Abstract
Neurologic injury is a devastating complication of cardiac surgery. Cerebral cooling is an important aspect of hypothermic cardiopulmonary bypass in some patients, because hypothermia is the only reliable method of neuroprotection against injuries related to cerebral ischemia. Hypothermia may afford neuroprotection by a variety of mechanisms, including reduction in cerebral metabolic rate, decreased excitatory transmitter release, reduced ion influx, and reduced vascular permeability.
Conversely, hyperthermia, even if mild (2–3 °C), is harmful; it aggravates ischemic neuronal injury and accelerates neuronal death. In patients with acute strokes, hyperthermia worsens prognosis with respect to stroke severity, infarct size, mortality, and outcome in survivors.
The degree of temperature discrepancy among standard monitoring sites in individual patients is often striking. The differences between jugular bulb temperature and rectal or bladder temperature are particularly large. Blood temperature in the arterial line leading from the oxygenator may be the most consistently accurate indicator of cerebral temperature.
When hypothermia is used to protect vital organs during cardiopulmonary bypass, the cooling phase should be adequate, and the rewarming phase must be carefully managed. Hyperthermia may be as hazardous during the postoperative period as during surgery, exacerbating the extent of tissue injury if an overt stroke has occurred. Postoperative hyperthermia correlates with a greater degree of cognitive dysfunction measured 6 weeks after cardiac surgery.
In conclusion, cardiac anesthesiologists can reduce the risk of inadvertent hyperthermia by selecting the best sites for temperature monitoring, carefully controlling the rewarming process, and continuing temperature monitoring during the postoperative period.
Key words: Cardiopulmonary bypass, cardiovascular surgical procedures, hyperthermia, hypothermia, induced, neuroprotection
For decades, anesthesiologists and other specialists have studied the effects of cerebral hypoxia and ischemia and have developed methods to protect the brain under these conditions.1,2 Cerebral cooling is an important aspect of hypothermic cardiopulmonary bypass (CPB), because deliberate hypothermia reliably protects the brain from ischemic injury.1,3–5 Even mild hypothermia—as little as 1 °C—will diminish the severity of cerebral ischemia. For every 1 °C that brain temperature is decreased, cerebral metabolic rate decreases by 7%.6
Benefits of Hypothermia
Hypothermia provides neuroprotection against ischemia by several mechanisms. First, it creates a favorable balance between oxygen supply and demand. Second, it decreases excitotoxic neurotransmitter release, which is believed to play an important role in delayed neuronal death.1,2 Third, hypothermia is a crucial factor in preventing blood-brain barrier dysfunction and decreasing permeability in brain arte-rioles.1,7 Fourth, hypothermia appears to decrease polymorphonuclear leukocyte adhesion in the damaged region.8
Harmful Effects of Hyperthermia
In contrast, even mild hyperthermia (1–2 °C) can be harmful. Increased cerebral temperature significantly aggravates ischemic neuronal injury and accelerates neuronal death.4,9 Thus, increased brain temperature exacerbates the effects of cerebral ischemia, including the histopathologic consequences, stroke severity, and risk of death. This is so for both intra-ischemic mild hyperthermia (that is, hyperthermia that is present when the injury occurs) and for delayed, postischemic hyperthermia.10 In addition, hyperthermia delays neuronal metabolic recovery9 and increases excitotoxic neurotransmitter release,11 oxygen free-radical production,12 intracellular acidosis,9 and blood-brain barrier permeability.7 Hyperthermia also modulates protein kinase activity13 and destabilizes the cytoskeleton.14
Several clinical studies have shown a strong association between body temperature and outcome after stroke. Reith and colleagues15 reported that in patients with acute strokes, low body temperature at hospital admission was independently and significantly related to lower initial stroke severity, lesion size, and mortality; and with a better outcome in survivors. An increase in body temperature of only 1 °C was associated with a 4-point difference in patients' Scandinavian stroke severity scores (SSS)16 at discharge, a 15-mm difference in infarct size, and an 80% difference in mortality rate. Azzimondi and coworkers17 showed that a fever of at least 37.9 °C, regardless of the cause, indicated a greater likelihood of death within 30 days. After an initial feasibility study was performed,18 a large, randomized clinical trial—the Copenhagen Stroke Study — showed that body temperature at admission predicts long-term mortality after acute stroke.19
Neuroprotection with Hypothermia
Because it affects the outcome of cerebral ischemic events, brain temperature may influence the extent of neurologic injury during and after cardiac surgery with CPB.20–22 Therefore, when hypothermia is used to protect vital organs, the cooling phase should be adequate and rewarming must be carefully managed. More than half of the overt strokes recognized after cardiac surgery probably occur during surgery itself.23 Salazar and colleagues23 reported that in the Johns Hopkins Hospital cardiac surgery database, 74% of strokes were identified on the day of surgery and 91% were identified within the first 3 postoperative days. The presence of cerebral hyperthermia during surgery can only aggravate the tissue injury that ensues. Furthermore, intraoperative hyperthermia has been associated with significantly worse postoperative neuropsychological performance.20,24
During hypothermic CPB, hypothermia is always initiated after aortic cannulation and the onset of bypass. Patients are rewarmed before bypass is terminated, usually before the aortic cross-clamp is removed. Cerebral embolism is unlikely during the hypothermic period because the heart is excluded from the circulation by the aortic cross-clamp throughout this period. Cerebral embolism most often occurs during periods when the brain is warm or warming, particularly during and immediately after removal of the aortic cross-clamp.25 Therefore, too aggressive rewarming in an attempt to avoid the “afterdrop” in temperature that usually occurs after discontinuation of CPB may cause cerebral hyperthermia during a period when cerebral embolism is likely. Although some investigators advocate the development of a pharmacologic agent to provide neuroprotection during these vulnerable periods, no such magic bullet has yet been discovered.26 Regardless of any agent that may be used or developed, clinicians should continue to avoid inducing hyperthermia.
The site of temperature monitoring during hypothermic CPB is crucial, because sites vary substantially in the readings they produce.27–29 Monitoring bladder or rectal temperatures is standard practice in many institutions, and many clinicians try to normalize temperature at these sites by aggressive rewarming before CPB is terminated. However, when deep hypothermia is rapidly induced, relatively noninvasive temperature measurements made at standard body monitoring sites do not approximate intracerebral temperature well.27 We and others27,29–32 have shown that, during rewarming from hypothermic CPB, temperatures measured in the nasopharynx, esophagus, and rectum differ substantially from those measured in the jugular bulb. In a recent study at the Texas Heart Institute*, we compared jugular bulb temperature—the best in vivo indicator of cerebral cortical temperature—throughout the rewarming phase of CPB in 16 patients undergoing hypothermic CPB for coronary artery bypass grafting. A 6-channel monitor continuously and simultaneously recorded all temperatures. We found that the bladder temperature was more than 4 °C lower than the jugular bulb temperature during some periods of rewarming, and nasopharyngeal and esophageal temperatures differed from jugular bulb temperature by as much as 2 °C during much of the rewarming phase (Fig. 1).
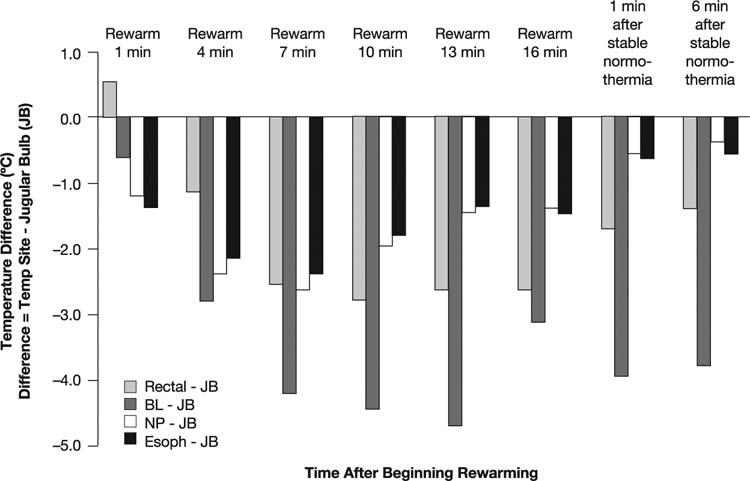
Fig. 1 Temperature differences between the jugular bulb (JB) site and the rectal, bladder (BL), nasopharyngeal (NP), and esophageal (Esoph) sites during rewarming in 16 patients undergoing cardiopulmonary bypass during cardiac surgery.
These findings have led us to modify our practice at the Texas Heart Institute. During CPB, we do not allow the nasopharyngeal temperature to go above 37 °C. We also start rewarming the patient much earlier and rewarm more slowly than we did previously. We never set the water bath above 38 °C. Importantly, we do not monitor bladder or rectal temperatures, because they do not accurately indicate cerebral temperature.
Our alterations in practice have improved our ability to avoid hyperthermia. In subsequent studies, the temperature difference between the jugular bulb and the bladder and rectum varied by about 3 °C during rewarming, which is better than the 5 °C difference we saw before these changes were put into practice.29 There is still a 1 to 2 °C gradient for nasopharyngeal and esophageal temperature, but these eventually equilibrate. The mean rewarming time is 38 minutes, which is a bit longer than it was before we instituted these changes; the rewarming rate is 0.2 °C per minute.29
Blood temperature in the arterial line leading from the oxygenator may be the most consistently accurate indicator of cerebral temperature.29 When we recorded pump outflow temperature with a temperature probe, we found that it closely approximated jugular bulb temperature within approximately 10 minutes (Fig. 2), leading us to use the arterial line temperature as our current gold standard monitor.
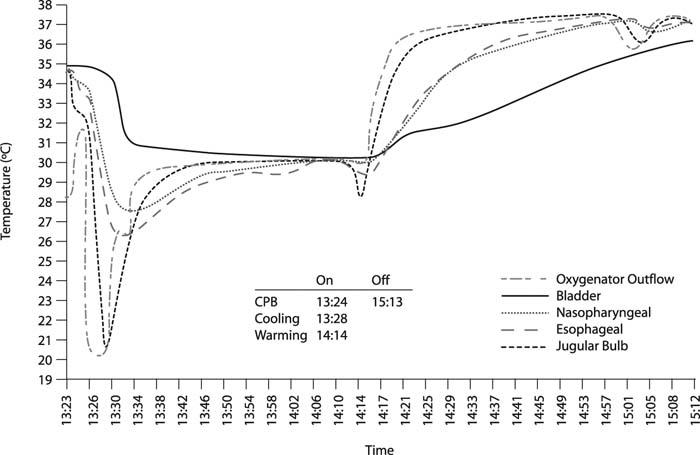
Fig. 2 Temperature monitoring during cardiopulmonary bypass (CPB) and rewarming in a patient undergoing cardiac surgery. Temperatures in the jugular bulb and oxygenator outflow are similar throughout and are virtually superimposable during rewarming.
Postoperative Hyperthermia
Hyperthermia that develops after surgery may be just as hazardous as intraoperative hyperthermia, and avoiding it requires fastidious attention. Postoperative hyperthermia is known to be correlated with a greater degree of cognitive dysfunction 6 weeks after cardiac surgery33 (Fig. 3). Temperatures exceeding 38.5 °C are common during the first 48 hours after cardiac surgery, occurring in nearly 40% of patients.34 Furthermore, fever can exacerbate the extent of tissue injury if an overt stroke occurs. Salazar's group23 and Hogue and co-authors35 have noted that approximately one fourth of strokes that occur after CPB occur during the postoperative period, usually in patients who initially had an uneventful recovery. These strokes may not be directly related to the CPB; other potential causes include circulatory failure or atrial fibrillation.35,36
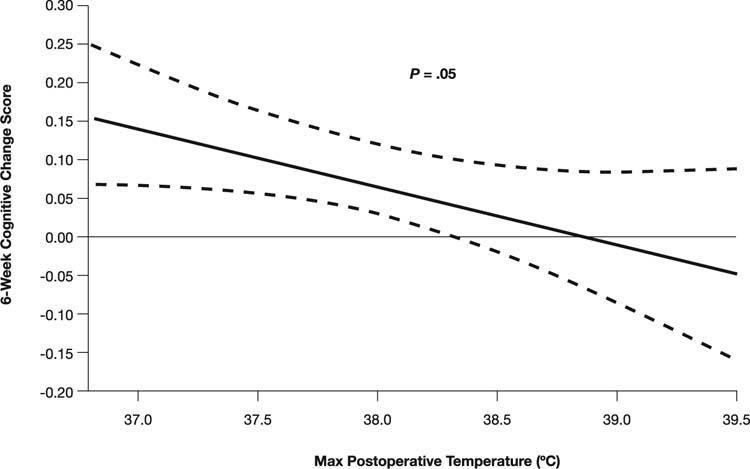
Fig. 3 The association between maximum postoperative temperature and cognitive change score 6 weeks after coronary artery bypass grafting.
(From: Grocott HP, Mackensen GB, Grigore AM, Mathew J, Reves JG, Phillips-Bute B, et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke 2002;33:537–41. Reproduced with permission from Lippincott Williams & Wilkins.)
These findings have led some clinicians to reduce the temperature at which they wean patients from bypass. In a randomized controlled study, Nathan and colleagues37 showed that patients rewarmed to a lower temperature (34 °C) before weaning were significantly less likely to have postoperative cognitive deficits than were patients rewarmed to 37 °C (48% vs 62% at 1 week, with the trend continuing to 3 months). It is important to note that this lower post-bypass temperature has the potential to cause hemodynamic instability or coagulopathy;5 the risks and benefits must therefore be weighed for each individual patient.
Summary
Hypothermia (33–35 °C) during cardiopulmonary bypass has well-documented neuroprotective benefits. Evidence suggests that hypothermia improves neurologic outcome even when induced after a cerebral ischemic event. Rewarming must proceed slowly, and the clinician may consider weaning at temperatures slightly below 37 °C in patients at high risk of an ischemic event. Hyperthermia—even if mild—can exacerbate any neurologic injury that occurs during surgery, so it is critical to avoid overheating of the brain during rewarming. Finally, physicians should continue temperature management into the postoperative period.
Acknowledgment
Stephen N. Palmer, PhD, ELS, provided editorial support.
Footnotes
*Unpublished data; 2001–2002.
Address for reprints: Nancy A. Nussmeier, MD, Department of Cardiovascular Anesthesiology, Texas Heart Institute, 6720 Bertner, Suite O-520, Houston, TX 77030
E-mail: nnussmeier@heart.thi.tmc.edu
This article is derived from a presentation at a symposium titled “Common Perioperative Problems for the Cardiac Anesthesiologist,” held in conjunction with the Society of Cardiovascular Anesthesiologists' 27th Annual Meeting and Workshops in Baltimore, Maryland, on 15 May 2005.
References
- 1.Ginsberg MD, Busto R. Combating hyperthermia in acute stroke: a significant clinical concern. Stroke 1998;29:529–34. [DOI] [PubMed]
- 2.Todd MM, Warner DS. A comfortable hypothesis reevaluated. Cerebral metabolic depression and brain protection during ischemia. Anesthesiology 1992;76:161–4. [PubMed]
- 3.Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J Cereb Blood Flow Metab 1987;7:729–38. [DOI] [PubMed]
- 4.Minamisawa H, Smith ML, Siesjo BK. The effect of mild hyperthermia and hypothermia on brain damage following 5, 10, and 15 minutes of forebrain ischemia. Ann Neurol 1990;28:26–33. [DOI] [PubMed]
- 5.Barone FC, Feuerstein GZ, White RF. Brain cooling during transient focal ischemia provides complete neuroprotection. Neurosci Biobehav Rev 1997;21:31–44. [DOI] [PubMed]
- 6.Wass CT, Lanier WL, Hofer RE, Scheithauer BW, Andrews AG. Temperature changes of > or = 1 degree C alter functional neurologic outcome and histopathology in a canine model of complete cerebral ischemia. Anesthesiology 1995; 83:325–35. [DOI] [PubMed]
- 7.Dietrich WD, Busto R, Halley M, Valdes I. The importance of brain temperature in alterations of the blood-brain barrier following cerebral ischemia. J Neuropathol Exp Neurol 1990; 49:486–97. [DOI] [PubMed]
- 8.Ishikawa M, Sekizuka E, Sato S, Yamaguchi N, Inamasu J, Bertalanffy H, et al. Effects of moderate hypothermia on leukocyte-endothelium interaction in the rat pial microvasculature after transient middle cerebral artery occlusion. Stroke 1999;30:1679–86. [DOI] [PubMed]
- 9.Chopp M, Welch KM, Tidwell CD, Knight R, Helpern JA. Effect of mild hyperthermia on recovery of metabolic function after global cerebral ischemia in cats. Stroke 1988;19:1521–5. [DOI] [PubMed]
- 10.Kawai N, Okauchi M, Morisaki K, Nagao S. Effects of delayed intraischemic and postischemic hypothermia on a focal model of transient cerebral ischemia in rats. Stroke 2000;31:1982–9. [DOI] [PubMed]
- 11.Castillo J, Davalos A, Noya M. Aggravation of acute ischemic stroke by hyperthermia is related to an excitotoxic mechanism. Cerebrovasc Dis 1999;9:22–7. [DOI] [PubMed]
- 12.Globus MY, Busto R, Lin B, Schnippering H, Ginsberg MD. Detection of free radical activity during transient global ischemia and recirculation: effects of intraischemic brain temperature modulation. J Neurochem 1995;65:1250–6. [DOI] [PubMed]
- 13.Busto R, Globus MY, Neary JT, Ginsberg MD. Regional alterations of protein kinase C activity following transient cerebral ischemia: effects of intraischemic brain temperature modulation. J Neurochem 1994;63:1095–103. [DOI] [PubMed]
- 14.Morimoto T, Ginsberg MD, Dietrich WD, Zhao W. Hyperthermia enhances spectrin breakdown in transient focal cerebral ischemia. Brain Res 1997;746:43–51. [DOI] [PubMed]
- 15.Reith J, Jorgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, Olsen TS. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet 1996;347:422–5. [DOI] [PubMed]
- 16.Lindenstrom E, Boysen G, Christiansen LW, Hansen BR, Neilsen PW. Reliability of Scandinavian neurological stroke scale. Cerebrovasc Dis 1991;1:103–7.
- 17.Azzimondi G, Bassein L, Nonino F, Fiorani L, Vignatelli L, Re G, D'Alessandro R. Fever in acute stroke worsens prognosis. A prospective study. Stroke 1995;26:2040–3. [DOI] [PubMed]
- 18.Kammersgaard LP, Rasmussen BH, Jorgensen HS, Reith J, Weber U, Olsen TS. Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: A case-control study: the Copenhagen Stroke Study. Stroke 2000;31:2251–6. [DOI] [PubMed]
- 19.Kammersgaard LP, Jorgensen HS, Rungby JA, Reith J, Nakayama H, Weber UJ, et al. Admission body temperature predicts long-term mortality after acute stroke: the Copenhagen Stroke Study. Stroke 2002;33:1759–62. [DOI] [PubMed]
- 20.Mora CT, Henson MB, Weintraub WS, Murkin JM, Martin TD, Craver JM, et al. The effect of temperature management during cardiopulmonary bypass on neurologic and neuropsychologic outcomes in patients undergoing coronary revascularization. J Thorac Cardiovasc Surg 1996;112:514–22. [DOI] [PubMed]
- 21.Gaudino M, Martinelli L, Di Lella G, Glieca F, Marano P, Schiavello R, Possati G. Superior extension of intraoperative brain damage in case of normothermic systemic perfusion during coronary artery bypass operations. J Thorac Cardiovasc Surg 1999;118:432–7. [DOI] [PubMed]
- 22.Cook DJ. Cerebral hyperthermia and cardiac surgery: consequences and prevention. Semin Thorac Cardiovasc Surg 2001;13:176–83. [DOI] [PubMed]
- 23.Salazar JD, Wityk RJ, Grega MA, Borowicz LM, Doty JR, Petrofski JA, Baumgartner WA. Stroke after cardiac surgery: short- and long-term outcomes. Ann Thorac Surg 2001;72:1195–202. [DOI] [PubMed]
- 24.Buss MI, McLean RF, Wong BI, Fremes SE, Naylor CD, Harrington EM, et al. Cardiopulmonary bypass, rewarming, and central nervous system dysfunction. Ann Thorac Surg 1996;61:1423–7. [DOI] [PubMed]
- 25.Barbut D, Hinton RB, Szatrowski TP, Hartman GS, Bruefach M, Williams-Russo P, et al. Cerebral emboli detected during bypass surgery are associated with clamp removal. Stroke 1994;25:2398–402. [DOI] [PubMed]
- 26.Patel P. No magic bullets: the ephemeral nature of anesthetic-mediated neuroprotection. Anesthesiology 2004;100:1049–51. [DOI] [PubMed]
- 27.Stone JG, Young WL, Smith CR, Solomon RA, Wald A, Ostapkovich N, Shrebnick DB. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology 1995;82:344–51. [DOI] [PubMed]
- 28.Grocott HP, Newman MF, Croughwell ND, White WD, Lowry E, Reves JG. Continuous jugular venous versus nasopharyngeal temperature monitoring during hypothermic cardiopulmonary bypass for cardiac surgery. J Clin Anesth 1997;9:312–6. [DOI] [PubMed]
- 29.Nussmeier NA, Li S, Strickler AG, Dragan E, Korkki RK, Cooper JR Jr. Temperature measurement during cardiopulmonary bypass [abstract]. Anesth Analg 2002;93:SCA35.
- 30.Bissonnette B, Holtby HM, Davis AJ, Pua H, Gilder FJ, Black M. Cerebral hyperthermia in children after cardiopulmonary bypass. Anesthesiology 2000;93:611–8. [DOI] [PubMed]
- 31.Cook DJ, Orszulak TA, Daly RC, Buda DA. Cerebral hyperthermia during cardiopulmonary bypass in adults. J Thorac Cardiovasc Surg 1996;111:268–9. [DOI] [PubMed]
- 32.Johnson RI, Fox MA, Grayson A, Jackson M, Fabri BM. Should we rely on nasopharyngeal temperature during cardiopulmonary bypass? Perfusion 2002;17:145–51. [DOI] [PubMed]
- 33.Grocott HP, Mackensen GB, Grigore AM, Mathew J, Reves JG, Phillips-Bute B, et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke 2002;33:537–41. [DOI] [PubMed]
- 34.Thong WY, Strickler AG, Li S, Stewart EE, Collier CL, Vaughn WK, Nussmeier NA. Hyperthermia in the forty-eight hours after cardiopulmonary bypass. Anesth Analg 2002;95:1489–95. [DOI] [PubMed]
- 35.Hogue CW Jr, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999;100:642–7. [DOI] [PubMed]
- 36.Slogoff S, Reul GJ, Keats AS, Curry GR, Crum ME, Elmquist BA, et al. Role of perfusion pressure and flow in major organ dysfunction after cardiopulmonary bypass. Ann Thorac Surg 1990;50:911–8. [DOI] [PubMed]
- 37.Nathan HJ, Wells GA, Munson JL, Wozny D. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: a random-ized trial. Circulation 2001;104(12 Suppl 1):I85–91. [DOI] [PubMed]


