Abstract
The Bacillus subtilis thermosensitive mutant ts-21 bears two C-G→T-A transitions in the mnaA gene. At the nonpermissive temperature it is characterized by coccoid cell morphology and reduced cell wall phosphate content. MnaA converts UDP-N-acetylglucosamine into UDP-N-acetylmannosamine, a precursor of the teichoic acid linkage unit.
In phosphate-replete conditions, Bacillus subtilis 168 cell walls are endowed with two teichoic acids: poly(glycerol phosphate) [poly(groP)], whose synthesis is indispensable for cell growth (20), and poly(glucosyl N-acetylgalactosamine 1-phosphate) [poly(GlcGalNAc 1-P)], a nonessential so-called minor polymer (3). Both polymers are attached to peptidoglycan via a linkage unit consisting of N-acetylglucosamine 1-phosphate, N-acetylmannosamine (ManNAc), and, most likely, one residue of glycerol phosphate (2, 18). Interference with poly(groP) synthesis, due to mutations in the teichoic acid gene tagB, tagD, or tagF (4, 21, 22) or to a limited expression of tagGH or tagO (16, 25), results (i) in a reduction of the cell wall phosphate content and (ii) in a rod-to-sphere change in cell shape.
Thermosensitive mutant ts-21 is due to mutations mapping to gene mnaA.
B. subtilis mutant L6571 (purA16 hisA35 pheA1 metB5) (ts-21) develops, at the nonpermissive temperature (47°C), a coccoid-like morphology, i.e., a phenotype associated with a deficient synthesis of poly(groP). This mutant was obtained by transforming strain L5047 (20) with chromosomal DNA of an N-methyl-N′-nitro-N-nitrosoguanidine-induced thermosensitive mutant of strain L5009 (4). The relevant mutation(s) was localized by PBS1 transduction (10) around 310° (data not presented), in the region encompassing nearly all known genes involved in the synthesis of strain 168 teichoic acids (18).
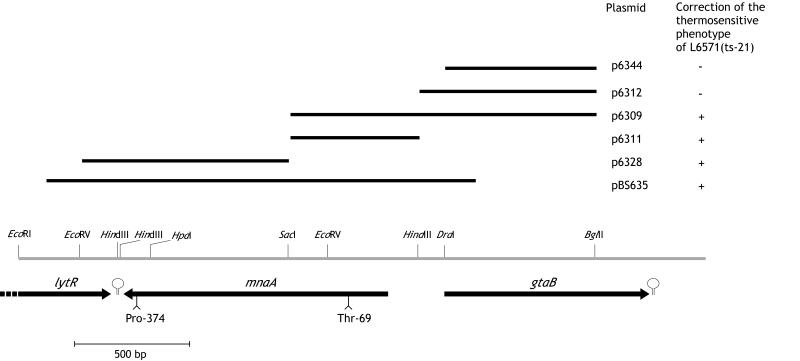
Transformation (11) of strain L6571 (ts-21) with p6311, pBS635, and p6328 (Fig. 1), nonreplicative plasmids in B. subtilis, yielded thermoresistant recombinants on LA-S (LB agar without NaCl) plates incubated for 24 h at 47°C. These plasmids cover orfX (yvyH) (15, 27), a gene previously shown to be essential for growth (27) and now renamed mnaA, in accordance with its function (see below) and the new bacterial polysaccharide gene nomenclature (23).
FIG. 1.
Correction of the strain ts-21 mutations by subclones of the mnaA-gtaB region. Plasmids correcting or not correcting the thermosensitive phenotype are indicated by + or −, respectively. The map corresponds to the region previously designated orfX-gtaB (27). Plasmids p6309, p6311, p6312, p6328, and p6344 were previously described (27). pBS635 was obtained by cloning into pBAD-TOPO vector (Invitrogen) the PCR product extending from nucleotide 639 of lytR to nucleotide 43 of gtaB and generated on strain 168 (5) DNA.
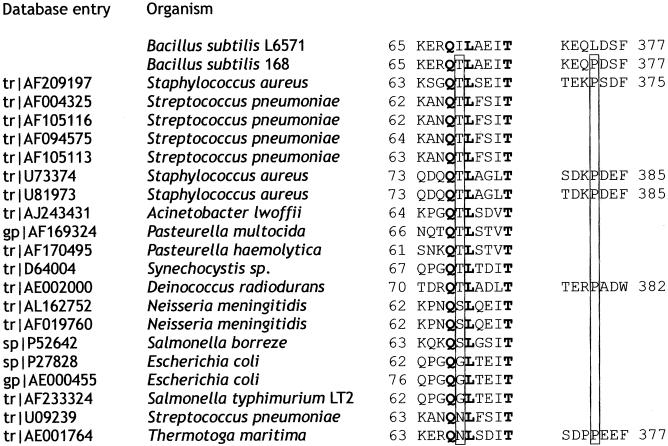
Sequencing of the mnaA region of strain L6571 (ts-21) and comparison to the wild-type sequence (27) revealed within gene mnaA two C-G→T-A transitions, converting codons ACA (Thr-69) and CCG (Pro-374) into ATA (Ile) and CTG (Leu), respectively. Knocking out either of these mutations by transformation with plasmid p6311 or p6328 (27), respectively (Fig. 1), restored temperature insensitivity and rod morphology as well as the wild-type level of cell wall phosphate (Fig. 2). Therefore, the thermosensitive phenotype of strain ts-21 requires the simultaneous presence of two mutations. Both amino acid substitutions in mutated MnaA correspond to the replacement of a neutral weakly hydrophobic residue by a more strongly hydrophobic one. In prokaryotic homologs of B. subtilis MnaA, the residue equivalent to Thr-69 is occupied by a hydrophilic (N, D, or E) or a weakly hydrophobic (T, S, or G) amino acid, and, as in the case of B. subtilis MnaA, preceded by a glutamine and followed by a leucine (Fig. 3). Therefore, Thr-69 forms part of a conserved and probably catalytically important domain. The behavior of the mutated protein suggests that the presence of Pro-374 somehow suppresses the phenotype generated by the Thr-69→Ile mutation at the putative catalytic site. Altering the protein configuration by replacing Pro-374 with Leu would allow the expression of the phenotype associated with the Thr-69→Ile substitution. Interestingly, the equivalent of the B. subtilis MnaA Pro-374 is not present in most MnaA homologs (Fig. 3). Alignment of the relevant C-terminal domains reveals that these proteins end 3 to 8 amino acids upstream of the missing B. subtilis MnaA Pro-374 equivalent.
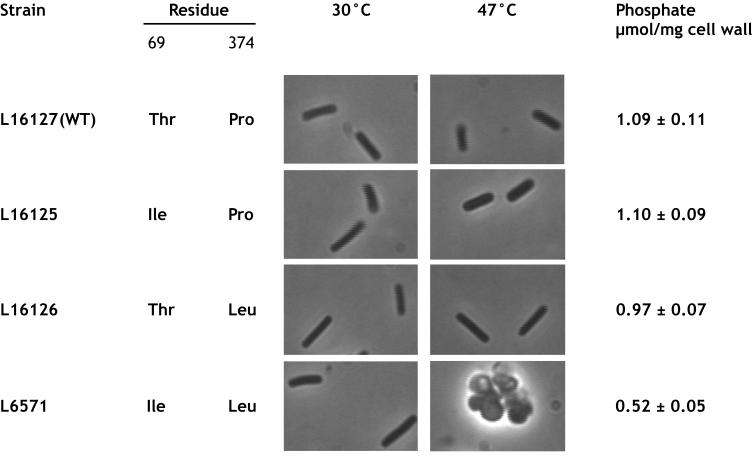
FIG. 2.
Cell morphology and cell wall phosphate content of mutant L6571 (ts-21) and its thermoresistant derivatives. Cells were grown for 20 h on LB agar plates at 30 and 47°C. Cell wall phosphate was determined for cells grown at 47°C. Residues at positions 69 and 374 in MnaA of each investigated strain are indicated. WT, wild type.
FIG. 3.
Alignment of the B. subtilis MnaA domains comprising Thr-69 and Pro-374 with their counterparts from different bacteria. All listed proteins are 43 to 64% identical to B. subtilis MnaA. The B. subtilis MnaA Thr-69 and Pro-374, as well as their equivalents in MnaA homologs, are boxed. Conserved residues are in boldface.
To determine whether the identified mutations in mnaA affect cell wall phosphate content at the nonpermissive temperature, cultures of strain L6571 (ts-21) and its thermoresistant derivatives grown in appropriately supplemented SA medium (10) at 32°C were shifted to 47°C at a nephelometric density of 45, corresponding to 4.5 × 107 cells/ml. Cells were harvested 100 min later, at a nephelometric density of 480, and, their walls were prepared essentially according to the method of Fein and Rogers (8). Lyophilized walls were mineralized (1), and their phosphate content was determined (6). Assay for the cell wall phosphate content from cultures of thermoresistant transformants L16125, L16126, and L16127 obtained with p6328, p6311, and pBS635, respectively, provided similar values (Fig. 2). However, the phosphate content of the thermosensitive mutant was nearly half that of the thermoresistant transformants (Fig. 2). This confirms that both amino acid substitutions in the ts-21 mutant are required for the temperature-sensitive phenotype associated with reduced cell wall teichoic acid content as well as morphological defects.
mnaA differs from several typical teichoic acid genes. First, its promoter, controlled by the σA factor (27), does not contain recognizable Pho boxes, as in the case of tagAB and tagDEF operons (19). Second, the 16-kb teichoic acid gene cluster extending from tagB to ggaB has an average GC content of 33% (17), i.e., significantly lower than the B. subtilis average of 43.5% (15), while mnaA and the divergently oriented gtaB (27) are characterized by GC contents of 46.4 and 43.1%, respectively, close to the B. subtilis 168 mean value. This suggests that the mnaA-gtaB divergon, like, for instance, the teichuronic acid genes tuaA to tuaF (26), was present in the B. subtilis chromosome before the acquisition of genes specifying poly(groP) and poly(GlcGalNAc 1-P) synthesis (18).
Purification and enzymatic activity of MnaA.
Comparison of the B. subtilis MnaA deduced amino acid sequence to those of Staphylococcus aureus proteins Cap5P and MnaA (14) reveals an overall homology of 58 and 61%, respectively, strongly suggesting that B. subtilis MnaA is involved in the formation of UDP-ManNAc, a precursor required for the teichoic acid linkage unit synthesis (13). To confirm this conclusion, we have assayed MnaA for UDP-GlcNAc 2-epimerase activity.
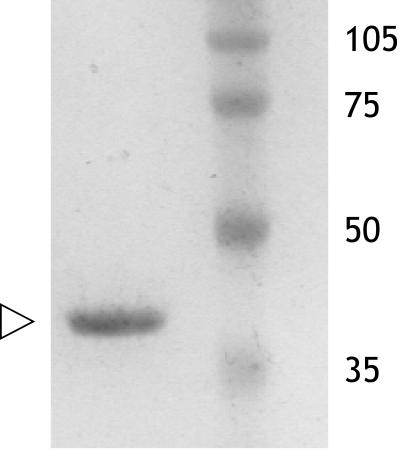
The entire mnaA gene, with the exception of its stop codon, was amplified by PCR and cloned in the expression vector pBAD-TOPO (Invitrogen), downstream of the araBAD promoter, and in frame with the distal His6 tag. In the resulting plasmid, designated pBS629, transcription of mnaA from the araBAD promoter can be induced by l-arabinose in a dose-dependent manner (9). E. coli TOP10 (Invitrogen) cells containing plasmid pBS629 were grown at 32°C with continuous shaking (200 rpm) in LB medium containing 50 μg of ampicillin/ml. At an optical density value at 600 nm of 0.5, synthesis of MnaA-His6 was induced with 0.2% arabinose, and the incubation continued for an additional 5 h under the same conditions. Cells were harvested from 25-ml cultures by centrifugation (3,000 × g, 10 min, 4°C) and stored at −80°C. Frozen cells were thawed for 15 min at room temperature, resuspended in 720 μl of lysis buffer (50 mM NaH2PO4 [pH 8], 300 mM NaCl, 10 mM imidazole) containing 1 mg of lysozyme/ml, and sonicated with six 10-s bursts alternating with 30 s of cooling in ice water. The lysate was cleared by centrifugation (10,000 × g, 20 min, 4°C) and applied to an Ni-nitrilotriacetic acid spin column (Qiagen) preequilibrated with lysis buffer. The column was washed (700 × g, 2 min, 4°C) three times with 600 μl of wash buffer (50 mM NaH2PO4 [pH 8], 300 mM NaCl, 20 mM imidazole). The protein, eluted (700 × g, 2 min, 4°C) with 150 μl of elution buffer (50 mM NaH2PO4 [pH 8], 300 mM NaCl, 250 mM imidazole), was aliquoted and kept at −80°C. Thawed aliquots were used as purified recombinant MnaA (Fig. 4).
FIG. 4.
Coomassie blue-stained gel of the MnaA-His6 fusion protein obtained following overexpression in E. coli TOP10(pBS629). Cells were induced with 0.2% l-arabinose and grown for 5 h. Purified protein is indicated by the arrowhead. At a higher protein loading, a few minor bands could be detected. Molecular weight markers are in thousands.
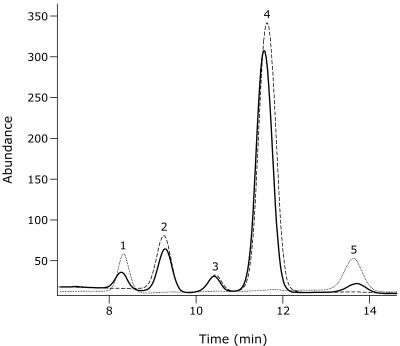
The 100-μl reaction mixture, containing 0.5 mM UDP-GlcNAc, 100 mM phosphate buffer (pH 7.0), and 1.5 μg of purified MnaA-His6 protein, was incubated for 2 h at 37°C. It was mixed with 100 μl of 1 M trifluoroacetic acid, hydrolyzed under vacuum at 100°C for 30 min, dried, and resuspended in 100 μl of water. Ten microliters of samples or 100 μM standards were injected onto a Dionex Series DX500 high-pressure liquid chromatography system with a Dionex CarboPAc PA1 anion-exchange column equilibrated in 8 mM NaOH. Separation of the components was achieved isocratically at a flow rate of 1 ml/min with 8 mM NaOH followed, at 26 min, by the application of a linear gradient from 0 to 450 mM sodium acetate in 100 mM NaOH for 40 min (7). The eluate was monitored with a pulse-electrochemical detector (Dionex), and the chromatograms were analyzed with the Igor Pro program (WaveMetrix Inc., Lake Oswego, Oreg.). Peaks 1, 2, 4, and 5 were present at positions characteristic of mannosamine, glucosamine, N-acetylglucosamine, and N-acetylmannosamine, respectively (Fig. 5). Glucosamine and N-acetylglucosamine represent the hydrolysis products of UDP-N-acetylglucosamine, the substrate. Peaks 1 and 5, corresponding to mannosamine and N-acetylmannosamine, respectively, were generated by the hydrolysis of UDP-ManNAc, the product of the enzymatic reaction. This was confirmed (i) by the absence of these peaks when the reaction was stopped at time zero (Fig. 5) or when the hydrolysis step was omitted (data not presented) and (ii) by the fact that they increased when N-acetylmannosamine was added at the beginning of the hydrolysis step (data not presented). Peak 3 is most likely a by-product of the acid hydrolysis of the substrate, the UDP-GlcNAc. Indeed, it was obtained in control reactions either arrested at time zero (Fig. 5) or containing no MnaA-His6 (data not presented).
FIG. 5.
MnaA-His6 assay. High-pressure liquid chromatography analysis was performed on trifluoroacetic acid hydrolysis products of ManNAc (dotted line), purified recombinant MnaA-His6 protein with UDP-GlcNAc without incubation (dashed line), and purified recombinant MnaA-His6 protein with UDP-GlcNAc incubated for 2 h (solid line). Under experimental conditions used, the equilibrium of interconversion between UDP-GlcNAc and UDP-ManNAc is reached in less than 10 min (data not presented). Peaks 1 to 5 are indicated.
At equilibrium, the ratio of substrate UDP-GlcNAc to UDP-ManNAc, the end product of the MnaA-mediated reaction, is about 12 to 1, i.e., not significantly different from 9 to 1 and 10 to 1, the figures previously reported for Bacillus cereus (12), E. coli (24), and S. aureus (14) enzymes. Such a bias is not surprising, since UDP-GlcNAc is, among others, massively channeled into peptidoglycan synthesis. In addition, following appropriate epimerization, UDP-GlcNAc is required for the synthesis of poly(GlcGalNAc 1-P), the minor teichoic acid of strain 168, and in low-phosphate media, for that of teichuronic acid (3, 18). However, the requirement of UDP-ManNAc, like that of UDP-GlcNAc for the teichoic acid linkage unit, is comparatively very low.
Acknowledgments
We are grateful to Jachen Barblan and Olivia Dénervaud-Ayer for competent technical help.
REFERENCES
- 1.Ames, B. N. 1966. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 8:115-118. [Google Scholar]
- 2.Araki, Y., and E. Ito. 1989. Linkage units in cell walls of gram-positive bacteria. Crit. Rev. Microbiol. 17:121-135. [DOI] [PubMed] [Google Scholar]
- 3.Archibald, A. R., I. C. Hancock, and C. R. Harwood. 1993. Cell wall structure, synthesis and turnover, p. 381-410. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, D.C.
- 4.Briehl, M., H. M. Pooley, and D. Karamata. 1989. Mutants of Bacillus subtilis 168 thermosensitive for growth and wall teichoic acid synthesis. J. Gen. Microbiol. 135:1325-1334. [DOI] [PubMed] [Google Scholar]
- 5.Burkholder, P. R., and N. H. Giles. 1947. Induced biochemical mutations in Bacillus subtilis. Am. J. Bot. 33:345-348. [PubMed] [Google Scholar]
- 6.Chen, P. S., T. Y. Toribara, and H. Warner. 1956. Microdetermination of phosphorus. Anal. Chem. 18:1756-1758. [Google Scholar]
- 7.Clarke, A. J. 1993. Compositional analysis of peptidoglycan by high-performance anion-exchange chromatography. Anal. Biochem. 212:344-350. [DOI] [PubMed] [Google Scholar]
- 8.Fein, J. E., and H. J. Rogers. 1976. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J. Bacteriol. 127:1427-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman, L., D. Belin, M. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karamata, D., and J. D. Gross. 1970. Isolation and genetic analysis of temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Mol. Gen. Genet. 108:277-287. [DOI] [PubMed] [Google Scholar]
- 11.Karamata, D., H. M. Pooley, and M. Monod. 1987. Expression of heterologous genes for wall teichoic acid in Bacillus subtilis 168. Mol. Gen. Genet. 207:73-81. [DOI] [PubMed] [Google Scholar]
- 12.Kawamura, T., M. Kimura, S. Yamamori, and E. Ito. 1978. Enzymatic formation of uridine diphosphate N-acetyl-d-mannosamine. J. Biol. Chem. 253:3595-3601. [PubMed] [Google Scholar]
- 13.Kaya, S., K. Yokoyama, Y. Araki, and E. Ito. 1984. N-Acetylmannosaminyl(1→4) N-acetylglucosamine, a linkage unit between glycerol teichoic acid and peptidoglycan in cell walls of several Bacillus strains. J. Bacteriol. 158:990-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiser, K. B., N. Bhasin, L. Deng, and J. C. Lee. 1999. Staphylococcus aureus cap5P encodes a UDP-N-acetylglucosamine 2-epimerase with functional redundancy. J. Bacteriol. 181:4818-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, G. Alloni, V. Azevedo, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 16.Lazarevic, V., and D. Karamata. 1995. The tagGH operon of Bacillus subtilis 168 encodes a two-component ABC transporter involved in the metabolism of two wall teichoic acids. Mol. Microbiol. 16:345-355. [DOI] [PubMed] [Google Scholar]
- 17.Lazarevic, V., C. Mauël, B. Soldo, P.-P. Freymond, P. Margot, and D. Karamata. 1995. Sequence analysis of the 308° to 311° segment of the Bacillus subtilis 168 chromosome, a region devoted to cell wall metabolism, containing non-coding grey holes which reveal chromosomal rearrangements. Microbiology 141:329-335. [DOI] [PubMed] [Google Scholar]
- 18.Lazarevic, V., H. M. Pooley, C. Mauël, and D. Karamata. 2002. Teichoic and teichuronic acids from gram-positive bacteria, p. 465-497. In A. Steinbüchel, E. J. Vandamme, and S. de Baets (ed.), Biopolymers, vol. 5. Polysaccharides I. Polysaccharides from prokaryotes. Wiley-VCH Verlag, Weinheim, Germany.
- 19.Liu, W., S. Eder, and F. M. Hulett. 1998. Analysis of Bacillus subtilis tagAB and tagDEF expression during phosphate starvation identifies a repressor role for PhoP-P. J. Bacteriol. 180:753-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauël, C., M. Young, P. Margot, and D. Karamata. 1989. The essential nature of teichoic acids in Bacillus subtilis as revealed by insertional mutagenesis. Mol. Gen. Genet. 215:388-394. [DOI] [PubMed] [Google Scholar]
- 21.Pooley, H. M., F.-X. Abellan, and D. Karamata. 1991. A conditional-lethal mutant of Bacillus subtilis 168 with a thermosensitive glycerol-3-phosphate cytidylyltransferase, an enzyme specific for the synthesis of the major wall teichoic acid. J. Gen. Microbiol. 137:921-928. [DOI] [PubMed] [Google Scholar]
- 22.Pooley, H. M., F.-X. Abellan, and D. Karamata,. 1992. CDP-glycerol:poly(glycerophosphate) glycerophosphotransferase, which is involved in the synthesis of the major wall teichoic acid in Bacillus subtilis 168, is encoded by tagF (rodC). J. Bacteriol. 174:646-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves, P. R., M. Hobbs, M. A. Valvano, M. Skurnik, C. Whitfield, D. Coplin, N. Kido, J. Klena, D. Maskell, C. R. Raetz, and P. D. Rick. 1996. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 4:495-503. [DOI] [PubMed] [Google Scholar]
- 24.Sala, R. F., P. M. Morgan, and M. E. Tanner. 1996. Enzymatic formation and release of a stable glycal intermediate: the mechanism of the reaction catalyzed by UDP-N-acetylglucosamine 2-epimerase. J. Am. Chem. Soc. 118:3033-3034. [Google Scholar]
- 25.Soldo, B., V. Lazarevic, and D. Karamata. 2002. Gene tagO is involved in the synthesis of all cell wall anionic polymers in Bacillus subtilis 168. Microbiology, 148:2079-2087. [DOI] [PubMed]
- 26.Soldo, B., V. Lazarevic, M. Pagni, and D. Karamata. 1999. Teichuronic acid operon of Bacillus subtilis 168. Mol. Microbiol. 31:795-805. [DOI] [PubMed] [Google Scholar]
- 27.Soldo, B., V. Lazarevic, P. Margot, and D. Karamata. 1993. Sequencing and analysis of the divergon comprising gtaB, the structural gene of UDP-glucose pyrophosphorylase of Bacillus subtilis 168. J. Gen. Microbiol. 139:3185-3195. [DOI] [PubMed] [Google Scholar]