Abstract
Six cases of Neisseria meningitidis serogroup W135 meningococcal infection have been reported in Turkey since 2003. Seven isolates recovered from four meningococcal meningitis patients and two asymptomatic carriers produced three distinct pulsed-field gel electrophoresis (PFGE) patterns. Multilocus sequence typing and antigen gene sequencing showed that five isolates were indistinguishable from ST-11 (ET-37) serogroup W135 meningococci, which were first isolated in Saudi Arabia and were responsible for the worldwide outbreak among Hajj pilgrims and their contacts in 2000. The remaining two isolates, which had related PFGE patterns, differed from each other at only one of the genetic loci characterized but were not related to the ST-11 clonal complex. None of the six individuals recalled contact with a pilgrim or had traveled on the Hajj. These six individuals exhibited no time or place relationships to each other, except for the two asymptomatic carriers, who were soldiers and served in the same military unit. These data demonstrate that serogroup W135 meningococci with different genotypes, including the Hajj epidemic strain, are endemic in Turkey.
Neisseria meningitidis is a common commensal bacterium of the human upper respiratory tract and can cause invasive infections such as septicemia or meningitis. Such colonization is known to be an epidemic threat in the countries of the meningitis belt (6). N. meningitidis strains have been classified into 13 serogroups (6, 13). Serogroup W135 meningococci were rarely associated with epidemiologically distinct outbreaks of meningococcal disease (2) until an international outbreak was reported following the annual Hajj seasons in Saudi Arabia in 2000 and 2001 (11, 12). The epidemic strain has been identified by multilocus sequence typing (MLST) as sequence type 11 (ST-11), which corresponds to electrophoretic type 37 (ET-37) as defined by multilocus enzyme electrophoresis (10). The Hajj pilgrimage provides ideal conditions for person-to-person transmission and acquisition of virulent meningococci, and high rates of carriage of serogroup W135 strains in susceptible individuals have been reported (4). Following the 2000 and 2001 Hajj seasons, N. meningitidis serogroup W135 infections were reported among pilgrims who had returned home, among their household contacts, and within the community at large worldwide (19).
In Turkey, limited epidemiologic studies of N. meningitidis have been reported (7, 9). In 1990 a carriage study in the Aegean region reported an N. meningitidis carriage rate of 28%, and the serogroups were identified as A (12%), B (9%), C (63%), D (0.4%), Y (0.6%), and X-Y (0.4%) (7). A recent study reported that the N. meningitidis carriage rate among primary school children was 6.2%, with A, B, and C as the dominant serogroups (9). In 2001 a serogroup W135 meningococcus was isolated for the first time in Turkey from a healthy preschool child (3), and the first case of invasive disease caused by a serogroup W135 organism was reported in 2003 (8). The isolate recovered in the first case was epidemiologically unrelated to the ST-11 epidemic strain that was originally isolated in Saudi Arabia and was responsible for the worldwide outbreak among Hajj pilgrims and their contacts in 2000 (8, 11).
In this study we describe the genetic and antigenic characterization of the first Turkish serogroup W135 isolate in 2003 (8) and of six serogroup W135 isolates recovered in 2004 from five individuals in Ankara, the capital city of Turkey, and we correlate these data with those of clinical and epidemiologic studies.
(This study was presented in part at the 7th International Meeting on Microbial Epidemiological Markers, Victoria, British Columbia, Canada, 11 to 14 May 2005.)
Isolate collection and identification.
N. meningitidis serogroup W135 isolates were recovered from the Gulhane Military Hospital in Ankara and the surrounding area in 2004. Clinical specimens, including throat swabs, blood, and cerebrospinal fluid (CSF), were immediately plated on BBL-modified Thayer-Martin medium (MTM II; Becton Dickinson Microbiology Systems). Plates were incubated for 2 days at 35°C under 5% CO2. Plates were examined for the presence of colonies showing the typical morphology of N. meningitidis. Suspect colonies were screened for oxidase reactivity, and positive samples were Gram stained. If gram-negative diplococci were present, a biochemical profile by the API NH system (bioMérieux, France) was used for confirmation. The isolates were stored at −70°C in brain heart infusion broth containing 15% glycerol until further analysis. An N. meningitidis strain isolated at Vanderbilt University Medical Center was used as a reference strain.
Serogrouping.
Serogrouping of the meningococcal isolates was performed by a slide agglutination technique as recommended by Difco Laboratories (Detroit, Mich.). The serogroup of these meningococcal isolates was further confirmed by the Tennessee Department of Health Laboratory Services by using monoclonal and polyclonal sera for groups A, B, C, D, X, Y, and W135, provided by both Difco Laboratories and Murex (Remel, Lenexa, Kans.) (14).
MLST and antigen gene sequencing.
Characterization of isolates by MLST and nucleotide sequencing of the antigen-encoding genes porA and porB were performed as described previously (5, 18), with allele, ST, and antigen type assignment carried out by interrogation of the appropriate Web-accessible databases (http://www.neisseria.org/nm/typing).
PFGE typing.
The relationship between strains was examined by genomic DNA pulsed-field gel electrophoresis (PFGE) as described previously with modifications (13). Briefly, genomic DNA was extracted from logarithmic-phase cultures grown on 5% blood agar, prepared in low-melting-point agarose plugs, and digested with the NheI enzyme (New England Biolabs, Beverly, MA) for 24 h, as previously described (16). The DNA size standard used was a bacteriophage lambda ladder consisting of concatemers starting at 48.5 kbp and increasing to approximately 1,000 kbp (Bio-Rad Laboratories, Hercules, CA). Electrophoresis was performed with a GenePath system (Bio-Rad) using a dedicated program two for 20 h. The gels were stained with ethidium bromide, rinsed, and photographed under UV light using the Gel Doc 2000 computerized documentation system (Bio-Rad). Each strain was classified as indistinguishable, closely related, possibly related, or different if the number of fragment differences compared with a reference strain was 0, 2 to 3, 4 to 6, or ≥7, respectively, according to the criteria recommended previously (17).
During 2004 at the Gulhane Military Hospital, a total of six N. meningitidis serogroup W135 isolates were identified from the Ankara area. Four were recovered from blood or CSF specimens from three patients with bacterial meningitis and two from throat swabs of two asymptomatic carriers. All individuals, who were males 6 to 21 years old, denied any history of contact with a pilgrim or of travel on the Hajj. There were no epidemiological links among the disease cases, but the two asymptomatic carriers were soldiers serving in the same military unit (Table 1). None of the individuals had received the N. meningitidis quadrivalent vaccine (A, C, Y, and W135), but they had previously received an A+C polysaccharide vaccine, with the exception of two children (isolates Nm118 and Nm121).
TABLE 1.
Clinical and epidemiologic characteristics of seven Neisseria meningitidis W135 isolates in Turkeya
| Isolate | Patient age (yr) | Site of isolation | Date isolatedb | Place isolated | Relation of patient to other patient(s) | Travel history | Clinical disease | PFGE type | Sequence type | porA | porB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nm118 | 20 | CSF | 03/26/2003 | Downtown Ankara | No | No | Meningitis | Turkey | ST-2754 | P1.5,2 | porB2-86 |
| Nm120 | 6 | CSF | 03/18/2004 | Sihhiye, Ankara | No | No | Meningitis | ET-37 | ST-11 | P1.5,2 | porB2-2 |
| Nm119 | 21 | CSF | 03/24/2004 | Downtown Ankara | No | No | Meningitis | ET-37 | ST-11 | P1.5,2 | porB2-2 |
| Nm121 | 21 | Blood | 03/24/2004 | Downtown Ankara | No | No | Meningitis | ET-37 | ST-11 | P1.5,2 | porB2-2 |
| Nm124 | 7 | CSF | 05/16/2004 | Sihhiye, Ankara | No | No | Meningitis | Turkey | ST-185 | P1.5,2 | porB2-86 |
| Nm123 | 20 | Throat | 08/12/2004 | Balgat, Ankara | Yesc | No | Asymptomatic | ET-37 | ST-11 | P1.5,2 | porB2-2 |
| Nm122 | 21 | Throat | 08/12/2004 | Balgat, Ankara | Yesc | No | Asymptomatic | ET-37 | ST-11 | P1.5,2 | porB2-2 |
All six individuals were male, and Nm118 was the first index case. Isolates Nm119 and Nm121 were recovered from the same patient.
Given as month/day/year.
These two cases served in the same military unit.
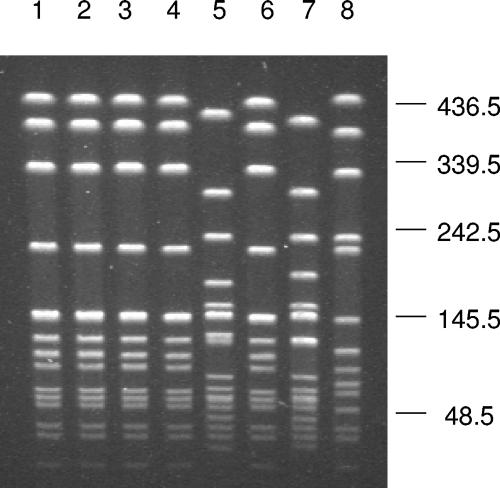
Three distinct PFGE patterns and three sequence types were identified among the seven isolates (Table 1; Fig. 1). Five isolates that were recovered from two patients and two carriers (Nm119, Nm120, Nm121, Nm122, and Nm123) possessed indistinguishable PFGE patterns (Fig. 1) and possessed a sequence type and antigen gene sequences identical to those identified for the ST-11 (ET-37) epidemic strain (Table 1) (11, 12). The other two PFGE patterns (isolates Nm118 and Nm124) were related to the PFGE pattern originally identified in Turkey (8) based on the Tenover criteria (17). MLST and antigen gene sequence analysis of Nm124 and Nm118 identified two closely related sequence types, ST-185 and ST-2754, respectively, and the two isolates also possessed identical serotype- and serosubtype-determining porB and porA sequences (Table 1). Interrogation of the Neisseria PubMLST isolate database (http://pubmlst.org/) revealed that ST-185 and ST-2754 organisms have both been isolated previously. ST-185 meningococci have been isolated from asymptomatic carriers in Germany and Japan, and also in one disease case in Israel. An ST-2754 organism was first isolated in a case of meningococcal disease in Germany. These data suggest that ST-11 serogroup W135 meningococci have spread in the population and, together with other, genetically unrelated serogroup W135 meningococci, are now responsible for cases of endemic disease in Turkey.
FIG. 1.
Pulsed-field gel electrophoresis patterns of NheI-digested genomic DNA of Neisseria meningitidis W135 isolates. Lanes 1 to 7, isolates Nm119, Nm123, Nm120, Nm121, Nm118, Nm122, and Nm124, respectively, recovered from Turkey. Lane 8, N. meningitidis W135 clinical isolate recovered at Vanderbilt University Medical Center and confirmed by the Tennessee Department of Health Laboratory Services. Molecular sizes are given in kilobases.
High rates of carriage and transmission of serogroup W135 ST-11 meningococci that occurred among pilgrims during the Hajj season have been implicated in the spread of serogroup W135 meningococcal disease in Africa, Asia, and the Middle East (2). Two million pilgrims from approximately 140 countries congregate annually in Mecca and Medina in Saudi Arabia for the Hajj and/or Umrah pilgrimages during Hajj seasons (4). Overcrowding is the most important factor for person-to-person transmission of meningococci and the spread of invasive disease (2). Most of the Turkish population is Muslim, and approximately 150,000 Turkish pilgrims make the annual Islamic pilgrimage to Mecca and Medina in Saudi Arabia (8). The first reported pandemic that was associated with the Hajj season began in 1987 in Saudi Arabia and was caused by hyperinvasive serogroup A meningococci. Following this outbreak, the Saudi government mandated vaccination with a bivalent serogroup A and serogroup C vaccine for all pilgrims entering the country for the Hajj season (4). Subsequently, the vaccination policy was extended further to include all Umrah visitors (1).
During April of 2000, approximately 400 cases of serogroup W135 meningococcal disease were reported in 10 countries among pilgrims returning from Hajj and their close contacts (2, 11). In 2001, the second international outbreak of serogroup W135 meningococcal disease was reported. This outbreak was smaller than the first, with 200 cases of meningococcal infection, associated with international travel or contact with travelers who had been to Saudi Arabia, identified in 10 different countries (20). It has also been reported that 55 deaths from meningococcal infection occurred during the Hajj pilgrimage (4). Following the 2001 outbreak, the Saudi Arabian government made it a requirement that all pilgrims entering the country should be vaccinated with a quadrivalent vaccine containing serogroups A, C, W135, and Y (2).
Although serogroup W135 ST-11 meningococci have been isolated worldwide since 1970, they have been reported rarely in European countries (15). In this study of W135-associated meningococcal disease in Turkey, W135 ST-11 meningococci have been isolated in disease cases and from healthy carriers, none of whom were linked epidemiologically to the Hajj pilgrimages of 2000 and 2001. These data suggest that carriage and transmission of the Hajj epidemic strain have led to the emergence of serogroup W135 ST-11 organisms as a cause of endemic meningococcal disease in Turkey. Moreover, these data highlight the need for continued epidemiological surveillance to identify any changes in the incidence of serogroup W135 meningococcal disease and to inform future public health strategies.
Acknowledgments
We thank the Tennessee Department of Health Laboratory Services for confirming the serogroup of the isolates; Levant Doganci, Mehmet Baysallar, Gulsen Hascelik, and Alaaddin Pahsa for excellent assistance; and Tanja Popovic and Leonard Mayer of the Centers for Disease Control and Prevention for helpful discussions. This publication made use of the Neisseria Multi Locus Sequence Typing website (http://pubmlst.org/neisseria/).
REFERENCES
- 1.Aguilera, J. F., A. Perrocheau, C. Meffre, and S. Hahne. 2002. Outbreak of serogroup W135 meningococcal disease after the Hajj pilgrimage, Europe, 2000. Emerg. Infect. Dis. 8:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apicella, M. A. 2004. Extrameningeal complications of Neisseria meningitidis serogroup W135 infection. Clin. Infect. Dis. 38:1638-1639. [DOI] [PubMed] [Google Scholar]
- 3.Bakir, M., A. Yagci, N. Ulger, C. Akbenlioglu, A. Ilki, and G. Soyletir. 2001. Asymptomatic carriage of Neisseria meningitidis and Neisseria lactamica in relation to Streptococcus pneumoniae and Haemophilus influenzae colonization in healthy children: apropos of 1400 children sampled. Eur. J. Epidemiol. 17:1015-1018. [DOI] [PubMed] [Google Scholar]
- 4.Balkhy, H. H., Z. A. Memish, and A. O. Osoba. 2003. Meningococcal carriage among local inhabitants during the pilgrimage 2000-2001. Int. J. Antimicrob. Agents 21:107-111. [DOI] [PubMed] [Google Scholar]
- 5.Bygraves, J. A., R. Urwin, A. J. Fox, S. J. Gray, J. E. Russell, I. M. Feavers, and M. C. Maiden. 1999. Population genetic and evolutionary approaches to analysis of Neisseria meningitidis isolates belonging to the ET-5 complex. J. Bacteriol. 181:5551-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caugant, D. A. 1998. Population genetics and molecular epidemiology of Neisseria meningitidis. APMIS 106:505-525. [PubMed] [Google Scholar]
- 7.Coskun, S., S. Yanikyurek, and M. Agzitemiz. 1990. Incidence of epidemiological meningitis in Aegean region. Turk. J. Infect. 4:431-435. [Google Scholar]
- 8.Doganci, L., M. Baysallar, M. A. Saracli, G. Hascelik, and A. Pahsa. 2004. Neisseria meningitidis W135, Turkey. Emerg. Infect. Dis. 10:936-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazi, H., S. Surucuoglu, B. Ozbakkaloglu, S. Akcali, N. Ozkutuk, K. Degerli, and S. Kurutepe. 2004. Oropharyngeal carriage and penicillin resistance of Neisseria meningitidis in primary school children in Manisa, Turkey. Ann. Acad. Med. Singapore 33:758-762. [PubMed] [Google Scholar]
- 10.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer, L. W., M. W. Reeves, N. Al-Hamdan, C. T. Sacchi, M. K. Taha, G. W. Ajello, S. E. Schmink, C. A. Noble, M. L. Tondella, A. M. Whitney, Y. Al-Mazrou, M. Al-Jefri, A. Mishkhis, S. Sabban, D. A. Caugant, J. Lingappa, N. E. Rosenstein, and T. Popovic. 2002. Outbreak of W135 meningococcal disease in 2000: not emergence of a new W135 strain but clonal expansion within the electophoretic type-37 complex. J. Infect. Dis. 185:1596-1605. [DOI] [PubMed] [Google Scholar]
- 12.Popovic, T., C. T. Sacchi, M. W. Reeves, A. M. Whitney, L. W. Mayer, C. A. Noble, G. W. Ajello, F. Mostashari, N. Bendana, J. Lingappa, R. Hajjeh, and N. E. Rosenstein. 2000. Neisseria meningitidis serogroup W135 isolates associated with the ET-37 complex. Emerg. Infect. Dis. 6:428-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popovic, T., S. Schmink, N. A. Rosenstein, G. W. Ajello, M. W. Reeves, B. Plikaytis, S. B. Hunter, E. M. Ribot, D. Boxrud, M. L. Tondella, C. Kim, C. Noble, E. Mothershed, J. Besser, and B. A. Perkins. 2001. Evaluation of pulsed-field gel electrophoresis in epidemiological investigations of meningococcal disease outbreaks caused by Neisseria meningitidis serogroup C. J. Clin. Microbiol. 39:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenqvist, E., E. Wedege, E. A. Hoiby, and L. O. Froholm. 1990. Serogroup determination of Neisseria meningitidis by whole-cell ELISA, dot-blotting and agglutination. APMIS 98:501-506. [PubMed] [Google Scholar]
- 15.Taha, M. K., M. Achtman, J. M. Alonso, B. Greenwood, M. Ramsay, A. Fox, S. Gray, and E. Kaczmarski. 2000. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet 356:2159. [DOI] [PubMed] [Google Scholar]
- 16.Tang, Y. W., M. G. Waddington, D. H. Smith, J. M. Manahan, P. C. Kohner, L. M. Highsmith, H. Li, F. R. Cockerill III, R. L. Thompson, S. O. Montgomery, and D. H. Persing. 2000. Comparison of protein A gene sequencing with pulsed-field gel electrophoresis and epidemiologic data for molecular typing of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 38:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urwin, R., J. E. Russell, E. A. Thompson, E. C. Holmes, I. M. Feavers, and M. C. Maiden. 2004. Distribution of surface protein variants among hyperinvasive meningococci: implications for vaccine design. Infect. Immun. 72:5955-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilder-Smith, A., T. M. Barkham, S. Ravindran, A. Earnest, and N. I. Paton. 2003. Persistence of W135 Neisseria meningitidis carriage in returning Hajj pilgrims: risk for early and late transmission to household contacts. Emerg. Infect. Dis. 9:123-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. 2001. Meningococcal disease, serogroup W135 (update). Wkly. Epidemiol. Rec. 76:213-214. [PubMed] [Google Scholar]