Abstract
Comamonas testosteroni strain R5 expresses a higher level of phenol-oxygenating activity than any other bacterial strain so far characterized. The expression of the operon encoding multicomponent phenol hydroxylase (mPH), which is responsible for the phenol-oxygenating activity, is controlled by two transcriptional regulators, PhcS and PhcR, in strain R5. In this study, we identified a third transcriptional regulator for the mPH operon (PhcT) that belongs to the AraC/XylS family. While the disruption of phcT in strain R5 significantly reduced the expression of the mPH operon, it did not eliminate the expression. However, the disruption of phcT in strain R5 increased the expression of phcR. The phenol-oxygenating activity was abolished by the disruption of phcR, indicating that PhcT alone was not sufficient to activate the expression of the mPH operon. The disruption of phcS has been shown in our previous study to confer the ability of strain R5 to express the mPH operon in the absence of the genuine substrate for mPH. PhcT was not involved in the gratuitous expression. Strain R5 thus possesses a more elaborate mechanism for regulating the mPH operon expression than has been found in other bacteria.
The expression of a bacterial catabolic pathway for aromatic compounds is often controlled by one or more transcriptional regulatory proteins (21), and sometimes, one transcriptional regulator controls the expression of another transcriptional regulator (24).
The expression of multicomponent phenol hydroxylase (mPH) (9, 11, 12, 19, 20, 36, 37) is generally thought to be controlled by a regulator of the XylR/DmpR subclass within the NtrC-type family of transcriptional regulators, resulting in the expression of phenol-metabolizing enzymes only in the presence of the pathway substrate or its structural analog (2, 12, 14, 18, 19, 22, 26, 30-33, 37). The regulators of this subclass are activated by direct interaction with an effector molecule which is normally the substrate for the catabolic pathway the regulators control (29).
Comamonas testosteroni R5 has been shown to exhibit an exceptionally high level of activity for phenol oxygenation (42). We have cloned a DNA fragment encoding mPH (phcKLMNOP) and its cognate transcriptional activator (phcR) of the XylR/DmpR subclass from strain R5 (37). This work (37) and an electrophoretic analysis in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (our unpublished data) indicated that the high activity of strain R5 was due to the high level of mPH expression, leading us to investigate its transcriptional mechanism. PhcR caused the expression of the mPH operon even in the absence of the genuine substrate for mPH, but this gratuitous expression was repressed by a member of the GntR family of transcriptional regulators named PhcS (38). This GntR family member for regulating the mPH operon has also been identified for C. testosteroni TA441. For strain TA441, the regulator named AphS repressed the transcription of the mPH operon even in the presence of phenol, which prevented strain TA441 from growing on phenol (1). In the present study, we found one open reading frame, named phcT, downstream of phcS. The physiological role of PhcT on the expression of phenol-metabolizing enzymes in strain R5 was studied.
(This work is taken from a thesis submitted by Maki Teramoto to Ochanomizu University, Tokyo, Japan, in partial fulfillment of the requirements for the degree of Doctor of Philosophy.)
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. The culture media used in this study were Luria-Bertani (LB) medium (25), M9 medium (3), and an inorganic medium called MP containing (per liter) 2.75 g of K2HPO4, 2.25 g of KH2PO4, 1.0 g of (NH4)2SO4, 0.2 g of MgCl2·6H2O, 0.1 g of NaCl, 0.02 g of FeCl3·6H2O, and 0.01 g of CaCl2 (pH 6.8 to 7.0). The Escherichia coli strains were grown at 37°C, while the C. testosteroni strains were grown at 30°C, unless otherwise stated. When required, the media were supplemented with the following antibiotics at the indicated concentrations: tetracycline, 12 μg/ml; ampicillin, 100 μg/ml; carbenicillin, 500 μg/ml; and chloramphenicol, 20 μg/ml (E. coli) or 80 μg/ml (C. testosteroni).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strain | ||
| C. testosteroni | ||
| R5 | Phl+, wild type, contains Phc mPH genes | 42 |
| R5T | Phl+, ΔphcT::Tcr of R5 | This study |
| R5TS | Phl+, Δ(phcT−phcS)::Tcr of R5 | This study |
| R5R | Phl−, ΔphcR::Tcr of R5 | This study |
| E. coli | ||
| DH5α | Host strain for DNA manipulation | Toyobo |
| S17-1 | Host strain for plasmid mobilization | 34 |
| Plasmid | ||
| pBluescriptIIKS(−) | Apr, cloning vector | Toyobo |
| pROR501 | Apr, 11.1-kb XbaI-SalI fragment of pLAFRR501 cloned into NheI-SalI-cleaved pRO1614 | 38 |
| pMT5059 | Apr, pBR322 derivative carrying multiple cloning sites and an NotI site | 41 |
| pMT5056 | Apr Tcr, pBR322 derivative carrying a Tcr gene cartridge flanked by EcoRV and PvuII sites | 41 |
| pMT5071 | Cmr, plasmid containing an NotI-flanked mobilization cassette (the cassette contains the Cmr gene, sacB, and the Mob region) | 40 |
| pSK1 | Apr, 3.9-kb KpnI-SmaI fragment of pROR501 carrying the phcT and phcS genes cloned into pMT5059 | 38 |
| pSK01T | Apr Tcr, 1.7-kb EcoRV fragment of pMT5056 carrying the Tcr gene cloned into a blunted SacII-SalI site of pSK1 | This study |
| pSK02T | Apr/Cbr Tcr Cmr, NotI fragment carrying the mobilization cassette of pMT5071 cloned into pSK01T | This study |
| pSK01TS | Apr Tcr, 1.7-kb PvuII fragment of pMT5056 carrying the Tcr gene cloned into a blunted SacII-ApaI site of pSK1 | This study |
| pSK02TS | Apr/Cbr Tcr Cmr, NotI fragment carrying the mobilization cassette of pMT5071 cloned into pSK01TS | This study |
| pBS2 | Apr, 4.0-kb BglII-SacII (blunted) fragment of pROR501 carrying the phcS and phcR genes cloned into a BglII-NruI site of pMT5059 | 38 |
| pBS01R | Apr Tcr, 1.7-kb PvuII fragment of pMT5056 carrying the Tcr gene cloned into a PvuII-PvuII site of pBS2 | This study |
| pBS02R | Apr/Cbr Tcr Cmr, NotI fragment carrying the mobilization cassette of pMT5071 cloned into pBS01R | This study |
| pRC50 | Tcr Cmr IncP, lacZ promoter-probe vector | 38 |
| pUC18 | Apr, cloning vector | Takara |
| pUC18Pr | Apr, 2.8-kb EcoRV-NheI fragment of pROR501 cloned into a HincII-XbaI site of pUC18 | This study |
| pRC50Pr | Tcr Cmr, 2.8-kb HindIII-BamHI fragment of pUC18Pr cloned into pRC50; phcR::lacZ transcriptional fusion | This study |
| pRC50Pk | Tcr Cmr, phcKL::lacZ transcriptional fusion | 38 |
| pUC18Pt | Apr, 2.5-kb XbaI-SalI fragment of pROR501 cloned into pUC18 | This study |
| pRC50Pt | Tcr Cmr, 2.5-kb EcoRI-HindIII fragment of pUC18Pt cloned into pRC50; phcT::lacZ transcriptional fusion | This study |
Abbreviations: Phl+, growth on phenol; Phl−, no growth on phenol; Apr, ampicillin resistant; Cbr, carbenicillin resistant; Apr/Cbr, resistant to both ampicillin and carbenicillin; Tcr, tetracycline resistant; Cmr, chloramphenicol resistant.
Genetic techniques.
Plasmid isolation, restriction endonuclease digestion, and transformation of the E. coli strains were conducted by the methods of Sambrook et al. (25). The C. testosteroni strains were transformed by the method of Chakrabarty et al. (6).
Nucleotide sequencing and computer analysis.
To determine the nucleotide sequence of the 2.5-kb XbaI-SalI fragment downstream of phcS (Fig. 1), subfragments were cloned into the multicloning site of pBluescript II KS(−). The nucleotide sequences of the subfragments were determined in both orientations by using M13 primers (Takara), a DNA sequencing kit (Dye Terminator Cycle Sequence; Perkin-Elmer) and a model 377 DNA sequencer (Perkin-Elmer) according to the manufacturer's instructions. The templates for the dideoxy chain-termination reactions were prepared by Wizard minipreps (Promega). The DNA sequence data were aligned by using version 1.7 of ClustalW (39).
FIG. 1.
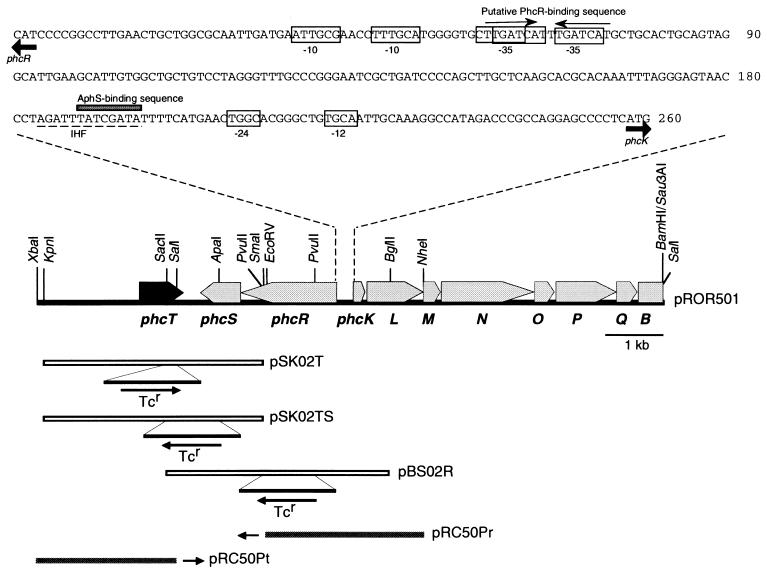
Genetic organization of the regulatory and structural genes for mPH in C. testosteroni R5. phcT (in black) was identified in this study. The other genes have been identified in our previous studies (37, 38). pSK02T carries the phcT gene which was disrupted by inserting a Tcr cassette, pSK02TS carries the phcT and phcS genes which were disrupted by inserting a Tcr cassette, and pBS02R carries phcR which was disrupted by inserting a Tcr cassette. The arrows indicate the direction of Tcr transcription. pRC50Pr and pRC50Pt are derivatives of pRC50, pRC50Pr carrying an EcoRV-NheI fragment which contains the phcR promoter region and pRC50Pt carrying an XbaI-SalI fragment which contains the phcT promoter region. The two small arrows indicate lacZ fused to these promoters on plasmids pRC50Pr and pRC50Pt. The nucleotide sequence of the phcR-phcK promoter region is also shown. Putative −35 and −10 sequences of the phcR promoter and putative −24 and −12 sequences of the phcK promoter are boxed (37). A putative IHF recognition sequence is indicated by the dashed underline (37). A putative PhcR-binding sequence, which is similar to the recognition sites of DmpR and XylR (30), is indicated by the pair of arrows. The shaded box indicates an AphS-binding sequence (1).
Construction of the phcT and phcR knockouts and of the knockout defective in both the phcT and phcS genes.
The phcT gene on pSK1 was disrupted by inserting a 1.7-kb EcoRV fragment of pMT5056, which carried a tetracycline resistance (Tcr) gene, into the blunted SacII-SalI site of pSK1 (pSK01T). A NotI fragment containing the mobilization cassette of pMT5071 was subsequently inserted into pSK01T. The plasmid thus constructed, pSK02T, was mobilized (7) from E. coli S17-1 to strain R5, and Tcr selection was done on an M9 agar plate containing 200 mg of phenol per liter, 5% (wt/vol) of sucrose, and tetracycline. The transconjugants (phcT knockouts: strain R5T) were chosen for their sensitivity to carbenicillin, and their chromosomal DNAs were analyzed by the PCR to confirm that gene replacement had indeed occurred (data not shown).
The phcR gene on pBS2 was disrupted by inserting a 1.7-kb PvuII fragment of pMT5056, which carried a Tcr gene, into the PvuII-PvuII site of pBS2 (pBS01R). The NotI fragment of pMT5071 was subsequently inserted into pBS01R. The plasmid thus constructed, pBS02R, was mobilized from E. coli S17-1 to strain R5, and Tcr selection was done on an M9 agar plate containing 600 mg of sodium acetate per liter, 5% (wt/vol) sucrose, and tetracycline. The transconjugants (phcR knockouts: strain R5R) were chosen and analyzed as just described above.
The phcT and phcS genes on pSK1 were disrupted by inserting the 1.7-kb PvuII fragment of pMT5056 into the blunted SacII-ApaI site of pSK1 (pSK01TS). The NotI fragment of pMT5071 was subsequently inserted into pSK01TS. The plasmid thus constructed, pSK02TS, was mobilized from E. coli S17-1 to strain R5, and Tcr selection was done on an M9 agar plate containing 200 mg of phenol per liter, 5% (wt/vol) sucrose, and tetracycline. The transconjugants (knockouts defective in both the phcT and phcS genes: strain R5TS) were chosen and analyzed as described above.
Assay for phenol-oxygenating activity.
The phenol-oxygenating activity (oxygen uptake rate) was measured at 25°C with a Clark-type oxygen electrode (5/6 Oxygraph; Gilson) as described previously (37). The activity, which was measured in the presence of 10 mM potassium cyanide following the addition of phenol (final concentration, 10 μM), represents the amount of oxygen consumed equally by phenol hydroxylase (PH) and catechol 2,3-dioxygenase (C23DOase). A previous study had indicated that the phenol-oxygenating activity was double the PH activity, showing that the activity of C23DOase is higher than that of PH (42). The cell weight (dry weight) was determined as described previously (37). Cells from a continuous culture were sampled immediately before the activity was measured.
Induction experiment.
The expression of mPH under batch culture conditions was examined as described below. C. testosteroni strains were grown in LB medium to the stationary phase, harvested, washed with MP medium, resuspended in the original culture volume of MP medium, and finally exposed to 2 mM phenol at 30°C for the indicated periods of time during shaking at 100 rpm. Before the phenol-oxygenating activity was measured, the culture was washed with MP medium and then resuspended in the same medium. C. testosteroni strains transformed with a pRC50 derivative were grown to the stationary phase in LB medium containing Cm and then washed and resuspended in MP medium containing phenol as just described above. Before the activity of β-galactosidase, the lacZ gene product, was measured (see below), the culture was washed with MP medium and then resuspended in the same medium. After each experiment, maintenance of the plasmid was checked by using nonselective and selective plates (supplemented with Cm).
Other methods.
The conditions used for continuous culture and sampling from the culture were as described previously (38). The C23DOase activity was measured by the method described previously (38). The protein concentration was determined by the method of Bradford (5) with a protein assay kit (Bio-Rad), using bovine serum albumin as the standard. The activity of β-galactosidase was determined by the protocol described by Miller (17).
Nucleotide sequence accession number.
The nucleotide sequence of the 2.5-kb XbaI-SalI region has been deposited in the DDBJ/EMBL/GenBank database under accession no. AB061422.
RESULTS AND DISCUSSION
Identification of the phcT gene.
We analyzed the DNA region downstream of phcS cloned in pROR501 (38). Sequencing of the 2.5-kb XbaI-SalI region identified a 771-bp open reading frame, named phcT, preceded by a putative Shine-Dalgarno sequence (27) (Fig. 1). This phcT gene encodes a protein of 257 amino acid residues with a predicted molecular mass of 28 kDa. The deduced product has 19% identity and 45% similarity to XylS, a transcriptional activator of the meta-cleavage pathway genes on Pseudomonas putida TOL plasmid pWW0 which are involved in the degradation of benzoate and substituted benzoates (13, 16, 35). The residues conserved in the AraC/XylS family of transcriptional regulators shown by Gallegos et al. (10) were well conserved in the PhcT sequence (Fig. 2). One characteristic of the AraC/XylS family is to show two putative helix-turn-helix (HTH) motifs within this conserved region that are likely to be involved in DNA binding (10). One HTH motif was indicated to be located between amino acid residues 175 and 196 in PhcT with the method described by Dodd and Egan (8). The other HTH motif conserved in the AraC/XylS family was predicted to be located between amino acid residues 211 and 252 in PhcT by a protein sequence motif search program (http://motif.genome.ad.jp/MOTIF.html). Therefore, PhcT was indicated to be a member of the AraC/XylS family of transcriptional regulators. A gene corresponding to phcT has not previously been found in the mPH gene clusters of other bacteria.
FIG. 2.
Comparison of the PhcT amino acid sequence with the consensus sequence for the AraC/XylS family (10). Hyphens indicate nonconserved amino acid residues. The position of the first amino acid residue in each of the XylS and PhcT proteins is indicated by the numbers in parentheses.
Enhancement of the mPH operon expression by PhcT.
We constructed a phcT knockout of C. testosteroni strain R5 (R5T) to examine the physiological role of phcT. Strain R5T was cultured in a chemostat with phenol as the sole carbon source, and the phenol-oxygenating activity and C23DOase activity of the culture of strain R5T were compared with those of parental strain R5 (Table 2). A C23DOase gene was found downstream of the phc mPH genes (phcB in Fig. 1) (37) and was thought to be transcribed in the same unit as mPH genes (28). Therefore, C23DOase activity was measured to monitor the transcriptional level of the mPH genes. The phenol-oxygenating activity of strain R5T was 55% of that of strain R5, and the level of C23DOase activity was positively correlated with that of the phenol-oxygenating activity (Table 2). These results suggest that PhcT promoted the expression of the mPH operon at the transcriptional level. The protein profiles in strains R5T and R5, which had been grown in a chemostat with phenol, were also analyzed by SDS-PAGE using mPH subunit proteins (PhcKLMNOP) overexpressed in E. coli as molecular markers. The quantities of the mPH subunits determined by SDS-PAGE showed good correlation with the phenol-oxygenating activity (data not shown).
TABLE 2.
Effect of disrupting phcT on the expression of the mPH operon in C. testosteroni R5 grown on phenol in a continuous culture
| Strain | Phenol-oxygenating activity (μmol min−1 [g of dry cells]−1) | C23DOase activity (μmol min−1 [mg of protein]−1) |
|---|---|---|
| R5a | 213 ± 5 (100) | 2.27 ± 0.14 (100) |
| R5T | 117 ± 7 (55) | 1.58 ± 0.11 (70) |
Data are from our previous study (38). Each value is the mean ± standard error from two or three independent cultures. Values in parentheses show the percentage of the value from strain R5.
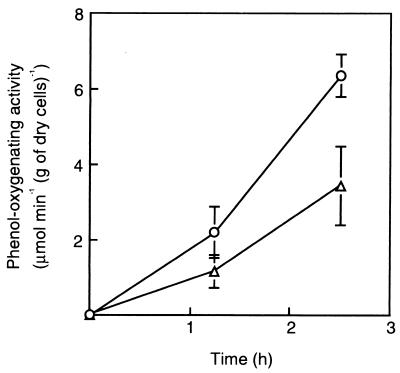
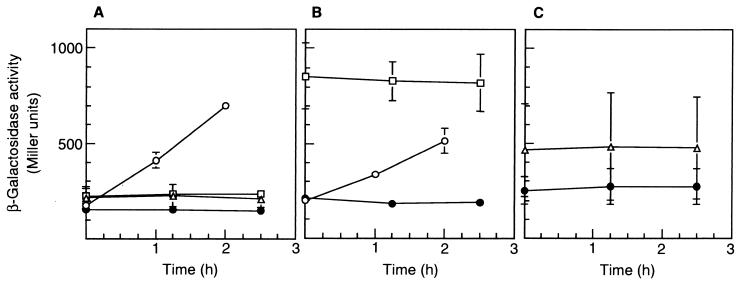
The expression of the mPH operon in response to phenol in strains R5T and R5 was also examined in batch cultures (Fig. 3). It was reduced upon introduction of the phcT disruption. The patterns of mPH expression matched the transcriptional patterns of the mPH operon monitored by phcKL::lacZ transcriptional fusion (Fig. 4A and B), suggesting that PhcT promoted mPH expression at the transcriptional level. Neither the transcriptional level of phcR, a transcriptional activator gene for the mPH operon, nor that of phcT was affected by phenol (Fig. 4). Interestingly, the transcription level of phcR was much higher in strain R5T than in strain R5 (Fig. 4A and B). In spite of the higher level of expression of phcR in strain R5T, the phenol-oxygenating activity was lower in this strain than in the wild-type strain. These results suggest that PhcT exerted positive control on the mPH operon and negative control on phcR and that PhcR was not rate limiting for the expression of the mPH operon. Similar regulation has been reported for the genes involved in the toluene/xylene degradation pathway encoded on the P. putida TOL plasmid. XylR activated the σ54-dependent promoter of xylS (Ps1) while repressing its own σ70 promoters (Pr1 and Pr2). Such regulation was observed because the σ70-RNA polymerase binding sites of Pr1 and Pr2 overlap the upstream activating sequences of the divergently organized σ54 promoter Ps1 (4, 15).
FIG. 3.
mPH expression in C. testosteroni strains R5 and R5T in response to phenol. Stationary-phase cultures of strains R5 (circles) and R5T (triangles) grown on LB medium were exposed to MP medium supplemented with 2 mM phenol (at time 0). Each value is the mean ± standard error from four independent experiments.
FIG. 4.
Transcriptional activities of the phcK, phcR, and phcT promoters in C. testosteroni R5 (A), of the phcK and phcR promoters in C. testosteroni R5T (B), and of the phcT promoter in C. testosteroni R5R (C) in response to phenol. The strains were transformed with pRC50Pk, which carries the transcriptional fusion of phcKL::lacZ (circles), pRC50Pr, which carries the transcriptional fusion of phcR::lacZ (squares), pRC50Pt, which carries the transcriptional fusion of phcT::lacZ (triangles), or pRC50 (control vector [solid circles]). The first and second genes of the mPH operon are phcK and phcL, respectively (Fig. 1). At time zero, each stationary-phase culture was transferred to MP medium supplemented with 2 mM phenol. Each value is the mean ± standard error from three or four independent experiments.
PhcT as an auxiliary factor for the PhcR-dependent transcriptional activation of the mPH operon.
We constructed a phcR knockout of C. testosteroni R5 (R5R) to examine whether PhcT alone would be sufficient to cause expression of the mPH operon. Strain R5R transformed with pRC50Pt showed higher β-galactosidase activity than strain R5R transformed with pRC50 (Fig. 4C), indicating that PhcT was expressed in the R5R strain. Strain R5R was unable to grow on phenol as the sole carbon source (data not shown). These results strongly suggest that PhcT alone was insufficient to induce expression of the Phc mPH operon.
Role of PhcT in the gratuitous expression of the mPH operon.
We have reported that a phcS knockout of C. testosteroni R5 (R5S) expressed the mPH operon even in the absence of the genuine substrate for mPH (38). To test the involvement of PhcT in this gratuitous expression, we constructed C. testosteroni R5 defective in both the phcS and phcT genes (R5TS). Strain R5TS was continuously cultured in a chemostat with acetate as the sole carbon source, and the phenol-oxygenating activity of the culture was measured. The activity of strain R5TS grown on acetate was at the same level as that of strain R5S grown on acetate (data not shown), indicating that PhcT was not involved in the gratuitous expression of the mPH operon.
Proposed model for the control of the phenol-oxidizing operon in Comamonas.
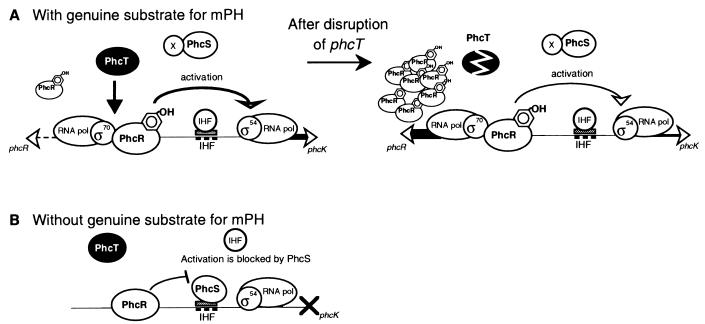
Together with our previous results (37, 38), a model for the novel transcriptional regulation of the mPH operon is proposed (Fig. 5). In the absence of the genuine substrate for mPH, PhcR-dependent gratuitous expression of the mPH operon was blocked by PhcS (38). As an AphS (PhcS-like protein)-binding site overlapped a putative integration host factor (IHF) recognition sequence (Fig. 1), bending of the promoter region for the mPH operon by the IHF for contact between PhcR and the σ54-RNA polymerase holoenzyme (23) might have been hampered by the binding of PhcS on the IHF recognition sequence. The hypothesis that the binding of IHF to the IHF recognition sequence is inhibited in a competitive manner by PhcS has not been experimentally tested. PhcT was not involved in the gratuitous expression (Fig. 5B). In the presence of the genuine substrate for mPH, the action of PhcS was prevented by an as yet uncharacterized factor, X, and PhcR could interact with the σ54-RNA polymerase holoenzyme. Phenol itself could not be the factor X, since PhcS was suggested to cause transcriptional repression of the Phc mPH genes in the presence of phenol in a heterologous Pseudomonas aeruginosa host (38). PhcT reduced the transcription of phcR but enhanced the PhcR-mediated transcriptional activation of the mPH promoter (Fig. 5A). We speculate that PhcR in strain R5 was enough to fully activate the mPH promoter and that excess PhcR in strain R5T was not used for this transcriptional activation. The mode of action of PhcT is not yet clear, but one possibility is that PhcT bound to the phcR promoter region decreased the transcription of phcR but interacted with PhcR to enhance the transcription of the mPH operon (Fig. 1 and 5A).
FIG. 5.
Model for the transcriptional regulation of the mPH operon in C. testosteroni R5. (A) Expression of the Phc mPH operon in the presence of the genuine substrate for mPH. PhcR-mediated transcriptional activation of the Phc mPH operon was enhanced by PhcT-mediated regulation, while transcription of phcR was largely inhibited by this regulation. The action of PhcS was prevented by factor X. In the absence of PhcT, PhcR-mediated transcriptional activation was not enhanced, and the inhibition of phcR transcription was relieved. (B) Expression of the Phc mPH operon in the absence of the genuine substrate for mPH. PhcR-mediated transcriptional activation of the Phc mPH operon was repressed by PhcS (38). PhcT was not involved in the gratuitous expression which occurred in strain R5S in the absence of the genuine substrate for mPH.
We have thus demonstrated in this study that acquisition of PhcT was largely responsible for the high phenol-oxygenating activity of strain R5. Acquiring PhcT may also be advantageous for limiting the excess expression of PhcR. These features must have been beneficial for strain R5 to predominate over other members of the microbial community and survive in the natural environment.
Acknowledgments
We thank Fusako Numazaki for technical assistance.
This work was supported by a grant from the New Energy and Industrial Technology Development Organization (NEDO).
REFERENCES
- 1.Arai, H., S. Akahira, T. Ohishi, and T. Kudo. 1999. Adaptation of Comamonas testosteroni TA441 to utilization of phenol by spontaneous mutation of the gene for a trans-acting factor. Mol. Microbiol. 33:1132-1140. [DOI] [PubMed] [Google Scholar]
- 2.Arai, H., S. Akahira, T. Ohishi, M. Maeda, and T. Kudo. 1998. Adaptation of Comamonas testosteroni TA441 to utilize phenol: organization and regulation of the genes involved in phenol degradation. Microbiology 144:2895-2903. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology. John Wiley and Sons, New York., N.Y.
- 4.Bertoni, G., S. Marqués, and V. de Lorenzo. 1998. Activation of the toluene-responsive regulator XylR causes a transcriptional switch between σ54 and σ70 promoters at the divergent Pr/Ps region of the TOL plasmid. Mol. Microbiol. 27:651-659. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarty, A. M., J. R. Mylroie, D. A. Friello, and J. G. Vacca. 1975. Transformation of Pseudomonas putida and Escherichia coli with plasmid-linked drug-resistance factor DNA. Proc. Natl. Acad. Sci. USA 72:3647-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 8.Dodd, I. B., and J. B. Egan. 1990. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 18:5019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrt, S., F. Schirmer, and W. Hillen. 1995. Genetic organization, nucleotide sequence and regulation of expression of genes encoding phenol hydroxylase and catechol 1,2-dioxygenase in Acinetobacter calcoaceticus NCIB8250. Mol. Microbiol. 18:13-20. [DOI] [PubMed] [Google Scholar]
- 10.Gallegos, M.-T., R. Schleif, A. Bairoch, K. Hofmann, and J. L. Ramos. 1997. AraC/XylS family of transcriptional regulators. Microbiol. Mol. Biol. Rev. 61:393-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrmann, H., C. Muller, I. Schmidt, J. Mahnke, L. Petruschka, and K. Hahnke. 1995. Localization and organization of phenol degradation genes of Pseudomonas putida strain H. Mol. Gen. Genet. 247:240-246. [DOI] [PubMed] [Google Scholar]
- 12.Hino, S., K. Watanabe, and N. Takahashi. 1998. Phenol hydroxylase cloned from Ralstonia eutropha strain E2 exhibits novel kinetic properties. Microbiology 144:1765-1772. [DOI] [PubMed] [Google Scholar]
- 13.Inouye, S., A. Nakazawa, and T. Nakazawa. 1986. Nucleotide sequence of the regulatory gene xylS on the Pseudomonas putida TOL plasmid and identification of the protein product. Gene 44:235-242. [DOI] [PubMed] [Google Scholar]
- 14.Jaspers, M. C. M., W. A. Suske, A. Schmid, D. A. M. Goslings, H.-P. E. Kohler, and J. R. van de Meer. 2000. HbpR, a new member of the XylR/DmpR subclass within the NtrC family of bacterial transcriptional activators, regulates expression of 2-hydroxybiphenyl metabolism in Pseudomonas azelaica HBP1. J. Bacteriol. 182:405-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marqués, S., M.-T. Gallegos, M. Manzanera, A. Holtel, K. N. Timmis, and J. L. Ramos. 1998. Activation and repression of transcription at the double tandem divergent promoters for the xylR and xylS genes of the TOL plasmid of Pseudomonas putida. J. Bacteriol. 180:2889-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mermod, N., J. L. Ramos, A. Bairoch, and K. N. Timmis. 1987. The xylS gene positive regulator of TOL plasmid pWWO: identification, sequence analysis and overproduction leading to constitutive expression of meta cleavage operon. Mol. Gen. Genet. 207:349-354. [DOI] [PubMed] [Google Scholar]
- 17.Miller, J. H. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Muller, C., L. Petruschka, H. Cuypers, G. Burchhardt, and H. Herrmann. 1996. Carbon catabolite repression of phenol degradation in Pseudomonas putida is mediated by the inhibition of the activator protein PhlR. J. Bacteriol. 178:2030-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng, L. C., V. Shingler, C. C. Sze, and C. L. Poh. 1994. Cloning and sequences of the first eight genes of the chromosomally encoded (methyl) phenol degradation pathway from Pseudomonas putida P35X. Gene 151:29-36. [DOI] [PubMed] [Google Scholar]
- 20.Nordlund, I., J. Powlowski, and V. Shingler. 1990. Complete nucleotide sequence and polypeptide analysis of multicomponent phenol hydroxylase from Pseudomonas sp. strain CF600. J. Bacteriol. 172:6826-6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsek, M. R., S. M. McFall, and A. M. Chakrabarty. 1996. Evolution of regulatory systems of biodegradative pathways, p. 135-152. In T. Nakazawa, K. Furukawa, D. Haas, and S. Silver (ed.), Molecular biology of pseudomonads. American Society for Microbiology, Washington, D.C.
- 22.Pavel, H., M. Forsman, and V. Shingler. 1994. An aromatic effector specificity mutant of the transcriptional regulator DmpR overcomes the growth constraints of Pseudomonas sp. strain CF600 on para-substituted methylphenols. J. Bacteriol. 176:7550-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-Martín, J., and V. de Lorenzo. 1997. Clues and consequences of DNA bending in transcription. Annu. Rev. Microbiol. 51:593-628. [DOI] [PubMed] [Google Scholar]
- 24.Salto, R., A. Delgado, M.-T. Gallegos, M. Manzanera, S. Marqués, and J. L. Ramos. 1996. Fine control of expression of the catabolic pathways of TOL plasmid of Pseudomonas putida for mineralization of aromatic hydrocarbons, p. 207-216. In T. Nakazawa, K. Furukawa, D. Haas, and S. Silver (ed.), Molecular biology of pseudomonads. American Society for Microbiology, Washington, D.C.
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Schirmer, F., S. Ehrt, and W. Hillen. 1997. Expression, inducer spectrum, domain structure, and function of MopR, the regulator of phenol degradation in Acinetobacter calcoaceticus NCIB8250. J. Bacteriol. 179:1329-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shine, J., and L. Dalgarno. 1975. Determination of cistron specificity in bacterial ribosomes. Nature 254:34-38. [DOI] [PubMed] [Google Scholar]
- 28.Shingler, V. 1996. Metabolic and regulatory check points in phenol degradation by Pseudomonas sp. strain CF600, p. 153-164. In T. Nakazawa, K. Furukawa, D. Haas, and S. Silver (ed.), Molecular biology of pseudomonads. American Society for Microbiology, Washington, D.C.
- 29.Shingler, V. 1996. Signal sensing by σ54-dependent regulators: derepression as a control mechanism. Mol. Microbiol. 19:409-416. [DOI] [PubMed] [Google Scholar]
- 30.Shingler, V., M. Bartilson, and T. Moore. 1993. Cloning and nucleotide sequence of the gene encoding the positive regulator (DmpR) of the phenol catabolic pathway encoded by pVI150 and identification of DmpR as a member of the NtrC family of transcriptional activators. J. Bacteriol. 175:1596-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shingler, V., C. H. Franklin, M. Tsuda, D. Holroyd, and M. Bagdasarian. 1989. Molecular analysis of a plasmid-encoded phenol hydroxylase from Pseudomonas CF600. J. Gen. Microbiol. 135:1083-1092. [DOI] [PubMed] [Google Scholar]
- 32.Shingler, V., and T. Moore. 1994. Sensing of aromatic compounds by the DmpR transcriptional activator of phenol-catabolizing Pseudomonas sp. strain CF600. J. Bacteriol. 176:1555-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shingler, V., and H. Pavel. 1995. Direct regulation of the ATPase activity of the transcriptional activator DmpR by aromatic compounds. Mol. Microbiol. 17:505-513. [DOI] [PubMed] [Google Scholar]
- 34.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 35.Spooner, R. A., K. Lindsay, and F. C. H. Franklin. 1986. Genetic, functional and sequence analysis of the xylR and xylS regulatory genes of the TOL plasmid pWWO. J. Gen. Microbiol. 132:1347-1358. [DOI] [PubMed] [Google Scholar]
- 36.Takeo, M., Y. Maeda, H. Okada, K. Miyama, K. Mori, M. Ike, and M. Fujita. 1995. Molecular cloning and sequencing of the phenol hydroxylase gene from Pseudomonas putida BH. J. Ferment. Bioeng. 79:485-488. [Google Scholar]
- 37.Teramoto, M., H. Futamata, S. Harayama, and K. Watanabe. 1999. Characterization of a high-affinity phenol hydroxylase from Comamonas testosteroni R5 by gene cloning, and expression in Pseudomonas aeruginosa PAO1c. Mol. Gen. Genet. 262:552-558. [DOI] [PubMed] [Google Scholar]
- 38.Teramoto, M., S. Harayama, and K. Watanabe. 2001. PhcS represses gratuitous expression of phenol-metabolizing enzymes in Comamonas testosteroni R5. J. Bacteriol. 183:4227-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuda, M. 1998. Use of a transposon-encoded site-specific resolution system for construction of large and defined deletion mutation in bacterial chromosome. Gene 207:33-41. [DOI] [PubMed] [Google Scholar]
- 41.Tsuda, M., H. Miyazaki, and T. Nakazawa. 1995. Genetic and physical mapping of genes involved in pyoverdin production in Pseudomonas aeruginosa PAO. J. Bacteriol. 177:423-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe, K., S. Hino, K. Onodera, S. Kajie, and N. Takahashi. 1996. Diversity in kinetics of bacterial phenol-oxygenating activity. J. Ferment. Bioeng. 81:560-563. [Google Scholar]