Abstract
To examine the association between colonization by two newly classified species of genital ureaplasmas (Ureaplasma parvum and U. urealyticum) in early pregnancy and subsequent late abortion or preterm birth at <34 weeks of gestation, four species of genital mycoplasmas—Mycoplasma genitalium, M. hominis, U. parvum, and U. urealyticum—as well as Chlamydia trachomatis and Neisseria gonorrhoeae were examined by PCR-based methods in a prospective cohort study of 877 women with singleton pregnancies at <11 weeks of gestation. Antibiotics were used only in cases in which C. trachomatis and/or N. gonorrhoeae was detected. Multivariate logistic-regression analysis was used to assess independent risk factors after taking maternal low body weight and past history of preterm birth into account. M. genitalium, M. hominis, U. parvum, U. urealyticum, C. trachomatis, and N. gonorrhoeae were detected in 0.8%, 11.2%, 52.0%, 8.7%, 3.2%, and 0.1% of these 877 women, respectively. Twenty-one (2.4%) women experienced late abortion or preterm birth at <34 weeks of gestation. Three factors—detection of U. parvum in the vagina (odds ratio [OR], 3.0; 95% confidence interval [CI], 1.1 to 8.5); use of antibiotics, such as penicillin and cefatrizine, for incidental inflammatory complications before 22 weeks of gestation (OR, 4.2; 95% CI, 1.6 to 10.0); and past history of preterm birth (OR, 10.4; 95% CI, 2.7 to 40.5)—were independently associated with late abortion and preterm birth. In conclusion, vaginal colonization with U. parvum, but not U. urealyticum, is associated with late abortion or early preterm birth.
Preterm birth and low birth weight are the leading causes of neonatal mortality and morbidity in the developed world. More than 60% of the mortality among infants without anatomic or chromosomal defects can be attributed to low birth weight (20). Ascending genital tract infections contribute to up to 50% of premature deliveries, particularly those occurring before 30 weeks of gestation (4, 15). Moreover, the rate of neonatal complications has been shown to be higher in neonates born to women with microbial invasion of the amniotic cavity than born to those women without infection (10).
Genital mycoplasmas, including Mycoplasma hominis, M. genitalium, and Ureaplasma spp., are suspected of contributing to a number of pathological conditions. M. hominis was isolated from the amniotic fluid in 30% of 404 women with intra-amniotic infection (21) and was shown to be associated with preterm birth at <33 weeks of gestation (23). M. genitalium was suggested to cause urethritis in men (11) and mucopurulent cervicitis in women (16), but its association with preterm birth has not been studied extensively. Ureaplasma has been implicated in infertility, spontaneous abortion, stillbirth, premature birth, low birth weight, and perinatal morbidity and mortality (3). Vaginal colonization with Ureaplasma has not been associated with preterm birth (3), while the presence of Ureaplasma in the amniotic fluid is associated with a robust host response in fetal, amniotic, and maternal compartments (24) and subsequent preterm birth (7). It is not known why this microorganism invades the amniotic cavity only in some women despite heavy colonization of the vagina by Ureaplasma.
Recently, the species previously classified as Ureaplasma urealyticum was separated into two new species: U. parvum (previously U. urealyticum biovar 1) and U. urealyticum (previously U. urealyticum biovar 2) (14, 19). Therefore, U. urealyticum organisms examined in previous studies (3, 7, 24) may have included both U. parvum and emended U. urealyticum. It is possible that the two new Ureaplasma species differ from each other in pathogenicity, as suggested in several studies (1, 18).
The purpose of the present prospective study was to examine the relationship between preterm birth and vaginal colonization with these four species of mycoplasmas in early pregnancy.
MATERIALS AND METHODS
Subjects and sample collection.
A total of 1,040 women with singleton pregnancies at <11 weeks of gestation were enrolled from Hokkaido University Hospital and nine affiliated hospitals after their fetuses were confirmed to have normal heartbeats between January 2002 and December 2002. A clean, unlubricated speculum was placed into the vagina. Sterile cotton swabs were used to obtain vaginal material from the posterior vaginal fornix. All the swab specimens obtained from pregnant women were subjected to alkaline denaturation.
Hybrid Capture test and PCR microtiter plate hybridization.
Alkaline-denatured samples were first subjected to both the Hybrid Capture 2 CT-ID test and the GC-ID test (Digene Corporation, Gaithersburg, MD) for Chlamydia trachomatis and Neisseria gonorrhoeae, respectively, in accordance with the manufacturer's instructions. DNA was extracted from the remainder of these alkaline-denatured samples. The DNA extracts were examined for four species of mycoplasmas (M. genitalium, M. hominis, U. parvum, and emended U. urealyticum) by using the mycoplasma species-specific PCR-microtiter plate hybridization assay of Yoshida et al. (25). Briefly, PCR was performed for the 16S rRNA gene by using primer pairs for both mycoplasmas and ureaplasmas. The amplified products were detected by hybridization with mycoplasma species-specific oligonucleotide probes immobilized on microtiter plates (25).
Antibiotics.
Antibiotics were administered to women in whom C. trachomatis and/or N. gonorrhoeae was detected but not to those in whom any mycoplasma was detected in the absence of C. trachomatis or N. gonorrhoeae.
Clinical profile and pregnancy outcome.
Gestational age was determined by a combination of the last menstrual period and ultrasonographic evaluation. Data regarding age, parity, body mass index (BMI), previous obstetric history related to preterm labor, use of antibiotics, and gestational week at delivery were obtained from the medical charts.
Informed consent.
This study was approved by each hospital's ethics committee, and all women gave written informed consent prior to participation.
Statistical analyses.
The Mann-Whitney U test, Fisher's exact probability test, and multivariate logistic-regression analysis (SPSS; SPSS Inc., Chicago, IL) were used for statistical analyses, with a P of <0.05 considered statistically significant.
RESULTS
A total of 877 women were analyzed in the present study after 163 women were excluded for the following reasons: induced abortion (n = 12), spontaneous preterm delivery due to a major anomaly of the fetus incompatible with life (n = 2), induced preterm delivery because of maternal breast cancer (n = 1), and unavailability for follow-up (n = 148).
The pregnancies of 21 (2.4%) of the 877 women ended in spontaneous abortion or preterm birth at <34 weeks of gestation (preterm birth group) (Table 1). Five of these 21 women experienced miscarriage between 11 and 15 weeks of gestation (Table 2). One woman experienced intrauterine fetal death at 24 weeks of gestation. Causes of preterm birth for the remaining 15 women were premature rupture of the membranes in 7 women at 26 to 33 weeks of gestation, failure to suppress uterine activity in 5 women at 28 to 33 weeks of gestation, preeclampsia in 1 woman at 30 weeks of gestation, fetal growth restriction in 1 woman at 30 weeks of gestation, and intrauterine infection in 1 woman at 32 weeks of gestation (Table 2). The 856 women who gave birth at or beyond 34 weeks of gestation served as a control group (Table 1).
TABLE 1.
Clinical profiles and outcomes
| Characteristic | Mean ± SD (%) for:
|
Pa | ||
|---|---|---|---|---|
| Total | Preterm birth group | Control group | ||
| No. of women | 877 | 21 | 856 | |
| Age (yr) | 28.9 ± 4.6 | 29.6 ± 6.1 | 28.9 ± 4.5 | 0.500 |
| No. with age of <25 yr | 147 (16.8) | 4 (19.0) | 143 (16.7) | 0.999 |
| Gestation wk at entry | 8.6 ± 1.1 | 8.8 ± 1.4 | 8.6 ± 1.1 | 0.348 |
| Nulliparous | 482 (55.0) | 14 (66.7) | 468 (54.7) | 0.375 |
| BMI at entry of <19.8b | 347 (39.6) | 5 (23.8) | 342 (40.0) | 0.176 |
| Past history of preterm birth | 18 (2.1) | 3 (14.3) | 15 (1.8) | 0.007 |
| Antibiotic use at <22 wk for: | ||||
| C. trachomatis | 28 (3.2) | 0 (0.0) | 28 (3.3) | 0.999 |
| Other reasons | 102 (11.6) | 7 (33.3) | 95 (11.1) | 0.006 |
| Cervical cerclage | 19 (2.2) | 1 (4.8) | 18 (2.1) | 0.372 |
| Vaginal colonization with: | ||||
| M. genitalium | 7 (0.8) | 0 (0.0) | 7 (0.8) | 0.999 |
| M. hominis | 98 (11.2) | 4 (19.0) | 94 (11.0) | 0.280 |
| U. parvum | 456 (52.0) | 16 (76.2) | 440 (51.4) | 0.027 |
| U. urealyticum | 76 (8.7) | 1 (4.8) | 75 (8.8) | 0.999 |
| Any species of mycoplasma | 564 (64.3) | 19 (90.5) | 545 (63.7) | 0.010 |
| More than one mycoplasma species | 70 (8.0) | 2 (9.5) | 68 (7.9) | 0.681 |
| C. trachomatis and any species of mycoplasma | 22 (2.5) | 0 (0.0) | 22 (2.6) | 0.999 |
| C. trachomatis | 28 (3.2) | 0 (0.0) | 28 (3.3) | 0.999 |
| N. gonorrhoeae | 1 (0.1) | 0 (0.0) | 1 (0.1) | 0.999 |
Comparison between the preterm birth and control groups.
BMI = body weight/body height squared (kg/m2).
TABLE 2.
Bacterial characteristics and cause of early delivery for the preterm birth group
| Patient | Gestational wk at delivery | Presence ofb:
|
Cause of preterm deliverya | |||
|---|---|---|---|---|---|---|
| M. genitalium | M. hominis | U. parvum | U. urealyticum | |||
| 1 | 11 | − | − | − | − | Miscarriage |
| 2 | 11 | − | − | + | − | Miscarriage |
| 3 | 12 | − | − | + | − | Miscarriage |
| 4 | 13 | − | − | − | + | Miscarriage |
| 5 | 15 | − | − | + | − | Miscarriage |
| 6 | 24 | − | − | + | − | Fetal death |
| 7 | 26 | − | − | + | − | PROM |
| 8 | 27 | − | − | + | − | PROM |
| 9 | 28 | − | + | − | − | FSUA |
| 10 | 29 | − | − | + | − | PROM |
| 11 | 30 | − | − | + | − | PROM |
| 12 | 30 | − | + | + | − | Fetal growth restriction |
| 13 | 30 | − | − | + | − | Preeclampsia |
| 14 | 31 | − | − | + | − | PROM |
| 15 | 31 | − | − | + | − | FSUA |
| 16 | 31 | − | − | − | − | FSUA |
| 17 | 32 | − | − | + | − | Intrauterine infection |
| 18 | 32 | − | + | + | − | FSUA |
| 19 | 33 | − | + | − | − | PROM |
| 20 | 33 | − | − | + | − | PROM |
| 21 | 33 | − | − | + | − | FSUA |
PROM, premature rupture of the membranes; FSUA, failure to suppress uterine activity.
+, present; −, absent.
There were no significant differences in mean maternal age, number of women aged <25 years, number of nulliparous women, number of women with BMI of <19.8, or number of women who had cervical cerclage in the current pregnancy between the two groups divided by the length of gestation (Table 1). A significantly larger number of women had a past history of preterm birth at <37 weeks of gestation in the preterm birth group. Antibiotic use before 22 weeks of gestation was confirmed for a total of 130 women. Antibiotics, such as clarithromycin, were used to eradicate C. trachomatis in 28 women. All of the 28 women with vaginal colonization by C. trachomatis were treated with antibiotics and did not give birth at <34 weeks of gestation. Antibiotics, such as penicillin and cefatrizine, were used in the remaining 102 women before 22 weeks of gestation for several reasons, including upper respiratory tract infection in 66 women (3/21 [14.3%] for the preterm birth group versus 63/856 [7.4%] for the control group), prophylactically after genetic amniocentesis in 13 women (1/21 [4.8%] versus 12/856 [1.4%]), prophylactically after cervical suture in 12 women (1/21 [4.8%] versus 11/856 [1.3%]), enteritis in 3 women (1/21 versus 2/856), urinary tract infection in 3 women (0/21 versus 3/856), suspected intrauterine infection in 2 women (0/21 versus 2/856), parotitis in 1 woman (0/21 versus 1/856), N. gonorrhoeae in 1 woman (0/21 versus 1/856), and cervicitis in 1 woman (1/21 versus 0/856). Thus, the reasons for antibiotic use did not differ largely between the two groups. However, a significantly larger number of women were treated with antibiotics before 22 weeks of gestation in the preterm birth group than in the control group for reasons other than the presence of C. trachomatis.
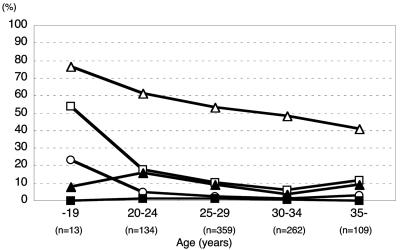
M. genitalium, M. hominis, U. parvum, U. urealyticum, C. trachomatis, and N. gonorrhoeae were detected in 0.8%, 11.2%, 52.0%, 8.7%, 3.2%, and 0.1% of the 877 women, respectively (Table 1). Five hundred sixty-four women (64.3%) harbored mycoplasma species (any of the four species), and 70 women (8.0%) harbored combinations of more than one mycoplasma species. Younger pregnant women seemed to have higher frequencies of colonization by U. parvum, M. hominis, and C. trachomatis than did older women (Fig. 1). The prevalences of M. genitalium, M. hominis, U. urealyticum, and C. trachomatis did not differ between the two groups, while mycoplasma species and U. parvum were detected in a significantly larger number of women in the preterm birth group than in the control group (Table 1).
FIG. 1.
Prevalence of vaginal mycoplasmas and C. trachomatis according to patient age. □, M. hominis; ▪, M. genitalium; ▵, U. parvum; ▴, U. urealyticum; ○, C. trachomatis.
Among the 16 women with U. parvum in the preterm birth group, two were coinfected with M. hominis (Table 2). Six of seven women (85.7%) who experienced preterm premature rupture of the membranes harbored U. parvum. One woman with U. urealyticum in the preterm birth group did not harbor other mycoplasma species. Neither C. trachomatis nor N. gonorrhoeae was detected in this group (Table 1).
U. parvum and/or U. urealyticum were detected in 523 (59.6%) of the 877 women. Nine of the 456 women with U. parvum were coinfected with U. urealyticum. Thus, among the 523 women with Ureaplasma infection, 447 (85.5%), 67 (12.8%), and 9 (1.7%) had U. parvum, U. urealyticum, and both, respectively. In these three groups, 16 (3.6%) of 447, 1 (1.5%) of 67, and 0 of 9 women subsequently developed late abortion or preterm birth at <34 weeks of gestation, respectively. Of 456 women with U. parvum infection, 49 (10.7%) were coinfected with M. hominis, while 49 (11.6%) of 421 women without U. parvum were infected with M. hominis, suggesting that M. hominis infection was independent of U. parvum infection. However, women with vaginal C. trachomatis were significantly more likely to have vaginal mycoplasmas other than U. urealyticum (Table 3).
TABLE 3.
Association of vaginal colonization by C. trachomatis with that by mycoplasmas
| Mycoplasma | No. (%) of patients with mycoplasmal colonization when C. trachomatis is:
|
Pa | |
|---|---|---|---|
| Present (n = 28) | Absent (n = 849) | ||
| M. genitalium | 2 (7.1) | 5 (0.6) | 0.0187 |
| M. hominis | 7 (25.0) | 91 (10.7) | 0.0287 |
| U. parvum | 21 (75.0) | 435 (51.2) | 0.0195 |
| U. urealyticum | 3 (10.7) | 73 (8.6) | 0.7278 |
Comparison between groups with and without the presence of C. trachomatis.
As past history of preterm birth at <37 weeks of gestation, antibiotic use for organisms other than C. trachomatis before 22 weeks of gestation, and vaginal colonization by U. parvum were possible candidate risk factors for late abortion or preterm birth (Table 1), these three factors were entered into the logistic-regression model to assess independent risk factors for late abortion or preterm birth at <34 weeks of gestation. All three factors remained independently associated with late abortion or preterm birth (Table 4).
TABLE 4.
Multivariate logistic-regression analysis for assessment of independent risk factors for late abortion or preterm birth at <34 weeks of gestation
| Risk factor | β | P | OR (95% CI)a |
|---|---|---|---|
| Constant | −0.02 | ||
| U. parvum | 1.11 | 0.035 | 3.04 (1.08-8.52) |
| Use of penicillin or cefatrizine at <22 wk | 1.44 | 0.003 | 4.24 (1.63-10.00) |
| Past history of preterm birth | 2.35 | 0.001 | 10.44 (2.69-40.49) |
OR, odds ratio; CI, confidence interval.
DISCUSSION
Although our cohort was relatively small for analysis of the role of mycoplasmas in preterm birth, the results of this prospective study demonstrated that women with vaginal U. parvum infection but not U. urealyticum infection, were at increased risk for late abortion or preterm birth at <34 weeks of gestation irrespective of past history of preterm birth, which is a well-known risk factor for preterm birth (2, 13). This was also confirmed in the present study. However, it is important to note that 440 of 456 women (96.5%) with U. parvum colonization gave birth to infants at or beyond 34 weeks of gestation.
Previous studies using methods that did not differentiate between U. urealyticum and U. parvum concluded that ureaplasmal colonization of the lower genital tract was not associated with adverse pregnancy outcome, while ureaplasmal infection of the chorioamnion was strongly associated with chorioamnionitis, preterm birth, and perinatal morbidity and mortality (3). There have been several previous studies of the association of the two Ureaplasma species with clinical disease and/or pregnancy outcome (1, 5, 9, 12, 18). Approximately 80% of Ureaplasma isolates from the vagina are U. parvum, while U. urealyticum is isolated less often, with frequencies ranging from only 7% to 30% (1, 6, 17, 18). Coinfection also occurs in some women (1, 17, 18), consistent with our results regarding distribution. Similarly, approximately 80% of Ureaplasma isolates from the amniotic fluid are U. parvum; U. urealyticum is isolated less often, with frequencies of approximately 20% (12, 17). These results suggest that vaginal U. parvum and U. urealyticum invade the amniotic cavity in equal frequencies.
Our results contradicted those of an earlier study (1) with regard to pregnancy outcome. Abele-Horn et al. (1) examined the association of vaginal colonization by U. parvum and U. urealyticum with pregnancy outcome in 174 women (148 with U. parvum and 26 with U. urealyticum) in whom Ureaplasma was isolated as the sole pathogenic microorganism. In their study, all specimens were collected after admission to hospital for delivery, and women colonized with vaginal Escherichia coli or other gram-negative bacteria, hemolytic streptococci, peptostreptococci, peptococci, N. gonorrhoeae, Candida albicans, Trichomonas vaginalis, C. trachomatis, Bacteroides spp., M. hominis, or Gardnerella vaginalis were excluded from the study population. Preterm birth was reported to occur more frequently in women with U. urealyticum than in those with U. parvum at <30 weeks of gestation (16/26 [62%] versus 24/148 [16%], respectively; P < 0.001) and at <37 weeks of gestation (20/26 [77%] versus 52/148 [35%], respectively; P < 0.05), suggesting that U. urealyticum has a more adverse effect on pregnancy outcome (1). In our prospective cohort study using a representative general population enrolled in early pregnancy, the pregnancies of 16 (3.6%) of 447 women with U. parvum and 1 (1.5%) of 67 women with U. urealyticum ended in late abortion or preterm birth at <34 weeks of gestation. Thus, our study population differed markedly from that examined by Abele-Horn et al. (1). Further, as we did not examine the presence or absence of bacterial vaginosis in our study population and because women with bacterial vaginosis may have been excluded in the study by Abele-Horn et al. (1), it is possible that other microorganisms not examined in this study were responsible for the conflicting results.
The association of vaginal M. hominis with preterm birth has been reported as positive in some studies (8, 23) but not in others (22). M. hominis was detected in 4 of 21 women (19.0%) in the preterm birth group and in 94 of 856 women (11.0%) in the control group and was not a risk factor for late abortion or preterm birth at <34 weeks of gestation in the present study. As vaginal colonization with M. hominis seemed to be independent of that with U. parvum, it is possible that M. hominis will become an independent risk factor for early preterm birth in future larger studies. Indeed, in our previous study (23), M. hominis was cultured from the vagina in 8 (6.8%) of 118 women, 7 (3.1%) of 224 women, and 47 (2.9%) of 1,616 women who gave birth at 22 to 32 weeks of gestation, at 33 to 36 weeks of gestation, and at or after 37 weeks of gestation, respectively, and was an independent risk factor for preterm birth at <33 weeks of gestation.
Effective antibiotics for Ureaplasma species, such as clarithromycin, were used in only 28 women with C. trachomatis infection. Twenty-one women with U. parvum who were coinfected with C. trachomatis (Table 3) did not develop late abortion or preterm birth at <34 weeks of gestation. Thus, among the 456 women with U. parvum infection, 0 of 21 (0.0%) women who were treated incidentally with effective antibiotics, versus 16 of 435 (3.7%) women who were not treated with effective antibiotics, developed late abortion or preterm birth at <34 weeks of gestation. Although these figures did not reach the level of significance, it is possible that antibiotics such as clarithromycin played a role in preventing late abortion or early preterm birth in these women infected with U. parvum. Further randomized and controlled studies are necessary to confirm this possibility.
Unexpectedly, the use of antibiotics that are not effective against mycoplasmas, such as penicillin and cefatrizine, was an independent risk factor for late abortion or early preterm birth in the present study. As the majority of women who were treated with these antibiotics were suffering from inflammatory diseases, it may be that these diseases that required antibiotic use, rather than the antibiotics themselves, were associated with late abortion or early preterm birth.
In conclusion, this prospective cohort study of women at an early stage of pregnancy and representative of the general population demonstrated that U. parvum, but not U. urealyticum, is an independent risk factor for late abortion or early preterm birth, apparently conflicting with the results of a previous study (1). Further studies are required to determine the reason for this discrepancy.
Acknowledgments
We thank the physicians at Tenshi Hospital, Hokkaido Social Insurance Central General Hospital, Kushiro Red Cross Hospital, Oji General Hospital, Kucchan-Kosei General Hospital, Hakodate Central General Hospital, Bibai Rousai Hospital, Sapporo Toho Hospital, and Iwaki Maternity Clinic for enrolling pregnant women in this prospective study.
REFERENCES
- 1.Abele-Horn, M., C. Wolff, P. Dressel, F. Pfaff, and A. Zimmermann. 1997. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J. Clin. Microbiol. 35:1199-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, M. M., L. D. Elam-Evans, H. G. Wilson, and D. A. Gilbertz. 2000. Rates of and factors associated with recurrence of preterm delivery. JAMA 283:1591-1596. [DOI] [PubMed] [Google Scholar]
- 3.Cassell, G. H., K. B. Waites, H. L. Watson, D. T. Crouse, and R. Harasawa. 1993. Ureaplasma urealyticum intrauterine infection: role in prematurity and disease in newborns. Clin. Microbiol. Rev. 6:69-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Challis, J. R., S. J. Lye, W. Gibb, W. Whittle, F. Patel, and N. Alfaidy. 2001. Understanding preterm labor. Ann. N. Y. Acad. Sci. 943:225-234. [DOI] [PubMed] [Google Scholar]
- 5.Deguchi, T., T. Yoshida, T. Miyazawa, M. Yasuda, M. Tamaki, H. Ishiko, and S. Maeda. 2004. Association of Ureaplasma urealyticum (biovar 2) with nongonococcal urethritis. Sex. Transm. Dis. 31:192-195. [DOI] [PubMed] [Google Scholar]
- 6.Domingues, D., L. Tavora Tavira, A. Duarte, A. Sanca, E. Prieto, and F. Exposto. 2002. Ureaplasma urealyticum biovar determination in women attending a family planning clinic in Guinea-Bissau, using polymerase chain reaction of the multiple-banded antigen gene. J. Clin. Lab. Anal. 16:71-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerber, S., Y. Vial, P. Hohlfeld, and S. S. Witkin. 2003. Detection of Ureaplasma urealyticum in second trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J. Infect. Dis. 187:518-521. [DOI] [PubMed] [Google Scholar]
- 8.Harrison, H. R., E. R. Alexander, L. Weinstein, M. Lewis, M. Nash, and D. A. Sim. 1983. Cervical Chlamydia trachomatis and mycoplasmal infections in pregnancy. Epidemiology and outcomes. JAMA 250:1721-1727. [PubMed] [Google Scholar]
- 9.Heggie, A. D., D. Bar-Shain, B. Boxerbaum, A. A. Fanaroff, M. A. O'Riordan, and J. A. Robertson. 2001. Identification and quantification of ureaplasmas colonizing the respiratory tract and assessment of their role in the development of chronic lung disease in preterm infants. Pediatr. Infect. Dis. J. 20:854-859. [DOI] [PubMed] [Google Scholar]
- 10.Hillier, S. L., M. A. Krohn, N. B. Kiviat, D. H. Watts, and D. A. Eschenbach. 1991. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am. J. Obstet. Gynecol. 165:955-961. [DOI] [PubMed] [Google Scholar]
- 11.Horner, P. J., C. B. Gilroy, B. J. Thomas, R. O. M. Naidoo, and D. Taylor-Robinson. 1993. Association of Mycoplasma genitalium with acute non-gonococcal urethritis. Lancet 342:582-585. [DOI] [PubMed] [Google Scholar]
- 12.Kim, M., G. Kim, R. Romero, S. S. Shim, E. C. Kim, and B. H. Yoon. 2003. Biovar diversity of Ureaplasma urealyticum in amniotic fluid: distribution, intrauterine inflammatory response and pregnancy outcomes. J. Perinat. Med. 31:146-152. [DOI] [PubMed] [Google Scholar]
- 13.Koike, T., H. Minakami, A. Iuzmi, T. Watanabe, S. Matsubara, and I. Sato. 2002. The recurrence risk of preterm birth due to preeclampsia. Gynecol. Obstet. Investig. 53:22-27. [DOI] [PubMed] [Google Scholar]
- 14.Kong, F., Z. Ma, G. James, S. Gordon, and G. L. Gilbert. 2000. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J. Clin. Microbiol. 38:1175-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lockwood, C. J., and E. Kuczynski. 1999. Markers of risk for preterm delivery. J. Perinat. Med. 27:5-20. [DOI] [PubMed] [Google Scholar]
- 16.Manhart, L. E., C. W. Critchlow, K. K. Holmes, S. M. Dutro, D. A. Eschenbach, C. E. Stevens, and P. A. Totten. 2003. Mucopurulent cervicitis and Mycoplasma genitalium. J. Infect. Dis. 187:650-657. [DOI] [PubMed] [Google Scholar]
- 17.Martinez, M. A., A. Ovalle, A. Santa-Cruz, B. Barrera, R. Vidal, and R. Aguirre. 2001. Occurrence and antimicrobial susceptibility of Ureaplasma parvum (Ureaplasma urealyticum biovar 1) and Ureaplasma urealyticum (Ureaplasma urealyticum biovar 2) from patients with adverse pregnancy outcomes and normal pregnant women. Scand. J. Infect. Dis. 33:604-610. [DOI] [PubMed] [Google Scholar]
- 18.Povlsen, K., P. Thorsen, and I. Lind. 2002. Relationship of Ureaplasma urealyticum biovars to the presence or absence of bacterial vaginosis in pregnant women and to the time of delivery. Eur. J. Clin. Microbiol. Infect. Dis. 20:65-67. [DOI] [PubMed] [Google Scholar]
- 19.Robertson, J. A., G. W. Stemke, J. W. Davis, Jr., R. Harasawa, D. Thirkell, F. Kong, M. C. Shepard, and D. K. Ford. 2002. Proposal of Ureaplasma parvum sp.nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974)Robertson et al. 2001. Int. J. Syst. Evol. Microbiol. 52:587-597. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro, S., M. C. McCormick, B. H. Starfield, J. P. Krischer, and D. Bross. 1980. Relevance of correlates of infant deaths for significant morbidity at 1 year of age. Am. J. Obstet. Gynecol. 136:363-373. [DOI] [PubMed] [Google Scholar]
- 21.Sperling, R. S., E. Newton, and R. S. Gibbs. 1988. Intraamniotic infection in low birth-weight infants. J. Infect. Dis. 157:113-117. [DOI] [PubMed] [Google Scholar]
- 22.Sweet, R. L., D. V. Landers, C. Walker, and J. Schachter. 1987. Chlamydia trachomatis infection and pregnancy outcome. Am. J. Obstet. Gynecol. 156:824-833. [DOI] [PubMed] [Google Scholar]
- 23.Usui, R., A. Ohkuchi, S. Matsubara, A. Izumi, T. Watanabe, M. Suzuki, and H. Minakami. 2002. Vaginal lactobacilli and preterm birth. J. Perinat. Med. 30:458-466. [DOI] [PubMed] [Google Scholar]
- 24.Yoon, B. H., R. Romero, J. S. Park, J. W. Chang, Y. A. Kim, J. C. Kim, and K. S. Kim. 1998. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am. J. Obstet. Gynecol. 179:1254-1260. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida, T., S. Maeda, T. Deguchi, T. Miyazawa, and H. Ishiko. 2003. Rapid detection of Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma parvum, and Ureaplasma urealyticum organisms in genitourinary samples by PCR-microtiter plate hybridization assay. J. Clin. Microbiol. 41:1850-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]