Abstract
In Escherichia coli, the use of carnitine as a terminal electron acceptor depends on a functional caiTABCDE operon. It had been suggested that the adjacent but divergent fixABCX operon is also required for carnitine metabolism, perhaps to provide electrons for carnitine reduction. We have constructed E. coli fixA and fixB mutants and find that they are unable to reduce carnitine to γ-butyrobetaine under anaerobic conditions.
During anaerobic growth, Escherichia coli is able to synthesize a number of respiratory chains (6, 13). In the absence of alternative electron acceptors, such as nitrate, trimethylamine N-oxide, or fumarate, growth of anaerobic E. coli cultures can be stimulated by adding carnitine or its dehydration product, crotonobetaine (11). Seim and colleagues showed that E. coli cells reduce crotonobetaine to γ-butyrobetaine during anaerobic growth (9, 11, 12) and thus use carnitine as a terminal electron acceptor.
The carnitine metabolic pathway in E. coli involves the products of the caiTABCDE operon located at the first minute on the E. coli chromosome. caiTABCDE is induced under anaerobic conditions by the presence of carnitine or crotonobetaine. Transcription is inhibited by adding γ-butyrobetaine to cultures and is also repressed in the presence of “better” electron acceptors, such as nitrate (5, 6).
In vivo overexpression of the DNA region 5′ to the cai operon resulted in four polypeptides with amino acid sequence similarity to polypeptides encoded by the Sinorhizobium meliloti fixABCX operon (4, 6). The FixA and FixB proteins of both E. coli and S. meliloti resemble the β- and α-subunits of electron transfer flavoproteins (14), which typically transfer electrons from a dehydrogenase to a ubiquinone oxidoreductase. FixC has regions that are similar to the family of ubiquinone oxidoreductases. The fourth protein produced by the operon, FixX, is predicted to be a novel type of ferredoxin (1). In S. meliloti, it has been suggested that the four Fix proteins act together to transfer electrons from a carbon source to nitrogenase (4).
In E. coli, it has been shown (2, 6) that, during anaerobic growth, the divergent fixABCX and caiTABCDE operons are coregulated in the presence of carnitine. However, they did not link the fix genes directly to carnitine metabolism, since they were unable to obtain stable chromosomal fix gene mutations (6). They observed a high mortality rate and chromosomal rearrangements in the fix region when they attempted to make mutants and suggested this was due to a crucial role for the fix gene products in E. coli (6). In this study, we have created stable in-frame mutations in E. coli fixA and fixB. These mutations alter carnitine metabolism in a way that is consistent with the idea that carnitine can be used by E. coli as a terminal electron acceptor under anaerobic conditions.
Bacterial strains and growth conditions.
The E. coli strains and plasmids used in this work are listed in Table 1. Cultures used for the growth experiments were grown aerobically in Luria-Bertani (LB) broth (10) at 37°C overnight. These cultures were then diluted 1:50 in 14-ml screw-top test tubes filled to the top with M9 broth (10) containing 0.2% glycerol as the sole carbon source and NH4 as the sole nitrogen source. As indicated, carnitine was added at a final concentration of 10 mM, and sodium nitrate was added at a final concentration of 40 mM. Sodium molybdate was added to all tubes at a concentration of 2 μM. The cultures were incubated in stoppered glass tubes at 30°C and allowed to become anaerobic over a 48-h period before culture densities were determined.
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Genotype or descriptiona | Source |
|---|---|---|
| Strains | ||
| BW25113 | lacIqrrnBT14 ΔlacZWJ16hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | B. Wanner (3) |
| AW2A | BW25113 fixA | This work |
| AW1B | BW25113 fixB | This work |
| Plasmids | ||
| pKD4 | oriR[R6Kγ] Kanr Ampr | B. Wanner (3) |
| pKD46 | Temperature-sensitive derivative of pSC101, AmpraraC-ParaB λ recombination genes (gam-β-exo) | B. Wanner (3) |
| Primers | ||
| 1A | 5′-GCGTGATATCTGTAATTAACACCACCGATATGAACGACGTGTAGGCTGGAGCTGCTTC-3′ | This work |
| 2A | 5′-GAGAAAACGTGTTCATAGCATCCCCTGTAATTAAATGACCATATGAATATCCTCCTTAG-3′ | This work |
| 1B | 5′-CGCAAAGTCATTTAATTACAGGGGATGCTATGAACACGGTGTAGGCTGGAGCTGCTTC-3′ | This work |
| 2B | 5′-GCCAAAATGCAGCCTTGCCAGAGTGGATCAACGCGCTAACATATGAATATCCTCCTTAG-3′ | This work |
In primers 1A and 2A, underlined sequence is identical to the E. coli fixA DNA sequences. In primers 1B and 2B, underlined sequence is identical to the E. coli fixB DNA sequences. Boldface sequence is identical to the kanamycin cassette DNA sequences on pKD4.
Construction of E. coli fixA and fixB mutants.
Since Eichler et al. had difficulties in constructing stable chromosomal insertion mutations in the E. coli fix genes (6), we decided to try to create in-frame mutations within the fix genes to eliminate possible negative polar effects of the mutations on downstream genes by using the PCR transformation method described by Datsenko and Wanner (3). Long primers containing sequences at the ends of the fix genes were used to amplify a kanamycin resistance DNA cassette. Primer 1 contained the 5′ fix DNA sequence and 5′ kanamycin cassette sequence, while primer 2 contained the 3′ fix DNA sequence and 3′ kanamycin cassette sequence (Table 1). PCR amplification of pKD4 with these primers generated PCR products with the 5′ and 3′ fix DNA sequences flanking the kanamycin cassette (3). These linear PCR products were electroporated into BW25113(pKD46) cells made competent as described previously (3). Mutants were selected by plating electroporated cells on LB agar containing 25 mg of kanamycin per liter and incubating them overnight at 37°C. Transformants were colony purified and tested for both the absence of pKD4 and the loss of the temperature-sensitive pKD46 plasmid by looking for penicillin sensitivity. The structure of the DNA in the fix region was examined by PCR to confirm replacement of the wild-type allele of the targeted fix gene with the kanamycin cassette (3). Constructs with fixA and fixB were successful, but despite several attempts, we were not able to construct the corresponding fixC or fixX mutants.
Growth of the fixA and fixB mutant cultures was not stimulated by the addition of carnitine.
Seim et al.. (11) showed that addition of carnitine or its dehydration product, crotonobetaine, stimulates the anaerobic growth of E. coli O44 K74 cultures. We confirmed that cultures of wild-type BW25113 cells grew to a higher density in the presence of carnitine than in media containing no terminal electron acceptor (Fig. 1). Addition of nitrate or carnitine plus nitrate to BW25113 also increased cell density. Growth of BW25113 with carnitine as the sole terminal electron acceptor was similar to growth of BW25113 when nitrate was provided (Fig. 1), indicating that carnitine was as effective as nitrate in stimulating wild-type BW25113 E. coli cultures.
FIG. 1.
Growth of E. coli cultures with various electron acceptors. Cultures were grown in M9 plus 0.2% glycerol under anaerobic conditions for 48 h in the presence of 10 mM carnitine, 40 mM nitrate, or carnitine plus nitrate as the available electron acceptors in the media. The data shown are representative of several experiments. Black bars, BW25113; gray bars; AW2A; open bars, AW1B.
In contrast, growth of the fixA and fixB mutants, AW2A and AW1B, was similar in the presence of carnitine to growth of wild-type BW25113 cells grown in the absence of a terminal electron acceptor. Addition of nitrate or carnitine plus nitrate to the fixA or fixB cultures resulted in culture densities and growth rates comparable to those seen in wild-type cells grown with carnitine, nitrate, or carnitine plus nitrate in the media (Fig. 1). Thus, both mutants could use another electron acceptor, and the lack of growth by the mutants was not due to carnitine toxicity. We concluded that the E. coli fixA and fixB genes were necessary for the metabolism of carnitine to γ-butyrobetaine in E. coli and suggest that these mutants are unable to use carnitine as a terminal electron acceptor.
fixA and fixB mutants do not metabolize carnitine to γ-butyrobetaine.
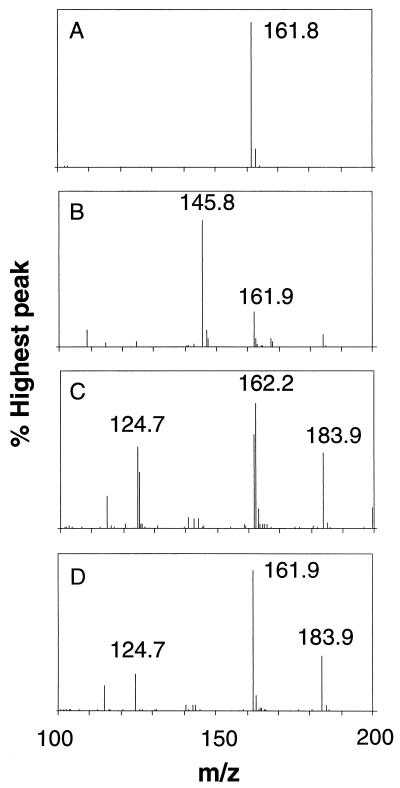
Supernatants from wild-type BW25113 and its fixA and fixB mutant derivatives grown in the presence of carnitine were analyzed by mass spectroscopy to determine whether carnitine was metabolized in the mutants. After incubation with carnitine, BW25113 supernatants showed only a small carnitine peak at 161.9 and a large peak of γ-butyrobetaine at 145.8, indicating almost complete conversion of carnitine to γ-butyrobetaine (Fig. 2B). In contrast, supernatants from fixA and fixB cultures contained no γ-butyrobetaine peak at 145.8, but still contained a large carnitine peak (Fig. 2C and D). The E. coli fix mutants were unable to metabolize carnitine to γ-butyrobetaine. Interestingly, two new peaks were observed in the mutants at 124.7 and 183.9. We are currently trying to determine the identities of these additional peaks.
FIG. 2.
Carnitine metabolism by wild-type and fix mutant strains. E. coli cultures were grown under anaerobic conditions for 48 h in the presence of 10 mM carnitine. Cultures were centrifuged to remove cells, and then supernatant samples were analyzed via mass spectroscopy. Positive ion atmospheric pressure ionization-electrospray mass spectroscopy was carried out with a Waters ZQ mass detector. The spray chamber parameters were optimized at 2.7 liters of nitrogen per min with a capillary voltage of 3.5 kV, cone voltage of 20 V, cone temperature of 520°C, and desolvation temperature of 125°C. Mass spectra were obtained from the medium control (A), BW25113 supernatant (B), AW2A supernatant (C), and AW1B supernatant (D).
Conclusion.
Using a newly developed technique for chromosomal mutagenesis, we isolated E. coli fixA and fixB mutants and verified an earlier hypothesis that components of the fixABCX operon were necessary for anaerobic carnitine reduction. The instability of these mutants in the previous work may have been due to the method of mutagenesis used or to the different E. coli strains mutated. With these in-frame mutations in E. coli fixA and fixB, we have shown that the fixA and fixB mutants were unable to use carnitine anaerobically as the sole terminal electron acceptor and that these mutants were unable to metabolize carnitine to γ-butyrobetaine.
Under anaerobic conditions, E. coli does not assimilate the carbon and nitrogen of carnitine. It has been suggested that the primary function of the carnitine metabolic pathway in E. coli cells may be to use carnitine or crotonobetaine as electron acceptors during anaerobic growth in the absence of the preferred substrates (8, 9, 11, 12). Elssner et al. (7) have suggested that γ-butyrobetainyl-CoA and crotonobetainyl-CoA are substrates necessary for the conversion of carnitine to crotonobetaine and that this pathway proceeds at the CoA level, concluding with the hydrolysis of γ-butyrobetainyl-CoA to give γ-butyrobetaine. This pathway, encoded by the cai operon, allows carnitine to be used as a terminal electron acceptor.
The divergent fixABCX operon is coregulated with the cai operon, and the products of the fix operon show homology to proteins found in known electron transport pathways (6, 14). Our results confirm that the E. coli FixA and FixB proteins are needed for carnitine reduction, and we hypothesize that they are involved in bringing reductant to CaiA, the postulated crotonobetainyl-CoA reductase (Fig. 3). Pathways that involve FixAB homologues have been shown to accept electrons from acyl-CoA dehydrogenases, pass these electrons to their FixC homologue, and finally donate the electrons to various respiratory chains through an electron transfer flavoprotein, ubiquinone oxidoreductase, homologous to FixC. If this is the mechanism of the FixABCX pathway in E. coli, we would expect FixX would interact with CaiA. It has been suggested that the FixABCX proteins in S. meliloti bring electrons to nitrogenase. In this case, it seems likely that reduced FixX is produced and is then able to transfer electrons to the nitrogenase proteins. However, FixAB homologues often interact with enoyl-CoA dehydrogenases, and we consider it possible that in E. coli, this pathway is run in the reverse direction, with the E. coli FixX protein being initially reduced and then passing electrons through the pathway to the electron transfer flavoprotein homologue encoded by the fixAB genes. Resolving how the Fix proteins move electrons to their substrates will require a more biochemical approach.
FIG. 3.
Suggested pathway for the metabolism of carnitine to γ-butyrobetaine in anaerobic E. coli cultures. Carnitine imported into the cells is converted to its carnitinyl-CoA thioester by CaiB in a transfer reaction with γ-butyrobetainyl-CoA that releases γ-butyrobetaine. CaiD converts carnitinyl-CoA to crotonobetainyl-CoA in a dehydration reaction, and the latter is reduced to γ-butyrobetainyl-CoA by CaiA. Electrons for this reaction are postulated to be contributed by the FixABCX proteins, which are reduced during the oxidation of other substrates available to the cell.
Acknowledgments
We thank Barry Wanner for providing us with the hyperrecombination strains and Adriana Parra-Colmenares for assistance with the mass spectrometer.
This work was supported by the Washington State University Agriculture Research Center and grants DE-FG06-93ER20119 and DE-FG03-96ER20225 from the United States Department of Energy—Energy Biosciences Program.
REFERENCES
- 1.Bruschi, M., and F. Guerlesquin. 1988. Structure, function and evolution of bacterial ferredoxins. FEMS Microbiol. Rev. 54:155-176. [DOI] [PubMed] [Google Scholar]
- 2.Buchet, A., K. Eichler, and M.-A. Mandrand-Berthelot. 1998. Regulation of the carnitine pathway in Escherichia coli: investigation of the cai-fix divergent promoter region. J. Bacteriol. 180:2599-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datsenko, K. A., and B. W. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Earl, C. D., C. W. Ronson, and F. M. Ausubel. 1987. Genetic and structural analysis of the Rhizobium meliloti fixA, fixB, fixC, and fixX genes. J. Bacteriol. 169:1127-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichler, K., F. Bourgis, A. Buchet, H.-P. Kleber, and M.-A. Mandrand-Berthelot. 1994. Molecular characterization of the cai operon necessary for carnitine metabolism in Escherichia coli. Mol. Microbiol. 13:775-786. [DOI] [PubMed] [Google Scholar]
- 6.Eichler, K., A. Buchet, F. Bourgis, H.-P. Kleber, and M.-A. Mandrand-Berthelot. 1995. The fix Escherichia coli region contains four genes related to carnitine metabolism. J. Basic Microbiol. 35:217-227. [DOI] [PubMed] [Google Scholar]
- 7.Elssner, T., L. Hennig, H. Frauendorf, D. Haferburg, and H.-P. Kleber. 2000. Isolation, identification, and synthesis of γ-butyrobetainyl-CoA and crotonobetainyl-CoA, compounds involved in carnitine metabolism of E. coli. Biochemistry 39:10761-10769. [DOI] [PubMed] [Google Scholar]
- 8.Jung, K., H. Jung, and H.-P. Kleber. 1987. Regulation of L-carnitine metabolism in Escherichia coli. J. Basic Microbiol. 27:131-137. [DOI] [PubMed] [Google Scholar]
- 9.Rebouche, C. J., and H. Seim. 1998. Carnitine metabolism and its regulation in microorganisms and mammals. Annu. Rev. Nutr. 18:39-61. [DOI] [PubMed] [Google Scholar]
- 10.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Seim, H., H. Loster, R. Claus, H.-P. Kleber, and E. Strack. 1982. Stimulation of the anaerobic growth of Salmonella typhimurium by reduction of L-carnitine, carnitine derivatives and structure-related trimethylammonium compounds. Arch. Microbiol. 132:91-95. [DOI] [PubMed] [Google Scholar]
- 12.Seim, H., H. Loster, R. Claus, H.-P. Kleber, and E. Strack. 1982. Formation of γ-butyrobetaine and trimethylamine from quaternary ammonium compounds structure-related to L-carnitine and choline by Proteus vulgaris. FEMS Microbiol. Lett. 13:201-205. [Google Scholar]
- 13.Stewart, V. 1998. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol. Rev. 52:190-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai, M. H., and M. H. Saier, Jr. 1995. Phylogenetic characterization of the ubiquitous electron transfer flavoprotein families ETF-α and ETF-β. Res. Microbiol. 146:397-404. [DOI] [PubMed] [Google Scholar]