Abstract
Hotez et al. argue that achieving success in the global fight against HIV/AIDS, tuberculosis, and malaria may well require a concurrent attack on the neglected tropical diseases.
The age of hypocrisy has been succeeded by that of indifference, which is worse, for indifference corrupts and appeases: it kills the spirit before it kills the body. It has been stated before, it bears repeating: the opposite of love is not hate, but indifference. —Elie Wiesel
The last five years have witnessed increased efforts by G8 nations and United Nations agencies to improve the health of the world's 3 billion people living on less than US$2 a day. Most of this attention has focused on efforts to intensify resources for fighting the three most devastating diseases: HIV/AIDS, tuberculosis, and malaria. Together, these “big three” account for a staggering 5.6 million deaths and the loss of 166 million disability-adjusted life years (DALYs) annually (see annex tables 2 and 3 in [ 1]). Prominent partnerships and initiatives are now devoted to the big three ( Box 1), and increased global attention to these diseases (and to the risks posed by avian influenza and other emerging viral infections) culminated in the November 2005 TIME Global Health Summit, branded by Bono as the “Woodstock of Global Health” ( http://www.time.com/time/2005/globalhealth).
Box 1. Prominent Partnerships and Initiatives Devoted to the “Big Three”
The Joint UN Programme on HIV/AIDS ( http://www.unaids.org)
Roll Back Malaria ( http://www.rollbackmalaria.org)
Stop Tuberculosis ( http://www.stoptb.org)
The Global Fund to Fight AIDS, Tuberculosis, and Malaria (So far has attracted US$4.7 billion in financing through 2008 [ http://www.theglobalfund.org])
The US Leadership against HIV/AIDS, Tuberculosis, and Malaria Act of 2003 (An emergency effort to provide US$15 billion over five years [ http://olpa.od.nih.gov/actions/public/108session1/pl108-25.asp]),
The President's Emergency Plan for AIDS Relief ( http://www.avert.org/pepfar.htm),
The Bill and Melinda Gates Foundation ( http://www.gatesfoundation.org), supporting the development of new technologies for disease control.
These new initiatives and “Woodstock” Global Health have done much to raise funds and elevate public awareness in order to launch a serious war on the big three. Conspicuously absent from these activities, however, has been commensurate advocacy for a group of diseases that exclusively affect the poor and the powerless in rural and impoverished urban areas of developing countries. An increasing body of evidence indicates that this group of “neglected tropical diseases” may not only threaten the health of the poor as much as HIV/AIDS, tuberculosis, or malaria, but even more importantly, may have effective treatment and prevention strategies that can be delivered for less than US$1 per capita per year. Furthermore, new evidence points to substantial geographic overlap between the neglected tropical diseases and the big three, with emerging data suggesting that control of the neglected tropical diseases could actually become a powerful tool for combating HIV/AIDS, tuberculosis, and malaria. Therefore, achieving success in the global fight against HIV/AIDS, tuberculosis, and malaria may well require a concurrent attack on the neglected tropical diseases and waging a larger battle against a new 21st century “gang of four.”
Global Burden of the Neglected Tropical Diseases
The most important neglected tropical diseases include three vector-borne protozoan infections—leishmaniasis, human African trypanosomiasis, and Chagas disease; three bacterial infections—trachoma, leprosy, and Buruli ulcer; and seven helminth infections—hookworm, ascariasis, trichuriasis, lymphatic filariasis, onchocerciasis, guinea worm (drancunculiasis), and schistosomiasis [ 2]. Cysticercosis, food-borne trematodiases, and some other parasitic infections could also be included in this list [ 3–5]. The common features of the neglected tropical diseases include high endemicity in rural and in impoverished urban areas of low-income countries, and an ability to impair childhood growth, intellectual development, and education, as well as worker productivity. In this way, the neglected tropical diseases are poverty-promoting conditions. Many of the neglected tropical diseases are disfiguring and stigmatizing—their characteristic features are described in the Bible and other ancient texts, affirming that they have affected humans for millennia [ 2]. However, because they affect the poorest of the poor, there are few or no commercial markets for drugs and vaccines against the neglected tropical diseases, and the pharmacopoeia for these diseases has remained essentially unchanged since the middle of the 20th century [ 2].
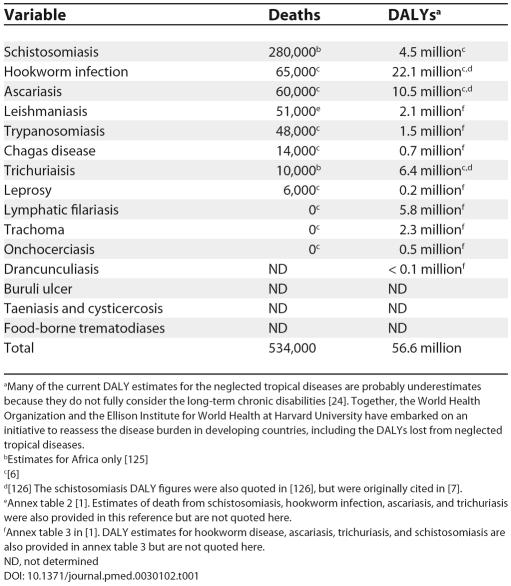
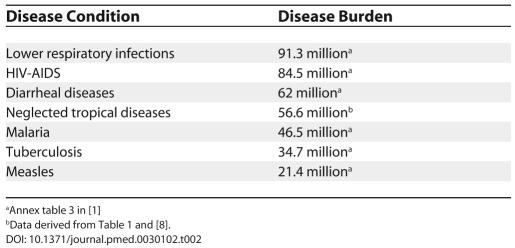
The burden of disease resulting from the neglected tropical diseases is huge. As shown in Table 1, they cause approximately 534,000 deaths annually, with five diseases—schistosomiasis, hookworm, ascariasis, leishmaniasis, and human African trypanosomiasis—accounting for more than 400,000 deaths. Even more significant than the mortality burden are the years of life lost that result from premature disability. By some estimates, the neglected tropical diseases are second only to HIV/AIDS as a cause of disease burden, resulting in approximately 57 million DALYs annually [ 8]. If considered together, the neglected tropical diseases would represent the fourth most important group of communicable diseases worldwide, behind lower respiratory infections, HIV/AIDS, and diarrheal diseases ( Table 2).
Table 1. Disease Burden of the Neglected Tropical Diseases in Deaths and DALYs.
aMany of the current DALY estimates for the neglected tropical diseases are probably underestimates because they do not fully consider the long-term chronic disabilities [ 24]. Together, the World Health Organization and the Ellison Institute for World Health at Harvard University have embarked on an initiative to reassess the disease burden in developing countries, including the DALYs lost from neglected tropical diseases.
bEstimates for Africa only [ 125]
c[ 6]
d[ 126] The schistosomiasis DALY figures were also quoted in [ 126], but were originally cited in [ 7].
eAnnex table 2 [ 1]. Estimates of death from schistosomiasis, hookworm infection, ascariasis, and trichuriasis were also provided in this reference but are not quoted here.
fAnnex table 3 in [ 1]. DALY estimates for hookworm disease, ascariasis, trichuriasis, and schistosomiasis are also provided in annex table 3 but are not quoted here.
ND, not determined
Table 2. Comparative Disease Burdens of Communicable Diseases Measured in DALYs.
Epidemiologic Overlap and Comorbidity of the Neglected Tropical Diseases, and Opportunities for Integrated Control
The neglected tropical diseases do not occur in isolation. In most countries of sub-Saharan Africa, and in many other tropical and subtropical countries, at least five to six neglected tropical diseases occur in the same region [ 9]. The implication of this geographic overlap is that a considerable proportion of the population of sub-Saharan Africa (and selected regions of Asia and the Americas) is polyparasitized with one or more soil-transmitted helminths (STHs), schistosomes, and filarial worms [ 10–14], particularly among the very poorest populations [ 15–17]. Each of the three major STHs ( Ascaris lumbricoides, Trichuris trichiura, and the hookworms, Necator americanus and Ancylostoma duodenale) and the two schistosomes ( Schistosoma haematobium and Schistosoma mansoni) are highly endemic to sub-Saharan Africa, where they adversely affect childhood growth and physical fitness [ 18–24]. Such polyparasitism has a substantial impact on the physical health of Africa's youth population [ 25], as well as on the impairment of their intellectual and cognitive development [ 26–30]. Polyparasitism also results in anemia [ 31], with hookworms being well-known causes of anemia because of intestinal blood loss [ 32], though T. trichiura and S. mansoni also cause some degree of intestinal blood loss [ 33–35], and S. haematobium causes anemia through hematuria [ 36]. In addition to host blood loss, the schistosomes also probably cause anemia through other mechanisms including hypersplenism, red blood cell sequestration, autoimmune hemolysis, and chronic inflammation [ 36, 37].
Documented improvements in childhood growth [ 18, 20], physical fitness [ 19, 21], cognition [ 26–30], school attendance [ 38], and hemoglobin and serum ferritin concentrations [ 39–41] following deworming, together with a theoretical framework of helminth transmission dynamics [ 42, 43], provided the basis for a World Health Assembly resolution adopted in 2001 urging member states to periodically deworm school-age children with a benzimidazole anthelmintic (either albendazole or mebendazole) and praziquantel as a means for reducing global disease burden ( http://www.who.int/wormcontrol) [ 44]. Periodic deworming also provides the basic strategy of the Schistosomiasis Control Initiative ( http://www.schisto.org) in Africa [ 13].
Lymphatic filariasis (LF), onchocerciasis, and trachoma exhibit considerable geographic overlap with the STHs and schistosomiasis [ 9]. Therefore, the combination of ivermectin and albendazole for the elimination of LF in Africa and ivermectin for onchocerciasis control—the cornerstones of the Global Programme to Eliminate Lymphatic Filariasis ( http://www.filariasis.org) and the African Programme for Onchocerciasis Control ( http://www.apoc.bf/en), respectively—add considerable benefits to the treatment of ascariasis and trichuriasis [ 45]. At the same time, combinations of ivermectin and azithromycin prevent blindness by reducing the incidence of onchocerciasis and trachoma, respectively [ 46]. Through its impact on ectoparasite infections, ivermectin also indirectly helps to reduce the frequency and severity of skin infections [ 45, 47].
Based on the substantial morbidity reductions afforded by reducing polyparasitism, Molyneux et al. [ 9] have recently put forward a comprehensive pro-poor strategy to integrate programs for either the control or the elimination of seven neglected tropical diseases—ascariasis, trichuriasis, hookworm, lymphatic filariasis, onchocerciasis, schistosomiasis, and trachoma—using existing drugs. Such integration efforts are particularly relevant to sub-Saharan Africa because the neglected tropical diseases in this region exhibit a high degree of geographic overlap [ 9]. It is proposed that such integrated control or elimination could be achieved with four drugs—ivermectin, albendazole, azithromycin, and praziquantel—of which three are currently donated by Merck and Company, GlaxoSmithKline, and Pfizer, respectively, while praziquantel is available at relatively low cost [ 12]. Each of the drugs has overlapping specificity so that multiple neglected pathogens would be concurrently targeted. In addition, the four-drug regimen would also target ectoparasite infections, such as scabies, pediculosis, tungiasis, and cutaneous larva migrans, and their resulting secondary bacterial skin infections [ 45, 47], and possibly also affect important respiratory bacterial pathogens including pneumococcus [ 9]. It has been estimated that for US$200 million annually, approximately 500 million Africans (US$0.40 per patient) could be treated in a four-drug integrated pro-poor package [ 9, 12]. That package could reduce tens of millions of DALYs annually and simultaneously address seven of the eight Millennium Development Goals (MDGs), including those related to poverty reduction, educational achievement, and child and maternal health [ 9]. This approach has been recommended by the Commission for Africa [ 48], and a Lancet editorial has emphasized that deworming is a prerequisite for improved health of the world's poorest children and is one of the “quick wins” identified in the Millennium Project Report [ 49, 50].
As scaling up integrated control moves forward, a number of research and monitoring questions will need to be addressed, including issues of compliance [ 51], drug interactions, emerging drug resistance [ 52], and sustainability. Moreover, proof of concept for the feasibility of integrated control will require attention to the specific populations (e.g., children versus adults) at risk for each of the neglected tropical diseases, and to the timing for administration of each of the four drugs. Equally important will be undertaking an economic analysis of the rapid-impact package. The studies on the economic rates of return of large-scale and successful neglected tropical disease control and elimination programs, such as onchocerciasis control in West Africa, guinea worm eradication, LF, and schistosomiasis control in Egypt and China, and Chagas disease control in South America, have shown that these are of the order of 15%–30% [ 53]. The World Bank and other agencies have recognized these programs as being among the most effective development investments in any sector [ 53], and it is hoped that an equal or greater rate of return could be achieved with a rapid-impact package targeting multiple neglected tropical diseases [ 9]. In the future, the concept of integration could be expanded beyond integrated chemotherapy-based morbidity control based largely on community-directed treatment to include access to clean water and improved sanitation; strengthening of surveillance, evaluation, and reporting systems; capacity building, deployment of new generation control tools; and education and communication strategies to address the root ecological and behavioral causes of the neglected tropical diseases (J. Utzinger, personal communication) [ 17, 54, 55].
Epidemiologic Overlap of the Neglected Tropical Diseases with HIV/AIDS, Tuberculosis, and Malaria
In many parts of the developing world, but especially in sub-Saharan Africa, the geographic overlap between HIV/AIDS, tuberculosis, and malaria is extensive. Indeed, Africa's catastrophic burden of disease resulting from HIV-associated tuberculosis [ 56] and severe malaria in individuals with HIV [ 57–59] is emerging as one of the first great human tragedies of the 21st century. Adding to the complexity of Africa's big three is the geographic and epidemiologic overlay of the neglected tropical diseases (S. Brooker, A. C. A. Clements, P. J. Hotez, S. I. Hay, A. Tatem, et al., unpublished data) [ 60, 61]. It has become increasingly clear that HIV/AIDS, tuberculosis, and malaria occur predominantly in populations who are polyparasitized [ 11, 62, 63]. Helminths are the most common parasites found in HIV-, tuberculosis-, and malaria-infected populations (S. Brooker, A. C. A. Clements, P. J. Hotez, S. I. Hay, A. Tatem, et al., unpublished data) [ 11, 62, 63], especially the three major STHs [ 64–66], the two major schistosomes [ 67–69], and the filariae [ 70]. Brooker and colleagues have recently demonstrated a close geographical overlap in Africa between hookworm and malaria (S. Brooker, A. C. A. Clements, P. J. Hotez, S. I. Hay, A. Tatem, et al., unpublished data). However, almost all of the major neglected tropical diseases have been linked with the big three.
Comorbidity of the Neglected Tropical Diseases with HIV/AIDS, Tuberculosis, and Malaria: Anemia
We are still in the early stages of appreciating the full extent of the comorbidity that occurs when the neglected tropical diseases are superimposed on HIV/AIDS, tuberculosis, and malaria [ 71]. Anemia has been revealed as perhaps the most important of the leading comorbid conditions. Among the neglected tropical diseases, hookworm, schistosomiasis, kala-azar, and trypanosomiasis are major causes of anemia [ 32, 35, 72, 73], with hookworm accounting for up to 35% and 73% of the iron-deficiency anemia and severe iron-deficiency anemia in Africa, respectively [ 74]. Children and pregnant women are particularly susceptible to anemia from hookworm and schistosomiasis [ 35, 74, 75]. As noted already, hookworm causes iron-deficiency anemia through intestinal blood loss, while the mechanisms of anemia from the other neglected tropical diseases are more complex. Each of the big three also results in anemia, with malaria responsible for the greatest burden. Malaria causes anemia by several different mechanisms, including increased destruction of both parasitized and nonparasitized red blood cells and dyserythropoiesis resulting from host production of inflammatory cytokines, especially tumor necrosis factor and macrophage migration inhibitory factor [ 76, 77]. The pathogenesis of anemia from HIV/AIDS and tuberculosis is also complex, most likely resulting from a state of chronic inflammation leading to bone marrow suppression [ 36].
Coinfection with the neglected tropical diseases also adversely affects the natural history and progression of the big three.
A major consequence of polyparasitism, therefore, is anemia from multiple infectious causes. In the case of hookworm and malaria, Brooker et al. (S. Brooker, A. C. A. Clements, P. J. Hotez, S. I. Hay, A. Tatem, et al., unpublished data) have shown that in Kenya the two types of anemia—anemia from hookworm and the anemia from malaria—can build on each other to produce profound reductions in hemoglobin. To make matters worse, many of these same African populations experience further reductions in hemoglobin because of sickle cell disease and thalassemia, as well as nutritional deficits in iron and folate. Fleming [ 78] put forward the concept of the “agriculture-related anemias” to describe this “perfect storm” confronting African populations—changes in diet, population growth with limited sanitation and with the consequences of endemic STH infections, an environment prone to the emergence of vector-borne disease, and natural selection of variants that offer partial protection against malaria—all of which resulted from the introduction of agriculture, beginning at the time of the Neolithic Revolution.
The severe anemia resulting from helminth polyparasitism and malaria produces several adverse health consequences among three particularly important African subpopulations: pregnant women, children, and individuals with HIV. In pregnancy, anemia is a leading contributor to maternal morbidity and mortality, and is associated with shock, risk of cardiac failure, decreased ability to work, and adverse perinatal outcomes [ 79]. In coastal Kenya, malaria was identified as the most important cause of anemia in primigravidae, whereas hookworm attained increased importance among multigravidae [ 79, 80]. Therefore, women are put at risk by the major consequences of anemia throughout their childbearing years. In young children, chronic iron deficiency and anemia are associated with increased child mortality, and impairments in physical growth, cognitive and motor development, immune function, and school performance [ 81–84]. Severe anemia is also a major contributor to mortality from malaria [ 85]. Just as women are at risk for anemia throughout their childbearing years, first from malaria and then from hookworm, so, too, are children whose first major experience with severe anemia is from malaria during early childhood, followed by hookworm and schistosomiasis in middle childhood and adolescence. Together, these infectious anemias account for an important component of the “silent burden of anemia” in African children [ 86]. Finally, among individuals with HIV, anemia has been shown to be an independent risk factor for early death, with correction of anemia associated with reversal of increased risk [ 87, 88].
Impact of the Neglected Tropical Diseases on Host Susceptibility to HIV/AIDS, Tuberculosis, and Malaria
Coinfection with the neglected tropical diseases also adversely affects the natural history and progression of the big three. Several studies (recently reviewed in [ 62]) point to the increasing severity of clinical malaria that results from helminth coinfection. These studies include those from Senegal showing enhanced risk or increased incidence of clinical malaria resulting from either STH [ 64, 66] or schistosome [ 68] infections, unpublished studies from Malawi showing that women infected with hookworms were at 1.8 times higher risk of having malaria than uninfected women [ 89], and studies from Thailand showing increased susceptibility to malaria in patients with STH infections [ 90–93].
However, not all studies investigating these relationships have identified deleterious interactions between helminths and malaria [ 63, 69, 94, 95]. Indeed, there may be a difference between helminthic effects on susceptibility to malaria and effects on the pathology induced by malaria infection. Druilhe et al. [ 62] have hypothesized that the STHs and schistosomes immunomodulate the host to increase malaria susceptibility by shifting their humoral responses from malaria-protective, cytophilic humoral antibodies (IgG1 and IgG3) to nonprotective, noncytophilic subclasses (IgG2, IgG4, and IgM). In addition, Diallo et al. [ 67] have proposed that schistosomes, and possibly other helminths, can unbalance the regulation of inflammatory cytokines in childhood malaria.
Another body of evidence links helminth coinfections with increased susceptibility to HIV/AIDS or worsening progression of HIV disease (reviewed in [ 65]). Borkow and Bentwich postulate that chronic helminth infections increase the risk of HIV infection through a process of chronic immune activation [ 96], which results in increased HIV plasma viral load [ 97–99]. Among a group of Kenyan car washers with high levels of exposure to S. mansoni infection, Secor et al. [ 100] have shown that patients with active schistosomiasis exhibit increased expression of the chemokine receptors and HIV-1 co-receptors, CCR5 and CXCR4, on peripheral blood CD4 T cells and monocytes. Gallagher et al. [ 101] observed increased risk of mother-to-child HIV transmission in pregnant women with helminth coinfection; increased risk correlated with cord blood lymphocyte production of interleukin-5 and interleukin-13 in response to helminth antigens. However, studies conducted in Uganda failed to identify an association between helminths and AIDS [ 102, 103]. In addition to promoting susceptibility to HIV/AIDS, one major neglected tropical disease in Africa, namely, visceral leishmaniasis, is an important opportunistic infection in individuals who are HIV-immunocompromised [ 72].
Finally, there is some evidence that helminth infections, especially hookworm and schistosomiasis, adversely affect the outcome of pulmonary tuberculosis or the progression to active tuberculosis [ 104, 105], and reduce the T cell responses in individuals receiving Bacillus Calmette–Gurerin (BCG) [ 106–108]. However, the data supporting this concept is still far from conclusive.
Taken together, this evidence indicates that coinfection with one or more neglected tropical disease may profoundly affect the outcome of one or more of the big three. Progression of disease from HIV/AIDS, tuberculosis, and malaria results from the comorbidity associated with anemia from hookworm, trichuriasis, schistosomiasis, trypanosomiasis, and leishmaniasis, and from the possible increase in susceptibility and worsening progression of disease that occurs with STH, schistosome, and filarial infections. However, the latter concept is still not without controversy and requires additional scientific investigations.
A Comprehensive Pro-Poor Health Policy
It is important to determine if there is a rationale for linking a pro-poor strategy for integrated neglected tropical disease control with ongoing programs that target the big three. Recent evidence of the extensive geographic overlap between the big three and the neglected tropical diseases, together with the deleterious interactions between both groups of infections, suggests significant, new opportunities to reduce the burden of disease in sub-Saharan Africa and elsewhere in the developing world. Success at integrating neglected tropical disease control into big three partnership programs could dramatically reduce the number of life years lost from premature death and disability in Africa [ 9]. The collateral benefits from including neglected tropical disease control under the umbrella of the HIV/AIDS, tuberculosis, and malaria global partnerships are potentially huge.
As noted above, integrated control of the neglected tropical diseases relies on the appropriate use of three anthelmintics—albendazole, ivermectin, and praziquantel—and the antibacterial agent azithromycin. In addition to affecting STH and schistosome deworming, ectoparasite control, and control or elimination of LF and onchocerciasis, the three anthelmintics would help to reduce anemia as well as the progression of disease resulting from HIV/AIDS, tuberculosis, and malaria [ 62, 65]. In Senegal, deworming for STH infections was shown to be equivalent to the protection from malaria conferred by the sickle cell trait [ 62, 64], and has the added effect of restoring T cell responses to mycobacterial antigens in helminth-exposed individuals before and after BCG vaccination [ 107, 108]. In Zimbabwe, treatment for schistosomiasis reduced the rate of HIV-1 viral replication and increased CD4 cell counts among individuals who were coinfected [ 99], although this phenomenon was not observed in Ugandans who were coinfected [ 109]. Therefore, it may be important to consider local and geographical differences when evaluating the impact of deworming on coinfections.
In addition to the beneficial effects of deworming on children already mentioned, antenatal anthelmintic treatment was shown recently to reduce maternal morbidity and mortality, and improve birth weight and infant survival [ 110]. The community-based health-care systems set up to deliver neglected tropical disease drugs could be well suited to administer antiretrovirals, directly observed tuberculosis therapy, and antimalarials and bed nets. Simultaneously, neglected disease control would benefit from big three control efforts. For example, insecticide-treated bed nets used in malaria control are highly effective at interrupting the transmission of LF [ 70], and many antiretrovirals would be expected to reduce the impact of opportunistic leishmaniasis.
New vaccines under development for HIV/AIDS, tuberculosis, and malaria also need to account for the influence of polyparasitism on vaccine immunogenicity [ 111]. In laboratory animals, the presence of adult hookworms in the intestine reduces vaccine-specific antibody titer and lymphoproliferation, with restoration occurring following deworming [ 112], and S. mansoni infection reduces the protective efficacy of BCG vaccination [ 113]. Studies in humans also show similar effects of concurrent helminth infections on immune responses to tetanus and cholera vaccines [ 114, 115]. However, a recently published study found that although maternal hookworm infection was associated with reduced maternal immune response to mycobacterial antigen, such maternal infection was also associated with an unexpected increase in their infants' response to BCG [ 116].
It is surprising that those aiming to control the big three have largely ignored these opportunities.
Since any vaccine trial that does not control for worm infections could be flawed, neglected tropical disease control should be linked with big three vaccine development efforts, such as those sponsored by the International AIDS Vaccine Initiative ( http://www.iavi.org), the Malaria Vaccine Initiative ( http://www.malariavaccine.org), and the Aeras Global Tuberculosis Vaccine Foundation ( http://www.aeras.org). Deworming prior to vaccination may also become a key component in the testing process of any new generation vaccine against the big three. Moreover, development of vaccines against the neglected tropical diseases themselves, including vaccines for hookworm [ 117], schistosomiasis [ 118], and leishmaniasis [ 119] is also underway. In the future, the neglected tropical disease vaccines are expected to become important control tools; in the case of hookworm and schistosomiasis vaccines, their deployment would reduce the frequency of chemotherapy and, therefore, possibly reduce the likelihood of emerging anthelmintic drug resistance [ 120].
Given the compelling logic and the very modest costs of embracing neglected tropical disease control efforts, it is surprising that those aiming to control the big three have largely ignored these opportunities and the collateral benefits from reductions in anemia, worm burdens, and susceptibility to HIV/AIDS, tuberculosis, and malaria morbidity. Bilateral donors and the major big three partnerships should expand their portfolios to incorporate deworming, LF, and onchocerciasis elimination efforts, and other neglected tropical disease control initiatives as a cost-effective means to reduce the morbidity and mortality of HIV/AIDS, tuberculosis, and malaria. Such interventions are inexpensive, effective, and fully compatible with the MDGs, the recommendations of the UN Millennium Project ( http://www.unmillenniumproject.org), the Commission for Africa report [ 48], and a Resolution of the Third Global Meeting of the World Health Organization Partners for Parasite Control [ 44]. While the recent report on health from the New Partnership for Africa's Development ( http://www.nepad.org), an initiative to develop an integrated socioeconomic development framework for Africa with a mandate from the Organisation of African Unity [ 121], and the policy statement on health from the European Parliament [ 122] refer to parasitic diseases, they do not emphasize the interrelationships between parasitic infections and HIV/AIDS, tuberculosis, and malaria, or the potential benefits of a more holistic approach to disease control. The excellent safety profile of the anthelmintics and azithromycin, and the fact that three of the four drugs are donated free of charge to those who need them, would almost certainly ensure that neglected tropical disease control could be incorporated without added risk and with minimal costs.
Scaling Up Disease Control
The international community has started to address issues of scaling up disease control in the developing world. HIV/AIDS, tuberculosis, and malaria have been given a financing mechanism for scale up through the establishment of the Global Fund to Fight AIDS, Tuberculosis, and Malaria. The scaling up of malaria control was explicitly featured in the final outcome document of the September 2005 UN World Summit ( http://www.un.org/ga/59/hlpm_rev.2.pdf). The World Bank and other donors are similarly identifying new channels and financing strategies for infectious disease control [ 123].
The scale up of control measures vis-à-vis the neglected tropical diseases can be readily added to these ongoing initiatives, possibly by first incorporating neglected tropical disease control into model health systems. Two great examples are the Tanzania Essential Health Interventions Project—a research and development partnership between Tanzania's Ministry of Health and Canada's International Development Research Centre ( http://www.idrc.ca)—and the Millennium Village Project, a new development project guided by a scientific council at the Earth Institute at Columbia University ( http://www.earth.columbia.edu/mvp) and based on the recommendations of the UN Millennium Project. The Millennium Village Project will provide a framework for scaling up control of the neglected tropical diseases alongside other health interventions in project sites in ten African countries, with rigorous surveying and monitoring of the MDG-based indicators. These issues will be subjects of important discussions at a January 2006 summit in Stockholm, Sweden, as both programs recognize that it is the countries themselves who must eventually define appropriate policy and priority, often through a decentralized system of district-level teams. Attending to the neglected tropical diseases will require committed support for baseline mapping of the prevalence of the individual neglected tropical diseases, scientifically vetted treatment protocols, and appropriate alignment of these efforts with regional and national health policies.
Conclusion
The neglected tropical diseases have joined the ranks of the big three to create a 21st century “gang of four.” For too long the big three partnerships have worked in isolation, as have the vertical programs against the individual neglected tropical diseases. The recent Paris Declaration emanating from the High-Level Forum on the Health MDGs calls for greater harmonization and collaboration across the major global health partnerships and the commended efforts by the smaller neglected tropical disease partnerships (the minor global health partnerships) to integrate these activities [ 124]. In the future, policy must be driven by the reality of the biological and epidemiological interactions between the big three and the neglected tropical diseases. Coordination of the major and minor global health partnerships and their associated research communities would give a significant thrust to the efforts now underway to reduce disease and poverty worldwide.
Acknowledgments
Competing Interests. PJH is partially supported by the Bill and Melinda Gates Foundation, Seattle, Washington, United States of America, through Human Hookworm Vaccine Initiative of the Albert B. Sabin Vaccine Institute, Washington, District of Columbia, United States of America. He is an inventor on an international patent application (PCT/US02/33106; filed November 11, 2002) entitled “Hookworm vaccine.” The patent was filed in the United States, Brazil, India, China, and Mexico. If awarded, the patent would belong to The George Washington University, with an exclusive license to the Human Hookworm Vaccine Initiative of the Albert B. Sabin Vaccine Institute, a nonprofit (501c3) organization devoted to increasing the use of vaccines worldwide. Because hookworm is a neglected disease afflicting the poorest of the poor in developing countries, a hookworm vaccine has no anticipated commercial value or income generating potential. The rationale for filing a patent is to ensure that the vaccine is developed for those who need it in developing countries, and to encourage vaccine manufacturers in developing countries to work with the Albert B. Sabin Vaccine Institute for manufacture of the hookworm vaccine. The first-generation hookworm vaccine, the Na-ASP-2 Hookworm Vaccine, was developed entirely in the nonprofit sector through the Human Hookworm Vaccine Initiative of the Albert B. Sabin Vaccine Institute. PJH is also Co-chair of the Scientific Advisory Council of the Albert B. Sabin Vaccine Institute (he receives no compensation for this activity), and is a member of the academic advisory board for the Pfizer Postdoctoral Fellowship in Infectious Diseases. DHM is partially supported by the UK Department for International Development and GlaxoSmithKline, London, United Kingdom, and participates in the Mectizan Expert Committee/Albendazole Coordination meetings, which are supported by Merck and Company, Whitehouse Station, New Jersey, United States of America, and GlaxoSmithKline, London, United Kingdom. AF is Director of the Schistosomiasis Control Initiative, which is supported by the Bill and Melinda Gates Foundation, Seattle, Washington, United States of America. EO is supported through the Task Force for Child Survival and Development and the Carter Center, Atlanta, Georgia, United States of America; the Bill and Melinda Gates Foundation, Seattle, Washington, United States of America; GlaxoSmithKline, London, United Kingdom; the Global Alliance to Eliminate Lymphatic Filariasis, Liverpool, United Kingdom. SES declares that she has no competing interests. JDS is partially supported by the UN, New York, New York, United States of America.
Abbreviations
- BCG
Bacillus Calmette–Gurerin
- DALY
disability-adjusted life year
- LF
lymphatic filariasis
- MDG
Millennium Development Goal
- STH
soil-transmitted helminth
Footnotes
Funding: The authors received no specific funding for this article.
Citation: Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Sachs SE, et al. (2006) Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med 3(5): e102.
References
- World Health Organization. World Health Report 2004: Changing history. Geneva: World Health Organization; 2004. Available: http://www.who.int/whr/2004/en. Accessed 5 January 2006 . [Google Scholar]
- Hotez PJ, Ottesen E, Fenwick A, Molyneux D. The neglected tropical diseases: The ancient afflictions of stigma and poverty and the prospects for their control and elimination. Adv Exp Med Biol. 2006 doi: 10.1007/0-387-33026-7_3. In press. [DOI] [PubMed] [Google Scholar]
- Pawlowski ZS. Role of chemotherapy of taeniasis in prevention of neurocysticercosis. Parasitol Int. Epub ahead of print. 2005 doi: 10.1016/j.parint.2005.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, Utzinger J. Emerging foodborne trematodiases. Emerg Infect Dis. 2005;11:1507–1114. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, et al. Clonorchiasis: A key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- WHO Expert Committee. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:i–vi. 1–57. [PubMed] [Google Scholar]
- Murray CJL, Lopez AD, editors. Global cooperative assessments in the health sector, disease burden, expenditures and intervention packages. Geneva: World Health Organization; 1994. 196 pp. [Google Scholar]
- Hotez P, Stoever K, Fenwick A, Molyneux D, Savioli L. The neglected epidemic of chronic diseases (letter) Lancet. 2006 doi: 10.1016/S0140-6736(06)68213-5. In press. [DOI] [PubMed] [Google Scholar]
- Molyneux DH, Hotez PJ, Fenwick A. “Rapid-impact interventions”: How a policy of integrated control for Africa's neglected tropical diseases could benefit the poor. PLoS Med. 2005;2:e336. doi: 10.1371/journal.pmed.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux DH, Nantulya HM. Linking disease control programmes in rural Africa: A pro-poor strategy to reach Abuja targets and millennium development goals. BMJ. 2004;328:1129–1132. doi: 10.1136/bmj.328.7448.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raso G, Luginbuhl A, Adjoua CA, Tan-Bi NT, Silue KD, et al. Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Cote d'Ivoire. Int J Epidemiol. 2004;33:1092–1102. doi: 10.1093/ije/dyh241. [DOI] [PubMed] [Google Scholar]
- Fenwick A, Molyneux D, Nantulya V. Achieving the Millennium Development Goals. Lancet. 2005;365:1029–1030. doi: 10.1016/S0140-6736(05)71134-X. [DOI] [PubMed] [Google Scholar]
- Fenwick A. New initiatives against Africa's worms. Trans R Soc Trop Med Hyg. 2006 doi: 10.1016/j.trstmh.2005.03.014. In press. [DOI] [PubMed] [Google Scholar]
- McKenzie FE. Polyparasitism. Int J Parasitol. 2005;34:221–222. doi: 10.1093/ije/dyh399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, et al. Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Raso G, Utzinger J, Silue KD, Ouattara M, Yapi A, et al. Disparities in parasitic infections, perceived ill health and access to health care among poorer and less poor schoolchildren of rural Cote d'Ivoire. Trop Med Int Health. 2005;10:42–57. doi: 10.1111/j.1365-3156.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- Ehrenberg JP, Ault SK. Neglected diseases of neglected populations: thinking to reshape the determinants of health in Latin America and the Caribbean. BMC Public Health. 2005;5:119. doi: 10.1186/1471-2458-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Kurz KM, Kinoti SN, Brigham H. Treatment with a single dose of albendazole improves growth of Kenyan schoolchildren with hookworm, Trichuris trichiura, and Ascaris lumbricoides infections . Am J Trop Med Hyg. 1989;41:78–87. [PubMed] [Google Scholar]
- Stephenon LS, Latham MC, Kinoti SN, Kurz KM, Brigham H. Improvements in physical fitness of Kenyan schoolboys infected with hookworm, Trichuris trichiura and Ascaris lumbricoides following a single dose of albendazole . Trans R Soc Trop Med Hyg. 1990;84:277–282. doi: 10.1016/0035-9203(90)90286-n. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Adams EJ, Kinoti SN, Pertet A. Weight gain of Kenyan school children infected with hookworm, Trichuris trichiura and Ascaris lumbricoides is improved following once- or twice-yearly treatment with albendazole . J Nutr. 1993;123:656–665. doi: 10.1093/jn/123.4.656. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Adams EJ, Kinoti SN, Pertet A. Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and Ascaris lumbricoides infections are improved four months after a single dose of albendazole . J Nutr. 1993;123:1036–1046. doi: 10.1093/jn/123.6.1036. [DOI] [PubMed] [Google Scholar]
- Richter J. The impact of chemotherapy on morbidity due to schistosomiasis. Acta Trop. 2003;86:161–183. doi: 10.1016/s0001-706x(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Lwambo NJ, Siza JE, Brooker S, Bunday DA, Guyatt H. Patterns of concurrent hookworm infection and schistosomiasis in schoolchildren in Tanzania. Trans R Soc Trop Med Hyg. 1999;93:497–502. doi: 10.1016/s0035-9203(99)90349-8. [DOI] [PubMed] [Google Scholar]
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: A meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- Beasley M, Brooker S, Ndinaromtan M, Madjiourroum EM, Baboguel M, Djenguinabe E, Bundy DA. First nationwide survey of the health of schoolchildren in Chad. Trop Med Int Health. 2002;7:625–630. doi: 10.1046/j.1365-3156.2002.00900.x. [DOI] [PubMed] [Google Scholar]
- Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Bundy DA. Parasitic helminth infection and cognitive function in school children. Proc Biol Sci. 1992;247:77–81. doi: 10.1098/rspb.1992.0011. [DOI] [PubMed] [Google Scholar]
- Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Robinson BA, et al. Moderate to heavy infections of Trichuris trichiura affect cognitive function in Jamaican school children . Parasitology. 1992;104:539–547. doi: 10.1017/s0031182000063800. [DOI] [PubMed] [Google Scholar]
- Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, et al. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum in Chinese primary schoolchildren . Am J Trop Med Hyg. 1999;60:556–565. doi: 10.4269/ajtmh.1999.60.556. [DOI] [PubMed] [Google Scholar]
- Sakti H, Nokes C, Hertanto WS, Hendratno S, Hall A, et al. Evidence for an association between hookworm infection and cognitive function in Indonesia school children. Trop Med Int Health. 1999;4:322–334. doi: 10.1046/j.1365-3156.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Jukes MC, Nokes CA, Alcock KJ, Lambo JK, Kihamia C, et al. Heavy schistosomiasis associated with poor short–term memory and slower reaction times in Tanzanian schoolchildren. Trop Med Int Health. 2002;7:104–117. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- Ezeamama AE, Friedman JF, Olveda RM, Acosta LP, Kurtis JD, et al. Functional significance of low–intensity polyparasite helminth infections in anemia. J Infect Dis. 2005;192:2160–2170. doi: 10.1086/498219. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, et al. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- Ramdath DD, Simeon DT, Wong MS, Grantham-McGregor SM. Iron status of schoolchildren with varying intensities of Trichuris trichiura infection . Parasitology. 1995;110:347–351. doi: 10.1017/s0031182000080938. [DOI] [PubMed] [Google Scholar]
- Robertson LJ, Crompton DW, Sanjur D, Nesheim MC. Haemoglobin concentrations and concomitant infections of hookworm and Trichuris trichiura in Panamanian primary schoolchildren . Trans R Soc Trop Med Hyg. 1992;86:654–656. doi: 10.1016/0035-9203(92)90176-d. [DOI] [PubMed] [Google Scholar]
- Ajanga A, Lwambo NJ, Blair L, Nyandindi U, Fenwick A, et al. Schistosoma mansoni in pregnancy and associations with anaemia in northwest Tanzania . Trans R Soc Trop Med Hyg. 2006;100:59–63. doi: 10.1016/j.trstmh.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Fleming AF, de Silva PS. Hematological diseases in the tropics. In: Cook GC, Zumla AI, editors. Manson's tropical diseases. 21st ed. Philadelphia: Saunders-Elsevier; 2004. pp. 169–244. [Google Scholar]
- Friedman JF, Kanzaria HK, McGarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends Parasitol. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- World Bank. School deworming at a glance. Washington (District of Columbia): World Bank; 2003. Available: http://www.worldbank.org/hnp. Accessed 4 January 2006 . [Google Scholar]
- Beasley NM, Tomkins AM, Hall A, Kihamia CM, Lorri W, et al. The impact of population level deworming on the haemoglobin levels of schoolchildren in Tanga, Tanzania. Trop Med Int Health. 1999;4:744–750. doi: 10.1046/j.1365-3156.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- Bhargava A, Jukes M, Lambo J, Kihamia CM, Lorri W, et al. Anthelmintic treatment improves the hemoglobin and serum ferritin concentrations of Tanzanian schoolchildren. Food Nutr Bull. 2003;24:332–342. doi: 10.1177/156482650302400403. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Chway HM, Montresor A, Tielsch JM, Jape JK, et al. Low dose daily iron supplementation improves iron status and appetite, but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. J Nutr. 2004;134:348–356. doi: 10.1093/jn/134.2.348. [DOI] [PubMed] [Google Scholar]
- Anderson RM, May RM. Population dynamics of human helminth infections: Control by chemotherapy. Nature. 1982;297:557–563. doi: 10.1038/297557a0. [DOI] [PubMed] [Google Scholar]
- Bundy DAP. Population ecology of intestinal helminth infections in human communities. Philos Trans R Soc Lond B Biol Sci. 1988;321:405–420. doi: 10.1098/rstb.1988.0100. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Report of the third global meeting of the partners for parasite control, Geneva, 29–30 November 2004. Geneva: World Health Organization; 2005. Deworming for health and development; 51 pp. [Google Scholar]
- Heukelbach J, Winter B, Wilcke T, Muehlen M, Albrecht S, et al. Selective mass treatment with ivermectin to control intestinal helminthiases and parasitic skin diseases in a severely affected population. Bull WHO. 2004;82:563–571. [PMC free article] [PubMed] [Google Scholar]
- Molyneux DH, Nantulya HM. Public–private partnerships in blindness prevention: reaching beyond the eye. Eye. 2005;19:1050–1056. doi: 10.1038/sj.eye.6701961. [DOI] [PubMed] [Google Scholar]
- Lawrence G, Leafasia J, Seridan J, Hills S, Wate J, et al. Control of scabies, skin sores and hematuria in children in the Solomon Islands: another role for ivermectin. Bull World Health Organ. 2005;83:34–42. [PMC free article] [PubMed] [Google Scholar]
- Commission for Africa. Our common interest: Report for the commission for Africa. Glasgow: Commission for Africa; 2005. Available: http://www.commissionforafrica.org/english/report/thereport/english/11-03-05_cr_report.pdf. Accessed 4 January 2006 . [Google Scholar]
- Savioli L, Engels D, Endo H. Extending the benefits of deworming for development. Lancet. 2005;365:1520–1521. doi: 10.1016/S0140-6736(05)66433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Anonymous] Thinking beyond deworming. Lancet. 2004;364:1993–1994. doi: 10.1016/S0140-6736(04)17521-1. [DOI] [PubMed] [Google Scholar]
- Guo JG, Cao CL, Hu GH, Lin H, Li D, et al. The role of ‘passive chemotherapy’ plus health education for schistosomiasis control in China during maintenance and consolidation phase. Acta Trop. 2005;96:177–183. doi: 10.1016/j.actatropica.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil–transmitted nematodes: A pressing public health agenda for helminth control. Int J Parasitol. 2004;34:1205–1210. doi: 10.1016/j.ijpara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Molyneux DH. “Neglected” diseases but unrecognized successes—Challenges and opportunities for infectious disease control. Lancet. 2004;364:380–383. doi: 10.1016/S0140-6736(04)16728-7. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Bergquist R, Xiao SH, Singer BH, Tanner M. Sustainable schistosomiasis control—the way forward. Lancet. 2003;362:1932–1934. doi: 10.1016/S0140-6736(03)14968-9. [DOI] [PubMed] [Google Scholar]
- Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139–146. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher D, Harries A, Getahun H. Tuberculosis and HIV interaction in sub–Saharan Africa: Impact on patients and programmes; implications for policies. Trop Med Int Health. 2005;10:734–742. doi: 10.1111/j.1365-3156.2005.01456.x. [DOI] [PubMed] [Google Scholar]
- Van Eijk AM, Ayisi JG, ter Kuile FO, Misore AO, Otieno JA, et al. Malaria and human immunodeficiency virus infection as risk factors for anemia in infants in Kisumu, western Kenya. Am J Trop Med Hyg. 2002;67:44–53. doi: 10.4269/ajtmh.2002.67.44. [DOI] [PubMed] [Google Scholar]
- Ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub–Saharan Africa. Am J Trop Med Hyg. 2004;71(Suppl 2):41–54. [PubMed] [Google Scholar]
- Cohen C, Karstaedt A, Frean J, Thomas J, Govender N, et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin Infect Dis. 2005;41:1641–1647. doi: 10.1086/498023. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Thomas CJ. Mapping and estimating the population at risk from lymphatic filariasis in Africa. Trans R Soc Trop Med Hyg. 2000;94:37–45. doi: 10.1016/s0035-9203(00)90431-0. [DOI] [PubMed] [Google Scholar]
- Brooker S. Schistosomes, snails and satellites. Acta Trop. 2002;82:207–214. doi: 10.1016/s0001-706x(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Druilhe P, Tall A, Sokhna C. Worms can worsen malaria: Towards a new means to role back malaria? Trends Parasitol. 2005;21:359–362. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Shapiro AE, Tukahebwa EM, Kasten J, Clarke SE, Magnussen P, et al. Epidemiology of helminth infections and their relationship to clinical malaria in southwest Uganda. Trans R Soc Trop Med Hyg. 2005;99:18–24. doi: 10.1016/j.trstmh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Tall A, Raphenon G, Trape JF, Druilhe P. Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria . Trans R Soc Trop Med Hyg. 2003;97:198–199. doi: 10.1016/s0035-9203(03)90117-9. [DOI] [PubMed] [Google Scholar]
- Fincham JE, Markus MB, Adams VJ. Could control of soil-transmitted helminthic infection influence the HIV/AIDS pandemic. Acta Trop. 2003;86:315–333. doi: 10.1016/s0001-706x(03)00063-9. [DOI] [PubMed] [Google Scholar]
- Le Hesran JY, Akiana J, Ndiaye EHM, Dia M, Senghor P, et al. Severe malaria attack is associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal . Trans R Soc Trop Med Hyg. 2004;98:397–399. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Diallo TO, Remoue F, Schacht AM, Charrier N, Dompnier JP, et al. Schistosomiasis co-infection in humans influences inflammatory markers in uncomplicated Plasmodium falciparum infection . Parasite Immunol. 2004;26:365–369. doi: 10.1111/j.0141-9838.2004.00719.x. [DOI] [PubMed] [Google Scholar]
- Sokhna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, et al. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal . Malar J. 2004;3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand V, Watier L, Le Hesran JY, Garcia A, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: Protective effect of schistosomiasis on malaria in Senegalese children . Am J Trop Med Hyg. 2005;72:702–707. [PubMed] [Google Scholar]
- Manga L. Vector-control synergies, between ‘roll back malaria’ and the Global Programme to Eliminate Lymphatic Filariasis, in the African region. Ann Trop Med Parasitol. 2002;96:S129–S132. doi: 10.1179/000349802125002473. [DOI] [PubMed] [Google Scholar]
- Harms G, Feldmeier H. HIV infection and tropical parasitic diseases—deleterious interactions in both directions. Trop Med Int Health. 2002;7:4770–4788. doi: 10.1046/j.1365-3156.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- Ali A. Leishmaniases and HIV/AIDS co-infections: Review of common features and management experiences. Ethiop Med J. 2002;40(Suppl 1):37–49. [PubMed] [Google Scholar]
- Woodruff AW, Ziegler JL, Hathaway A, Gwata T. Anaemia in African trypanosomiasis and ‘big spleen disease’ in Uganda. Trans R Soc Trop Med Hyg. 1973;67:329–337. doi: 10.1016/0035-9203(73)90109-0. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Chwaya HM, Tielsch J, Schulze KJ, Albonico M, et al. Epidemiology of iron deficiency anaemia in Zanzibari schoolchildren: The importance of hookworms. Am J Clin Nutr. 1997;65:153–159. doi: 10.1093/ajcn/65.1.153. [DOI] [PubMed] [Google Scholar]
- Brooker S, Peshu N, Warn PA, Mosobo M, Guyatt HL, et al. The epidemiology of hookworm infection and its contribution to anaemia among pre-school children on the Kenyan coast. Trans R Soc Trop Med Hyg. 1999;93:240–246. doi: 10.1016/s0035-9203(99)90007-x. [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Xie JL, Gordeuk V, Bucala R. The anemia of malaria infection: role of inflammatory cytokines. Curr Hematol Rep. 2004;3:97–106. [PubMed] [Google Scholar]
- Crawley J. Reducing the burden of anemia in infants and young children in malaria-endemic countries of Africa: From evidence to action. Am J Trop Med Hyg. 2004;71(Suppl 2):25–34. [PubMed] [Google Scholar]
- Fleming AF. Agriculture-related anemias. Br J Biomed Sci. 1994;31:345–357. [PubMed] [Google Scholar]
- Shulman CE, Graham WJ, Jilo H, Lowe BS, New L, et al. Malaria is an important cause of anaemia in primigravidae: Evidence from a district hospital in coastal Kenya. Trans R Soc Trop Med Hyg. 1996;90:535–539. doi: 10.1016/s0035-9203(96)90312-0. [DOI] [PubMed] [Google Scholar]
- Guyatt HL, Brooker S, Peshu N, Shulman CE. Hookworm and anaemia prevalence. Lancet. 2000;356:2101. doi: 10.1016/s0140-6736(05)74313-0. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years alter treatment for iron deficiency in infants. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;13:S636–S645. doi: 10.1093/jn/131.2.636S. [DOI] [PubMed] [Google Scholar]
- Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:S649–S666. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- Umbreit J. Iron deficiency: A concise review. Am J Hematol. 2005;78:225–231. doi: 10.1002/ajh.20249. [DOI] [PubMed] [Google Scholar]
- Mackintosh CL, Beeson JG, Marsh K. Clinical features and pathogenesis of severe malaria. Trends Parasitol. 2004;20:597–603. doi: 10.1016/j.pt.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Schellenberg D, Arstrong Schellenberg JRM, Mushi A, de Savigny D, Mgalula L, et al. The silent burden of anaemia in Tanzanian children: A community–based study. Bull World Health Organ. 2003;81:581–590. [PMC free article] [PubMed] [Google Scholar]
- Moore RD. Human immunodeficiency virus infection, anemia and survival. Clin Infect Dis. 1999;29:44–49. doi: 10.1086/520178. [DOI] [PubMed] [Google Scholar]
- Moore RD. Anemia and human immunodeficiency virus disease in the era of highly active antiretroviral therapy. Semin Hematol. 2000;37:18–23. doi: 10.1016/s0037-1963(00)90064-7. [DOI] [PubMed] [Google Scholar]
- Thigpen MC, Filler SJ, Hamel M, Kazembe P, Parise ME. Association of helminthiasis with peripheral malaria parasitemia among pregnant women. Am J Trop Med Hyg. 2004;71(Suppl 4):80. doi: 10.4269/ajtmh.2011.10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher M, Singhasivanon P, Gay F, Silachromroon U, Phumratanaprapin W, et al. Contemporaneous and successive mixed Plasmodium falciparum and Plamodium vivax infections are associated with Ascaris lumbricoides: An immunomodulating effect? . J Parasitol. 2001;87:912–915. doi: 10.1645/0022-3395(2001)087[0912:CASMPF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nacher M, Singhasivanon P, Gay F, Phumratanaprapin W, Silachromroon U, et al. Association of helminth infection with decreased reticulocyte counts and hemoglobin concentration in Thai falciparum malaria. Am J Trop Med Hyg. 2001;97:199–202. doi: 10.4269/ajtmh.2001.65.335. [DOI] [PubMed] [Google Scholar]
- Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, et al. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand . J Parasitol. 2002;88:55–58. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Chaorattannakawee S, Natalang O, Hananantachai H, Nacher M, Brockman A, et al. Trichuris trichiura infection is associated with the multiplicity of Plasmodium falciparum infections in Thailand . Ann Trop Med Parasitol. 2003;97:199–202. doi: 10.1179/000349803125002968. [DOI] [PubMed] [Google Scholar]
- Nacher M. Interactions between worm infections and malaria. Clin Rev Allergy Immunol. 2004;26:85–92. doi: 10.1007/s12016-004-0003-3. [DOI] [PubMed] [Google Scholar]
- Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, et al. Association of Schistosoma haematobium infection with protectin against acute Plasmodium falciparum malaria in Malian children . Am J Trop Med Hyg. 2005;73:1124–1130. [PMC free article] [PubMed] [Google Scholar]
- Borkow G, Bentwich Z. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: Role of hyporesponsiveness and anergy. Clin Microbiol Rev. 2004;17:1012–1030. doi: 10.1128/CMR.17.4.1012-1030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolday D, Mayaan S, Mariam ZG, Berhe N, Seboxa T, et al. J Acquir Immune Defic Syndr. 2002;31:56–62. doi: 10.1097/00126334-200209010-00008. [DOI] [PubMed] [Google Scholar]
- Bentwich Z. Concurrent infections that rise the HIV viral load. J HIV Ther. 2003;8:72–75. [PubMed] [Google Scholar]
- Kallestrup P, Zinyama R, Gomo E, Butterworth AE, Mudenge B, et al. Schistosomiasis and HIV-1 infection in rural Zimbabwe: Effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J Infect Dis. 2005;192:1956–1961. doi: 10.1086/497696. [DOI] [PubMed] [Google Scholar]
- Secor WE, Shah A, Mwinzi PM, Ndenga BA, Watta CO, et al. Increased density of human immunodeficiency virus type 1 coreceptors CCR5 and CXCR4 on the surfaces of CD4(+) T cells and moncytes of patients with Schistosoma mansoni infection. Infect Immun. 2003;71:6668–6671. doi: 10.1128/IAI.71.11.6668-6671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Malhotra I, Mungai PL, Wamachi AN, Koko JM, et al. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS. 2004;19:1849–1855. doi: 10.1097/01.aids.0000189846.90946.5d. [DOI] [PubMed] [Google Scholar]
- Elliott AM, Mawa PA, Joseph S, Namujju PB, Kizza M, et al. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans R Soc Trop Med Hyg. 2003;97:103–108. doi: 10.1016/s0035-9203(03)90040-x. [DOI] [PubMed] [Google Scholar]
- Brown M, Kizza M, Watera C, Quigley MA, Rowland S, et al. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. J Infect Dis. 2004;190:1869–1879. doi: 10.1086/425042. [DOI] [PubMed] [Google Scholar]
- Borkow G, Weisman Z, Leng Q, Stein M, Kalinkovich A, et al. Helminths, human immunodeficiency virus and tuberculosis. Scand J Infect Dis. 2001;33:568–571. doi: 10.1080/00365540110026656. [DOI] [PubMed] [Google Scholar]
- Elliott AM, Kyosiimire J, Quigley MA, Nakiyingi J, Watera C, et al. Eosinophilia and progression to active tuberculosis in HIV-1 infected Ugandans. Trans R Soc Trop Med Hyg. 2003;97:477–480. doi: 10.1016/s0035-9203(03)90096-4. [DOI] [PubMed] [Google Scholar]
- Barreto ML, Rodrigues LC, Silva RC, Assis AM, Reis MG, et al. Lower hookworm incidence, prevalence, and intensity of infection in children with a Bacillus Calmette–Guerin vaccination scar. J Infect Dis. 2000;182:1800–1803. doi: 10.1086/317627. [DOI] [PubMed] [Google Scholar]
- Elias D, Akuffo H, Pawlowski A, Haile M, Schon T, et al. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against virulent Mycobacterium tuberculosis . Vaccine. 2005;23:1326–1334. doi: 10.1016/j.vaccine.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Elias D, Wolday D, Akuffo H, Petros B, Boronner U, et al. Effect of deworming on human T cell responses to mycobacterial antigens in helminth–exposed individuals before and after bacilli Calmette–Guerin (BCG) vaccination. Clin Exp Immunol. 2001;123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M, Mawa PA, Joseph S, Bukusuba J, Watera C, et al. Treatment of Schistosoma mansoni infection increases helminth-specific type 2 cytokine responses and HIV-1 loads in coinfected Ugandan adults. J Infect Dis. 2005;191:1648–1657. doi: 10.1086/429668. [DOI] [PubMed] [Google Scholar]
- Christian P, Khatry SK, West KP. Antenatal anthelmintic treatment, birthweight, and infant survival. Lancet. 2004;364:981–983. doi: 10.1016/S0140-6736(04)17023-2. [DOI] [PubMed] [Google Scholar]
- Fincham, Markus MB, Brombacher F. Vaccination against helminths: Influence on HIV/AIDS and TB. Trends Parasitol. 2002;18:385–386. doi: 10.1016/s1471-4922(02)02300-0. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Wu WH, Antoine AD, Bottazzi ME, Valenzuela JG, et al. The impact of concurrent and treated Ancylostoma ceylancium hookworm infections on the immunogenicity of a recombinant hookworm vaccine in hamsters . J Infect Dis. 2006;193:155–162. doi: 10.1086/498528. [DOI] [PubMed] [Google Scholar]
- Elias D, Akuffo H, Pawlowski A, Haile M, Schon T, et al. Schistosoma mansoni infection reduces the protective efficacy of BCG vaccination against Mycobacterium tuberculosis . Vaccine. 2005;23:1326–1334. doi: 10.1016/j.vaccine.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Espinel I, Wieseman M, Paredes W, Espinel M, et al. Human onchocerciasis and tetanus vaccination: Impact on the postvaccination antitetanus antibody response. Infect Immun. 1999;67:5951–5957. doi: 10.1128/iai.67.11.5951-5957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine (CV 103-HgR) . Infect Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AM, Namujju PB, Mawa PA, Quigley MA, Nampijja M, et al. A randomised controlled trial of the effects of albendazole in pregnancy on maternal responses to mycobacterial antigens and infant responses to bacilli Calmette–Guerin (BCG) immunisation. BMC Infect Dis. 2005;5:115. doi: 10.1186/1471-2334-5-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Buss P. Hookworm—“The great infection of mankind”. PLoS Med. 2005;2:e67. doi: 10.1371/journal.pmed.0020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron A, Riveau G, Carpon M, Trottein F. Schistosomes: The road from host–parasite interactions to vaccines in clinical trials. Trends Parasitol. 2005;21:143–149. doi: 10.1016/j.pt.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Coler RN, Reed SG. Second generation vaccines against leishmaniasis. Trends Parasitol. 2005;21:244–249. doi: 10.1016/j.pt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Brooker S, Bethony JM, Rodrigues LC, Alexander N, Geiger SM. Epidemiologic, immunologic and practical considerations in developing and evaluating a human hookworm vaccine. Expert Rev Vaccines. 2005;4:35–50. [Google Scholar]
- New Partnership for Africa's Development. Health strategy. Midrand (South Africa): New Partnership for Africa's Development; 2003. Available: http://www.sarpn.org.za/documents/d0000612/index.php. Accessed 4 January 2006 . [Google Scholar]
- European Parliament, Committee on Development. Report on major and neglected diseases in Developing Countries. A6. 2005 February 15 Available: http://www.europarl.eu.int/news/expert/briefing_page/35-248-9-36-20050907BRI00034-05-09-2005-2005/default_p001c009_en.htm. Accessed 4 January 2006 . [Google Scholar]
- World Bank. Rolling back malaria, the World Bank Strategy and Booster Programme. Washington (District of Columbia): The World Bank; 2005. 193 pp. [Google Scholar]
- [Anonymous] Best practice principles for Global Health Partnership activities at country level. High-Level Forum on the Health MDGs; 2005 14–15 November; Paris, France. High-Level Forum on the Health MDGs. 2005:11. Available: http://www.hlfhealthmdgs.org/Documents/GlobalHealthPartnerships.pdf. Accessed 4 January 2006 . [Google Scholar]
- Van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, et al. Quantification of clinical morbidity associated with schistosome infection in sub–Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Chan MS. The global burden of intestinal nematode infections—Fifty years on. Parasitol Today. 1997;13:438–443. doi: 10.1016/s0169-4758(97)01144-7. [DOI] [PubMed] [Google Scholar]