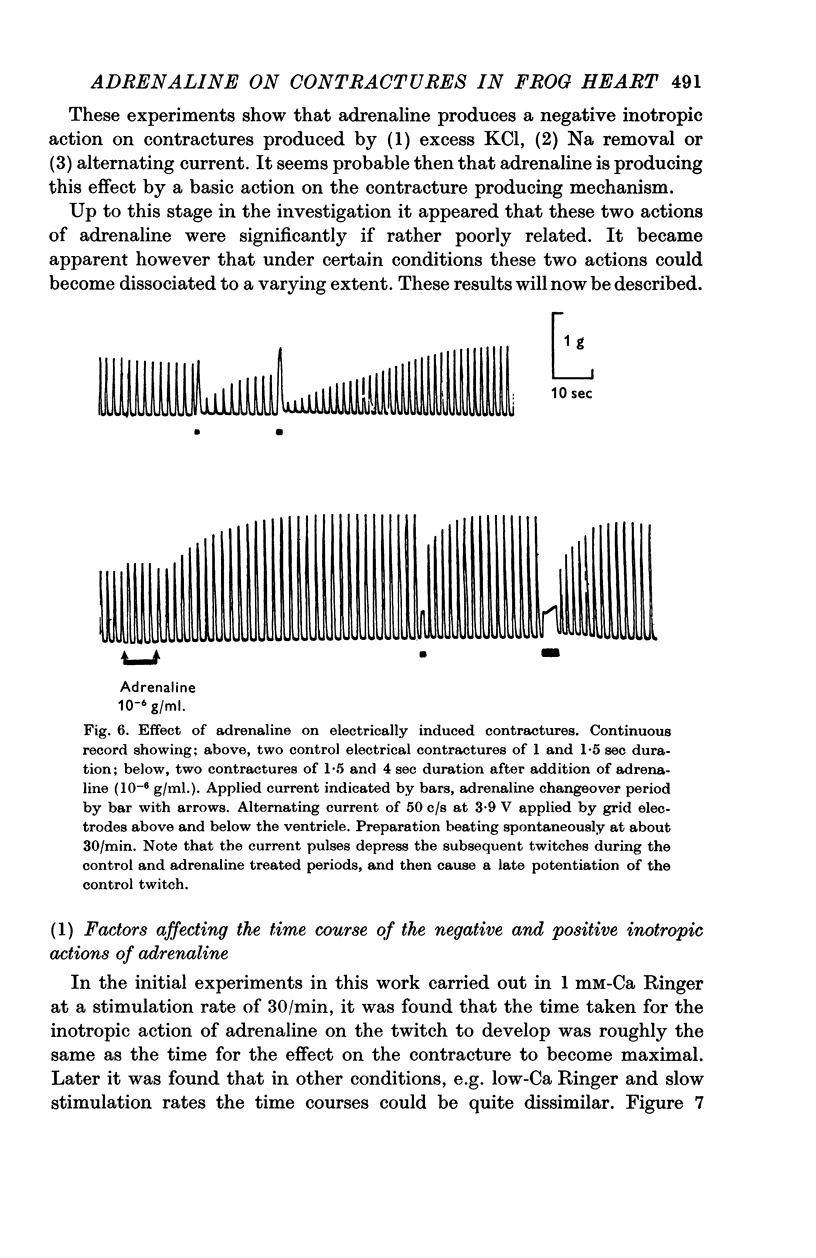
4. In 1 mM-Ca Ringer, Na-free contractures are reduced to 0·72 of controls by 1 × 10-6 g/ml. adrenaline. Adrenaline also significantly reduces tension in contractures induced by 50 c/s alternating current.
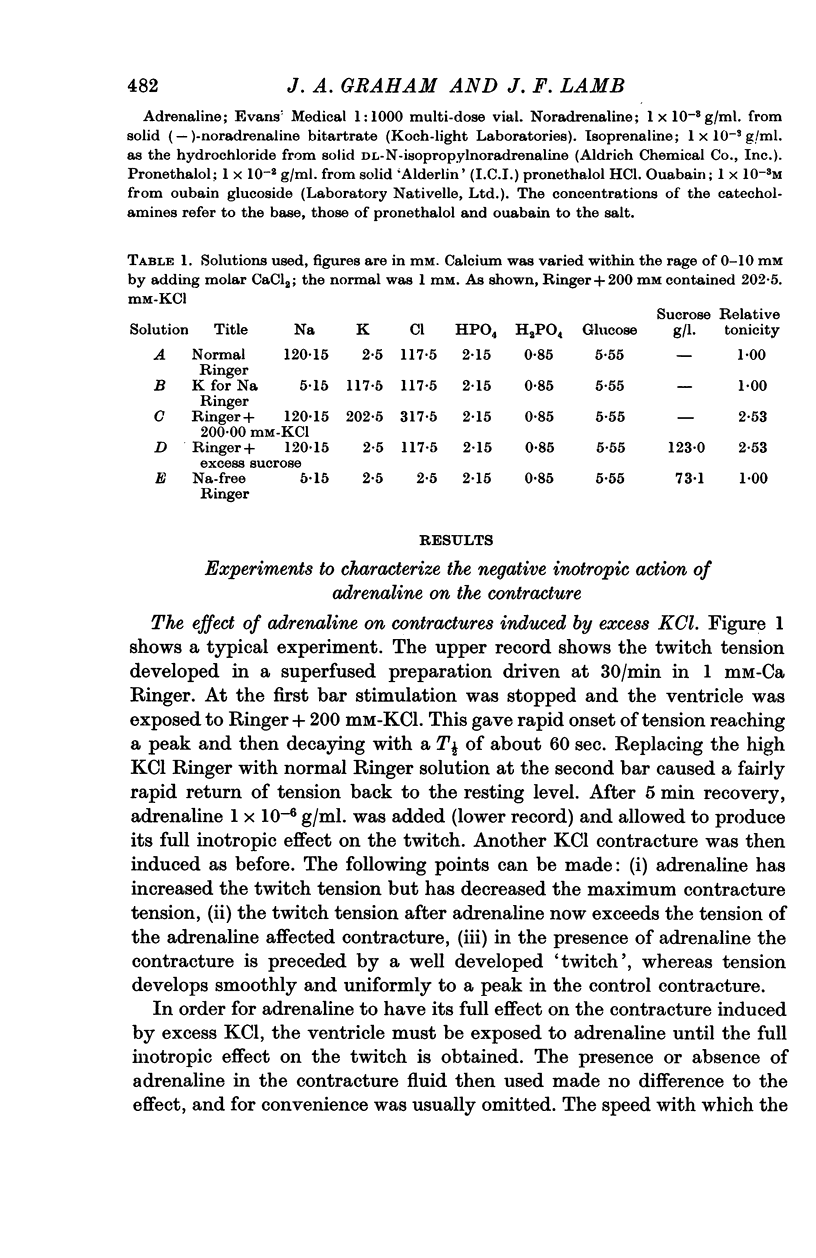
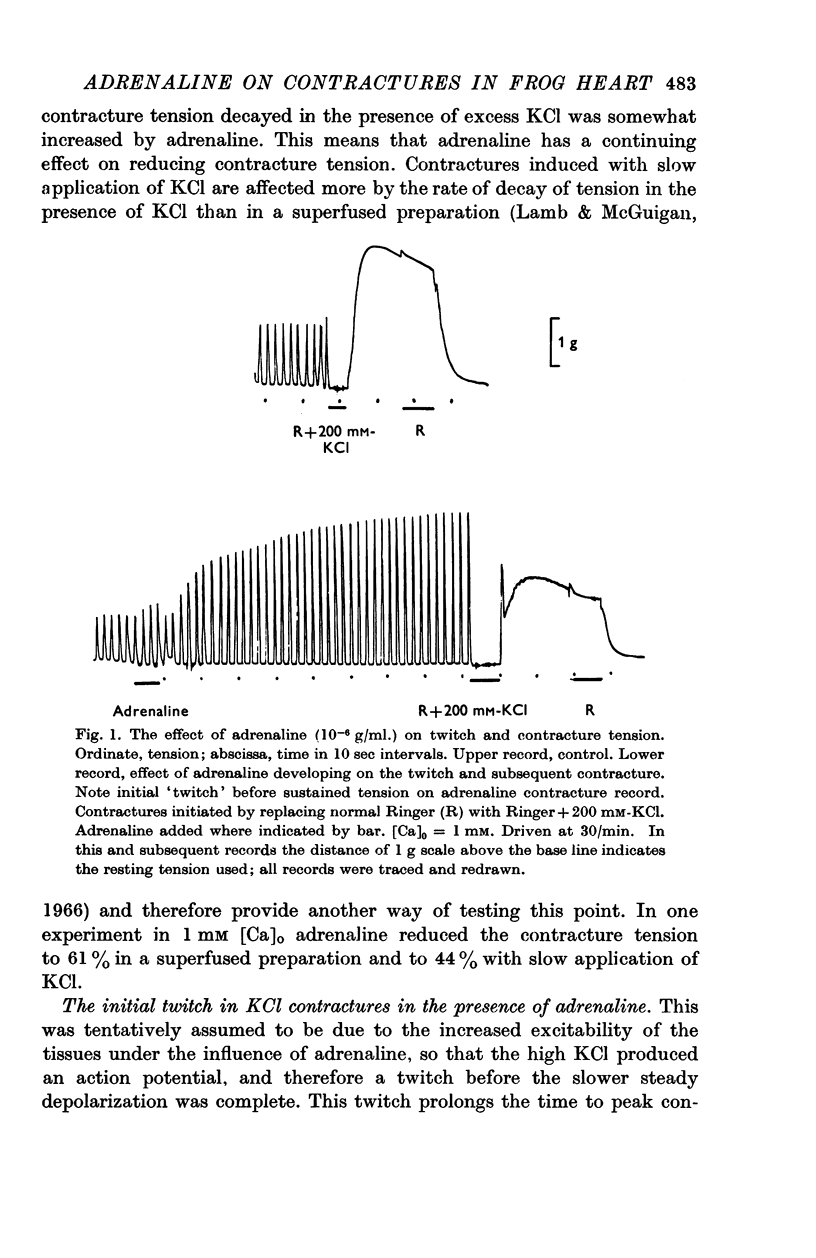
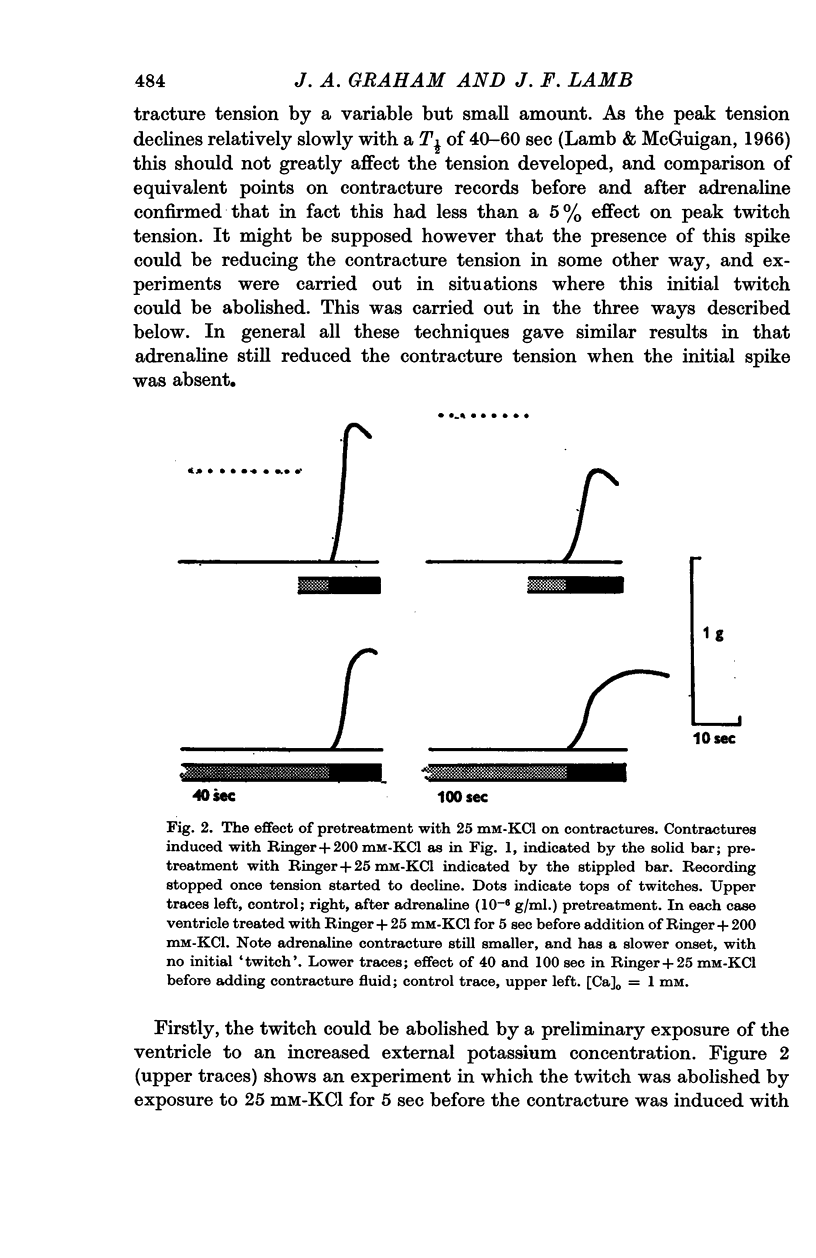
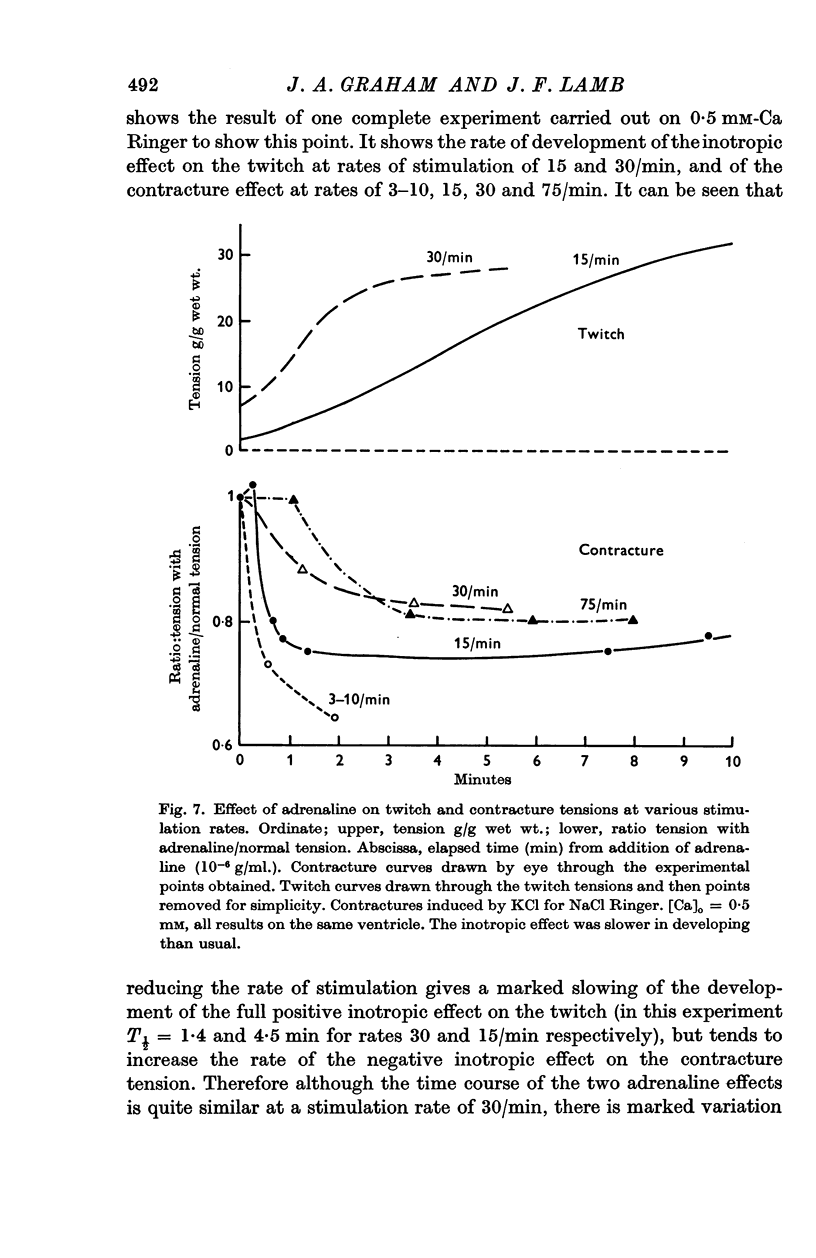
5. The action of adrenaline on contracture tension is largely complete in 1-2 min at various rates of stimulation and calcium concentrations. A similar time course has been found for the effect of adrenaline on membrane potential.
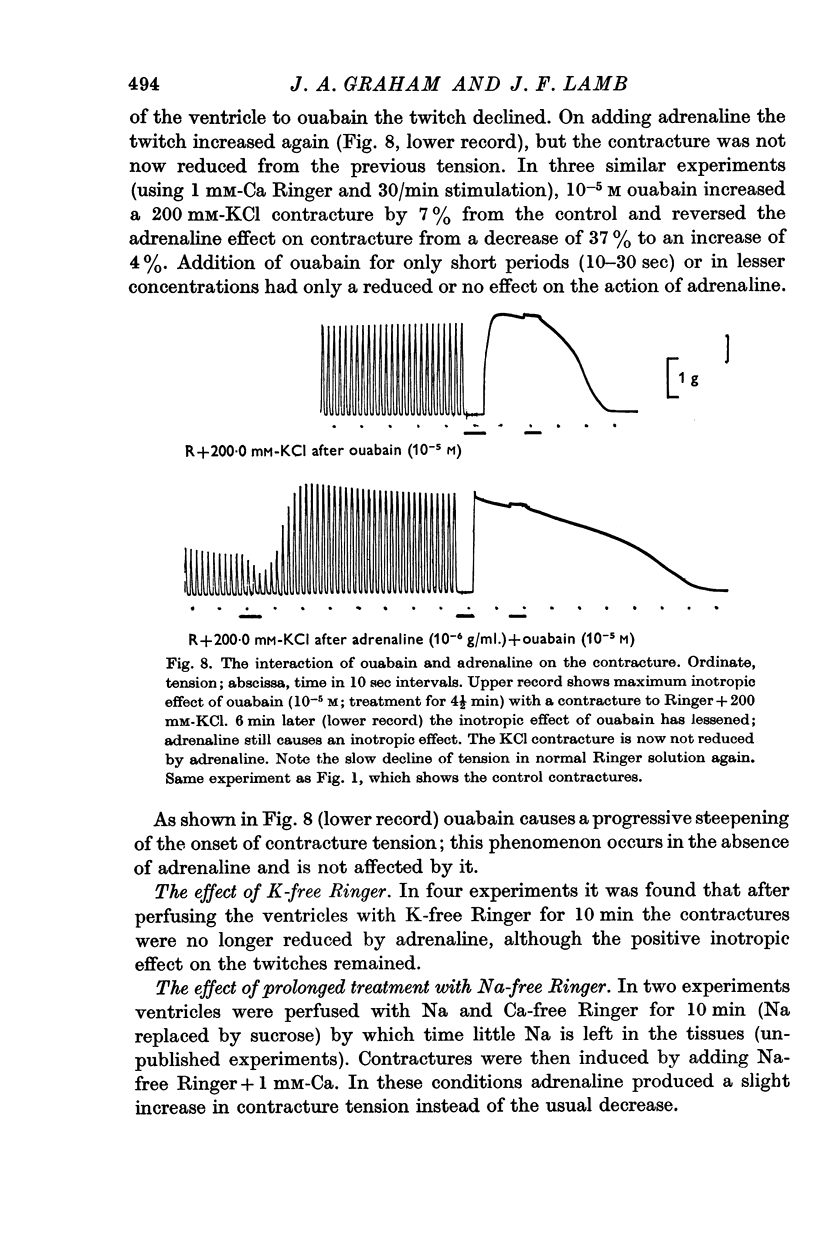
6. Pronethalol blocks the action of adrenaline on both twitch and contracture. The action on the contracture can also be blocked by ouabain (1 × 10-5 M), and exposure of the tissue to K-free or Na-free Ringer solution.
7. Adrenaline hyperpolarizes the membrane potential with a range of [K]o from 0 to 200 mM. This effect is blocked by pronethalol and ouabain. After exposure to ouabain, adrenaline causes a significant decrease in the membrane potential. This may be due to an increase in the sodium permeability.
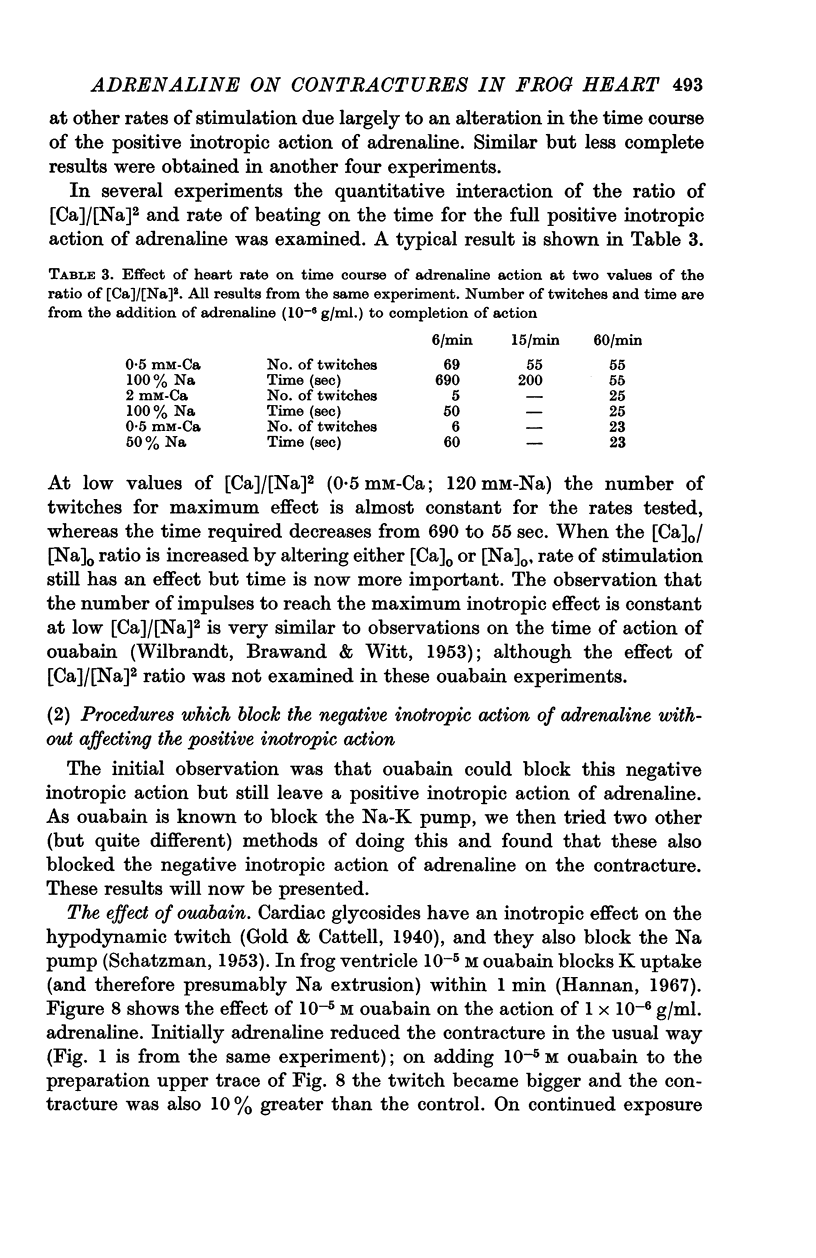
8. At low values of the [Ca]/[Na]2 ratio, adrenaline takes a relatively constant number of beats for full action, but at high values of the ratio the development of full effect is largely time dependent.
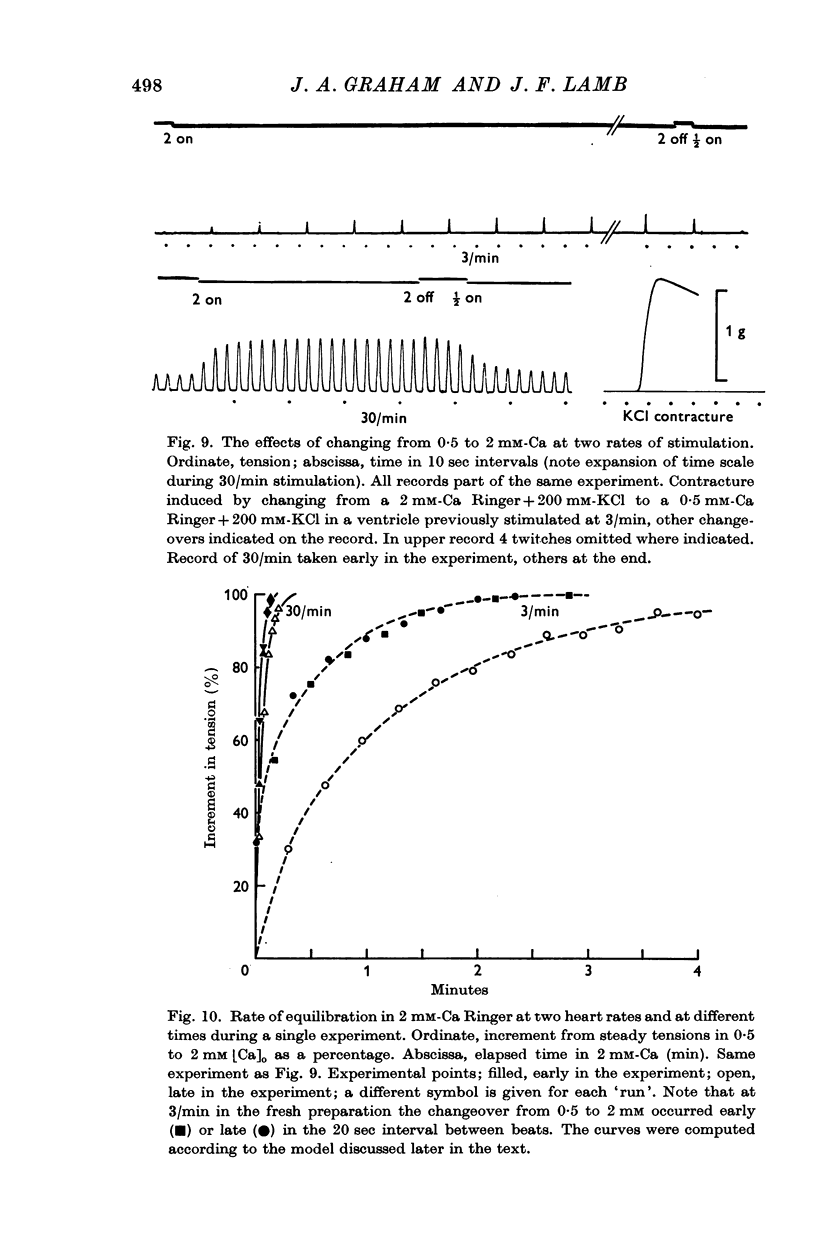
9. The time course of the effect on the twitch of changing from 0·5 to 2 mM-Ca Ringer has been studied at various rates of stimulation. The equilibration time has been found to depend on the heart rate.
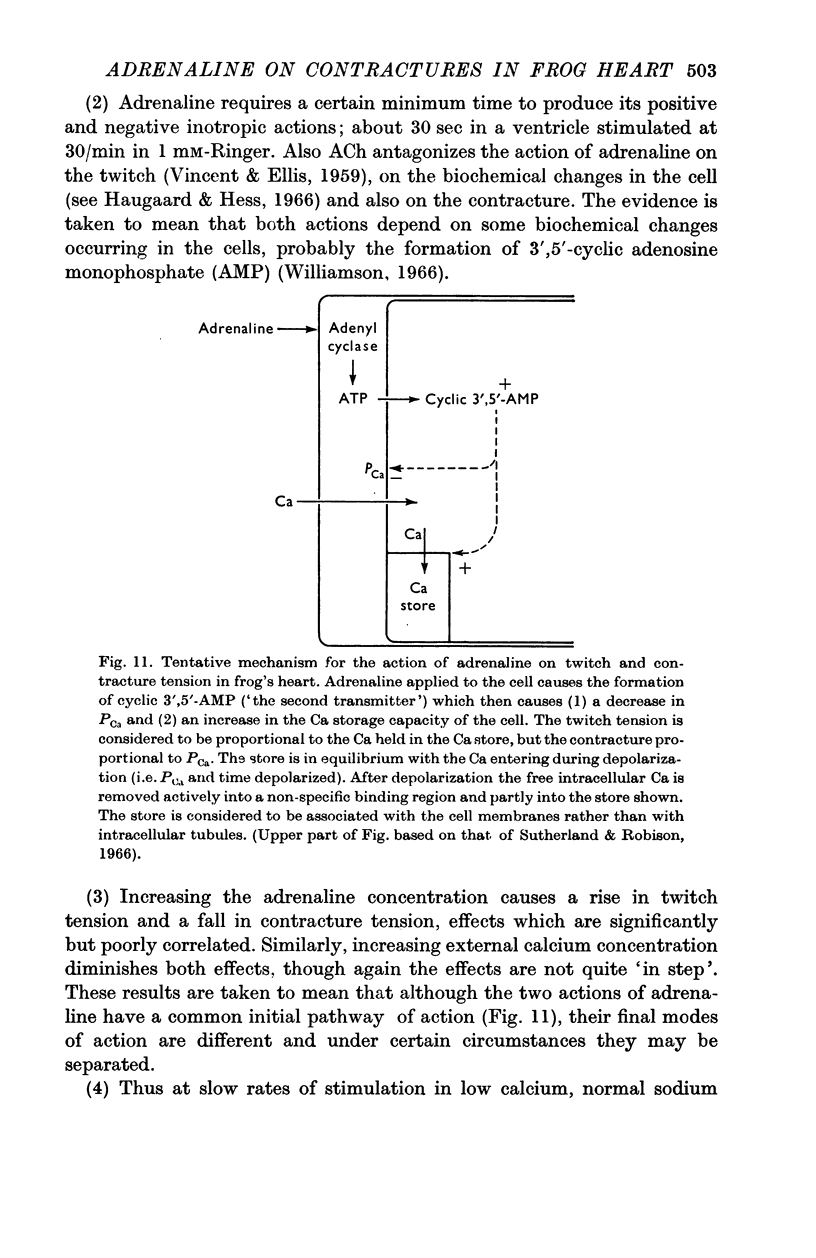
10. The effect on the contracture suggests that adrenaline decreases the calcium permeability. It is further suggested that the development of twitch tension is not due to direct Ca entry but is due to the release of Ca from a local store within or between the cells. The inotropic action of adrenaline is explained in terms of this store.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHLQUIST R. P. A study of the adrenotropic receptors. Am J Physiol. 1948 Jun;153(3):586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Slayman C. L. Membrane potential and conductance during transport of sodium, potassium and rubidium in frog muscle. J Physiol. 1966 Jun;184(4):970–1014. doi: 10.1113/jphysiol.1966.sp007961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK J. W., STEPHENSON J. S. Pharmacology of a new adrenergic beta-receptor-blocking compound (Nethalide). Lancet. 1962 Aug 18;2(7251):311–314. doi: 10.1016/s0140-6736(62)90103-4. [DOI] [PubMed] [Google Scholar]
- Barcroft J., Dixon W. E. The gaseous metabolism of the mammalian heart: Part I. J Physiol. 1907 Mar 25;35(3):182–204. doi: 10.1113/jphysiol.1907.sp001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. L., Goffart M., Dias M. V. The effects of adrenaline and of sympathetic stimulation on the demarcation potential of mammalian skeletal muscle. J Physiol. 1950 Apr 15;111(1-2):184–194. doi: 10.1113/jphysiol.1950.sp004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge W. Observations on the rôle of potassium salts in frog's muscle. J Physiol. 1911 May 22;42(4):359–382. doi: 10.1113/jphysiol.1911.sp001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSTEN M. E., MOMMAERTS W. F. THE ACCUMULATION OF CALCIUM IONS BY SARCOTUBULAR VESICLES. J Gen Physiol. 1964 Nov;48:183–197. doi: 10.1085/jgp.48.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsten M. E. Cardiac sarcotubular vesicles. Effects of ions, ouabain and acetylstrophanthidin. Circ Res. 1967 Jun;20(6):599–605. doi: 10.1161/01.res.20.6.599. [DOI] [PubMed] [Google Scholar]
- Clark A. J. The action of ions and lipoids upon the frog's heart. J Physiol. 1913 Oct 17;47(1-2):66–107. doi: 10.1113/jphysiol.1913.sp001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J., TRAUTWEIN W. Die Wirkung von Adrenalin auf das Ruhepotential von Myokardfasern des Vorhofs. Experientia. 1956 Oct 15;12(10):396–398. doi: 10.1007/BF02157290. [DOI] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. R. The action of adrenalin. J Physiol. 1905 Jul 13;32(5-6):401–467. doi: 10.1113/jphysiol.1905.sp001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. L., Ogawa S. The effect of adrenalin on the gaseous metabolism of the isolated mammalian heart. J Physiol. 1914 Feb 27;47(6):446–459. doi: 10.1113/jphysiol.1914.sp001636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell W. H. On the Augmentor (Accelerator) Nerves of the Heart of Cold-blooded Animals. J Physiol. 1884 Apr;5(1):46–48. doi: 10.1113/jphysiol.1884.sp000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz E. W., Hess M. L., Lain R. F., Briggs F. N. Activity of the vesicular calcium pump in the spontaneously failing heart-lung preparation. Circ Res. 1967 May;20(5):477–484. doi: 10.1161/01.res.20.5.477. [DOI] [PubMed] [Google Scholar]
- Graham J. A., Lamb J. F. The effect of adrenaline on action potential and twitch in frog ventricular muscle. J Physiol. 1967 Jan;188(2):25P–26P. [PubMed] [Google Scholar]
- HAAS H. G., TRAUTWEIN W. Increase of sodium efflux induced by epinephrine in the heart of the frog. Nature. 1963 Jan 5;197:80–81. doi: 10.1038/197080a0. [DOI] [PubMed] [Google Scholar]
- HAUGAARD N., HESS M. E. ACTIONS OF AUTONOMIC DRUGS ON PHOSPHORYLASE ACTIVITY AND FUNCTION. Pharmacol Rev. 1965 Mar;17:27–69. [PubMed] [Google Scholar]
- HESS M. E., SHANFELD J., HAUGAARD N. The role of the autonomic nervous system in the regulation of heart phosphorylase in the open-chest rat. J Pharmacol Exp Ther. 1962 Feb;135:191–196. [PubMed] [Google Scholar]
- HOLLAND W. C., SEKUL A. A. Effect of ouabain on Ca-45 and C1-36 exchange in isolated rabbit atria. Am J Physiol. 1959 Oct;197:757–760. doi: 10.1152/ajplegacy.1959.197.4.757. [DOI] [PubMed] [Google Scholar]
- Haugaard N., Hess M. E. The influence of catecholamines on heart function and phosphorylase activity. Pharmacol Rev. 1966 Mar;18(1):197–203. [PubMed] [Google Scholar]
- KAVALER F. Membrane depolarization as a cause of tension development in mammalian ventricular muscle. Am J Physiol. 1959 Nov;197:968–970. doi: 10.1152/ajplegacy.1959.197.5.968. [DOI] [PubMed] [Google Scholar]
- Kavaler F., Morad M. Paradoxical effects of epinephrine on excitation-contraction coupling in cardiac muscle. Circ Res. 1966 May;18(5):492–501. doi: 10.1161/01.res.18.5.492. [DOI] [PubMed] [Google Scholar]
- Keenan M. J., Niedergerke R. Intracellular sodium concentration and resting sodium fluxes of the frog heart ventricle. J Physiol. 1967 Jan;188(2):235–260. doi: 10.1113/jphysiol.1967.sp008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., McGuigan J. A. Contractures in a superfused frog's ventricle. J Physiol. 1966 Oct;186(2):261–283. doi: 10.1113/jphysiol.1966.sp008033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., McGuigan J. A. The efflux of potassium, sodium, chloride, calcium and sulphate ions and of sorbitol and glycerol during the cardiac cycle in frog's ventricle. J Physiol. 1968 Mar;195(2):283–315. doi: 10.1113/jphysiol.1968.sp008459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Ladinsky H., Choi S. J., Kasuya Y. Studies on the in vitro interaction of electrical stimulation and Ca++ movement in sarcoplasmic reticulum. J Gen Physiol. 1966 Mar;49(4):689–715. doi: 10.1085/jgp.49.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAD F., CHI Y. M., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. III. The effect of catecholamines and choline esters on the formation of adenosine 3',5'-phosphate by preparations from cardiac muscle and liver. J Biol Chem. 1962 Apr;237:1233–1238. [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. Movements of Ca in beating ventricles of the frog heart. J Physiol. 1963 Jul;167:551–580. doi: 10.1113/jphysiol.1963.sp007167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. The potassium chloride contracture of the heart and its modification by calcium. J Physiol. 1956 Dec 28;134(3):584–599. doi: 10.1113/jphysiol.1956.sp005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. The rate of action of calcium ions on the contraction of the heart. J Physiol. 1957 Oct 30;138(3):506–515. doi: 10.1113/jphysiol.1957.sp005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. The staircase phenomenon and the action of calcium on the heart. J Physiol. 1956 Dec 28;134(3):569–583. doi: 10.1113/jphysiol.1956.sp005666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORKAND R. K., NIEDERGERKE R. HEART ACTION POTENTIAL: DEPENDENCE ON EXTERNAL CALCIUM AND SODIUM IONS. Science. 1964 Nov 27;146(3648):1176–1177. doi: 10.1126/science.146.3648.1176. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., NONOMURA Y. The influence of ouabain on the relation between membrane potential and tension in frog heart muscle. J Pharmacol Exp Ther. 1963 Jul;141:1–5. [PubMed] [Google Scholar]
- Oliver G., Schäfer E. A. The Physiological Effects of Extracts of the Suprarenal Capsules. J Physiol. 1895 Jul 18;18(3):230–276. doi: 10.1113/jphysiol.1895.sp000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oye I. The action of adrenaline in cardiac muscle. Dissociation between phosphorylase activation and inotropic response. Acta Physiol Scand. 1965 Nov;65(3):251–258. doi: 10.1111/j.1748-1716.1965.tb04268.x. [DOI] [PubMed] [Google Scholar]
- Palmer R. F., Posey V. A. Ion effects on calcium accumulation by cardiac sarcoplasmic reticulum. J Gen Physiol. 1967 Sep;50(8):2085–2095. doi: 10.1085/jgp.50.8.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONNENBLICK E. H. Force-velocity relations in mammalian heart muscle. Am J Physiol. 1962 May;202:931–939. doi: 10.1152/ajplegacy.1962.202.5.931. [DOI] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- THOMAS L. J., Jr Increase of labeled calcium uptake in heart muscle during potassium lack contracture. J Gen Physiol. 1960 Jul;43:1193–1206. doi: 10.1085/jgp.43.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUTWEIN W., SCHMIDT R. F. [On the membrane effect of adrenalin on the myocardial fiber]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:715–726. [PubMed] [Google Scholar]
- WILBRANDT W., BRAWAND K., WITT P. N. Die quantitative Abhängigkeit der Strophanthosidwirkung auf das Froschherz von der Tätigkeit des Herzens und von der Glykosidkonzentration. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1953;219(5):397–407. [PubMed] [Google Scholar]
- WILBRANDT W., KOLLER H. Die Calciumwirkung am Froschherzen als Funktion des Ionengleichgewichts zwischen Zellmembran und Umgebung. Helv Physiol Pharmacol Acta. 1948;6(2):208–221. [PubMed] [Google Scholar]
- WINEGRAD S., SHANES A. M. Calcium flux and contractility in guinea pig atria. J Gen Physiol. 1962 Jan;45:371–394. doi: 10.1085/jgp.45.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R. Kinetic studies of epinephrine effects in the perfused rat heart. Pharmacol Rev. 1966 Mar;18(1):205–210. [PubMed] [Google Scholar]


