Abstract
Streptomyces ramocissimus, the producer of elongation factor Tu (EF-Tu)-targeted antibiotic kirromycin, contains three divergent tuf-like genes, with tuf1 encoding regular kirromycin-sensitive EF-Tu1; the functions of tuf2 and tuf3 are unknown. Analysis of the tuf gene organization in nine producers of kirromycin-type antibiotics revealed that they all contain homologues of tuf1 and sometimes of tuf3 but that tuf2 was found in S. ramocissimus only. The tuf2-flanking regions were sequenced, and the two tuf2-surrounding open reading frames were shown to be oriented in opposite directions. In vivo transcription analysis of the tuf2 gene displayed an upstream region with bidirectional promoter activity. The transcription start site of tuf2 was located approximately 290 nucleotides upstream of the coding sequence. Very small amounts of tuf2 transcripts were detected in both liquid- and surface-grown cultures of S. ramocissimus, consistent with the apparent absence of EF-Tu2 in total protein extracts. The tuf2 transcript level was not influenced by the addition of kirromycin to exponentially growing cultures. To assess the function of S. ramocissimus EF-Tu2, the protein was overexpressed in Streptomyces coelicolor LT2. This strain is a J1501 derivative containing His6-tagged EF-Tu1 as the sole EF-Tu species, which facilitated the separation of EF-Tu2 from the interfering EF-Tu1. S. ramocissimus EF-Tu1 and EF-Tu2 were indistinguishable in their ability to stimulate protein synthesis in vitro and exhibited the same kirromycin sensitivity, which excludes the possibility that EF-Tu2 is directly involved in the kirromycin resistance mechanism of S. ramocissimus.
Elongation factor Tu (EF-Tu), a member of the family of GTPase switch proteins, plays a pivotal role in the elongation process of bacterial protein synthesis (for a review see reference 15). The GTP-bound form of EF-Tu is responsible for delivery of aminoacyl-tRNA to the mRNA-programmed ribosomal A site. Cognate codon-anticodon recognition triggers the GTPase center on EF-Tu, causing the dissociation of inactive EF-Tu·GDP from the ribosome. Reactivation of the factor occurs via a nucleotide exchange reaction catalyzed by EF-Ts. EF-Tu is specifically affected by four different types of antibiotics of which kirromycin is the first identified and best studied (for references see reference 25). The binding of this antibiotic to EF-Tu still allows the factor to interact sequentially with aminoacyl-tRNA and the ribosomal A site. However, after GTP hydrolysis, EF-Tu·GDP is no longer ejected from the ribosome, thus immobilizing this and all following ribosomes on the mRNA, which explains the recessive character of kirromycin resistance in a mixed population of resistant and sensitive EF-Tu species. Certain error-restrictive mutations in ribosomal protein S12 (encoded by rpsL) overcome this recessivity; the mutant ribosomes will preferentially use the kirromycin-resistant EF-Tu for translation (33).
Polyketide antibiotic kirromycin and related compounds, called elfamycins, are produced by actinomycetes. These gram-positive mycelial soil bacteria undergo a complex process of morphological differentiation and produce a wide variety of secondary metabolites (12). Antibiotic production is generally confined to stationary phase in liquid culture and usually coincides with the onset of morphological differentiation in surface-grown cultures. Various mechanisms are exploited by antibiotic-producing microorganisms to protect themselves from the toxic action of their own products (5); these include the use of an efficient drug efflux system, intracellular storage of the antibiotic in an inactive form, modification of an otherwise sensitive target, and (temporary) expression of a resistant target. The mechanism used by producers of kirromycin-type antibiotics to protect themselves against their own products is only partially known; some producers contain an intrinsically kirromycin-resistant EF-Tu (4, 9).
Kirromycin producer Streptomyces ramocissimus contains three divergent tuf genes, which are designated tuf1, tuf2, and tuf3 and which code for EF-Tus that are surprisingly heterogeneous: EF-Tu2 displays 88% amino acid identity with EF-Tu1, and EF-Tu3 shows only about 65% amino acid identity with both EF-Tu1 and EF-Tu2 (37). The tuf1 gene encodes the major, kirromycin-sensitive EF-Tu (37) and is the promoter-distal gene in the rpsL operon, which also includes the genes for ribosomal proteins S12 (rpsL) and S7 (rpsG) and EF-G (fus). tuf1 is transcribed at a very high level during exponential growth from both the rpsL operon promoter and a tuf1-specific promoter (32). The roles of S. ramocissimus tuf2 and tuf3 are not yet clear; the gene products could not be detected under normal growth conditions, and overexpression in Escherichia coli yielded inactive products, deposited in inclusion bodies (37). Studies of the genetically well-characterized Streptomyces coelicolor revealed that this strain contains both tuf1 and tuf3 homologues (35) but lacks a tuf2 equivalent. Transcription of S. coelicolor tuf3 is subject to positive stringent control (36), and the tuf3 gene product can function as a real EF-Tu in a Streptomyces in vitro translation system (24).
The lack of tuf2 homologues in all Streptomyces species studied so far (35, 37; L. N. Olsthoorn-Tieleman, unpublished results) and the apparent absence of a tuf2 gene product in S. ramocissimus (37) raised the question of whether tuf2 encodes an EF-Tu with a general or specialized function. In this paper we provide the sequences of the flanking genes of S. ramocissimus tuf2 and perform a transcriptional analysis of tuf2 and describe the overexpression and purification of its gene product. The actual functioning of EF-Tu2 as an EF-Tu and its interaction with kirromycin were studied by using a recently developed Streptomyces in vitro translation system (24).
MATERIALS AND METHODS
Bacterial strains, culture conditions, and vectors.
Elfamycin-producing Actinomyces strains used are listed in Table 1. E. coli JM101 (26) and ET12567 (18), grown and transformed by standard procedures (26), were used for routine subcloning. All DNA manipulations were performed by following standard protocols given by Sambrook et al. (26). pUSRT2 was constructed by cloning the 2.9-kb BamHI fragment from pASRT2 (37) in BamHI-digested pUC18 (41). pUSRT2-U1 and pUSRT2-5 contain the 0.4-kb BamHI/SacII fragment of pUSRT2-1 (37) cloned into the BamHI/SacI sites of pUC18 and the 0.6-kb BamHI/BclI fragment of pUSRT2-1 cloned in BamHI-digested pUC18, respectively.
TABLE 1.
Elfamycin-producing actinomycetes used in this study
| Straina | Antibiotic produced | tuf gene(s) | EF-Tu phenotypesb (reference) |
|---|---|---|---|
| S. cinnamoneus Tü89 | Kirrothricin | tuf1 | Kirr (4, 9) |
| S. collinus Tü365 | Kirromycin | tuf1, tuf3 | Kirs (9, 20) |
| S. diastatochromogenes Tü1062 | Kirromycin | tuf1 | Kirs (9) |
| S. filipinensis NRRL 11044 | Heneicomycin | tuf1, tuf3 | Not known |
| S. fradiae Tü1222 | Kirromycin | tuf1, tuf3 | Kirs (9) |
| S. goldiniensis ATCC 21386 | Aurodox | tuf1, tuf3 | Kirs (9) |
| N. lactamdurans ATCC 27382 | Efrotomycin | tuf1 | Kirr (9) |
| S. ramocissimus CBS 190.6 | Kirromycin | tuf1, tuf2, tuf3 | Kirs (9, 37) |
| Streptomyces sp. strain NNRL 15496 | SB22484 factors 1-4 | tuf1 | Not known |
S., Streptomyces; N., Nocardia.
Kirr, kirromycin resistant; Kirs, kirromycin sensitive.
S. ramocissimus strains B7 and CBS 190.6 (wild-type), both obtained from Gist-brocades NV (Delft, The Netherlands), were grown as liquid cultures in S medium for the isolation of chromosomal DNA and EF-Tu1. SFM medium (containing, per liter, 20 g of mannitol, 20 g of soy flour, and 20 g of agar dissolved in tap water and autoclaved twice) is a modified version of that reported by Hobbs et al. (10) and was used to make high-titer spore suspensions of S. ramocissimus B7. Conditions for reproducibly dispersed growth of S. ramocissimus B7 in NMMP medium (11) containing 1% (wt/vol) glucose were as described by Tieleman et al. (32). Kirromycin response was induced in liquid cultures by adding kirromycin to a final concentration of 5 μM at an optical density at 450 nm (OD450) of 0.6, after which the cultures were allowed to continue growing. S. ramocissimus B7 spores were plated on cellophane disks on AMMAT medium (32) to facilitate the harvesting of the mycelium for RNA isolation. Morphology of the surface-grown cultures was determined by phase-contrast microscopy, while kirromycin secretion into the agar was detected by using E. coli JM101 as the indicator strain.
S. coelicolor M145 (11) was obtained from the John Innes Centre, Norwich, United Kingdom; the construction of S. coelicolor J1501 derivative LT2 is described by Olsthoorn-Tieleman et al. (24). S. coelicolor strains were grown in YEME medium (11) and on R5 plates (11), when necessary supplemented with 1% (wt/vol) mannitol, 7.5 μg of uracil/ml, and 50 μg of histidine/ml, as described previously (11). MSP (2% [wt/vol] mannitol, 2% [wt/vol] soy peptone) was used to grow S. coelicolor LT2 for in vitro translation experiments. Protoplast preparation and transformation were performed as described by Hopwood et al. (11).
Southern hybridization.
Chromosomal DNA from the different elfamycin-producing actinomycetes was isolated from liquid cultures grown in S medium according to the method described by Hopwood et al. (11) and digested with the appropriate enzymes. Southern blotting and hybridization were performed under conditions described previously (24). The 1.0-kb MluI/NcoI, 1.0-kb NarI, and 0.65-kb SalI fragments of S. ramocissimus tuf1, tuf2, and tuf3, respectively, were used as probes after 32P labeling by random priming (7). The hybridization stringency was set at 6× SSC (20× SSC is 3 M NaCl plus 0.3 M sodium citrate, pH 7)-0.6% sodium dodecyl sulfate (SDS) at 65°C, and final washes were with 2× SSC-0.1% SDS at the same temperature.
DNA sequence analysis.
The nucleotide sequence of the tuf2 downstream region was determined by dideoxy sequencing using the Pharmacia T7 sequencing kit and single-stranded DNA templates derived by subcloning DNA fragments from pUSRT2 in M13mp18 and M13mp19 (41). Synthetic oligonucleotides were used to close gaps in the sequence. Sequence analyses were performed using the Wisconsin GCG package (6). BLAST search engines BlastN, BlastP, and BlastX (2) were used to perform database searches.
Promoter probing experiments.
pISRT2xylE-1 and pISRT2xylE-1i were constructed by cloning the BamHI/SacII fragment of tuf2-containing plasmid pUSRT2-1 (37) via pUC18 into xylE-based promoter probe vector pIJ4083 (4a) in both orientations. Transformants containing either pISRT2xylE-1 or pISRT2xylE-1i were grown on R5 in the presence of 5 μg of thiostrepton (a gift from Squibb, Princeton, N.J.)/ml. Plates were sprayed with 0.5 M catechol after 2 to 5 days of growth, and the amount of catechol converted into yellow 2-hydroxymuconic semialdehyde by catechol 2,3-dioxygenase was assessed visually.
Nuclease S1 protection assays.
RNA was isolated from liquid- and surface-grown S. ramocissimus B7 cultures as described by Hopwood et al. (11), except that DNase I treatment was used in addition to salt precipitation to eliminate DNA from the nucleic acid preparations. RNA concentrations were determined spectrophotometrically, and the quality of the preparations was checked by gel electrophoresis. Hybridization of 30 μg of RNA with the appropriate DNA probes was performed in sodium trichloroacetate-based buffer (22) at 45°C overnight after denaturation at 70°C for 15 min. All subsequent steps were carried out as described previously (30) with an excess of probe. The 510-bp Bsp120I/PvuII fragment from pUSRT2-5, 32P end labeled at the 5′ end of the Bsp120I site, was used for mapping tuf2 transcripts. The tuf1 and tuf3 genes have no homology with this probe, thus excluding the possibility that these mRNAs would contribute to the protection pattern. The 600-bp BamHI/PvuII fragment from pUSRT2-U1, 32P end labeled at the 5′ end of the BamHI site, was used for mapping orfQ transcripts. Products were analyzed on denaturing 6% polyacrylamide gels, using 32P-end-labeled HpaII fragments of pBR322 as size markers.
Construction of tuf2 overexpression vector pISRT2-1.
The S. ramocissimus tuf2 gene was isolated from plasmid pUSRT2-3 (37) as a 1.5-kb BamHI/HgiAI fragment and ligated into the BamHI/PstI sites of vector pUC18 (41), thereby creating pUSRT2-4. The tuf2 gene was isolated from this vector as a BamHI-HindIII fragment and cloned into the corresponding restriction sites of pIJ4070 (a kind gift from M. J. Bibb, Norwich, United Kingdom), resulting in pUSRT2ermE. In this way the gene was placed under the control of the strong and constitutive Streptomyces ermE promoter. Finally pISRT2-1 was constructed by inserting the tuf2 gene and upstream ermE promoter as a 1.7-kb KpnI/PstI fragment into the KpnI/PstI sites of Streptomyces high-copy-number vector pIJ487 (40).
Production and purification of S. ramocissimus EF-Tu species.
Exponentially growing S. ramocissimus B7, cultured in S medium, was used as a source for EF-Tu1. EF-Tu2 overproduction was achieved by inoculation of fresh spores from S. coelicolor LT2 harboring pISRT2-1 into YEME containing 5 μg of thiostrepton/ml and growth for 64 h at 30°C. The mycelium was washed twice with ice-cold TuGly buffer (50 mM Tris-HCl [pH 7.6], 7 mM MgCl2, 60 mM NH4Cl, 1 mM dithiothreitol, 10 μM GDP, 10 μM phenylmethylsulfonyl fluoride, 10% [vol/vol] glycerol) and kept frozen at −80°C. For the purification of S. ramocissimus EF-Tu1 and EF-Tu2, the method described by Olsthoorn-Tieleman et al. (24) for S. coelicolor EF-Tu1 was used with the following modifications. Before applying the EF-Tu2-containing S100 fraction on the DEAE-Sepharose column, it was first subjected to chromatography on a Ni2+-nitrilotriacetic acid (NTA) agarose column to remove S. coelicolor His6-tagged EF-Tu1 (EF-Tu1His). In addition to the described purification protocol, the EF-Tu1 and EF-Tu2 protein solutions were applied to an fast protein liquid chromatography Mono Q column and eluted using a linear gradient of KCl (140 to 500 mM) in TuGly buffer for further purification. The protein solutions were concentrated over Amicon Centriflo ultrafiltration cones and stored at −80°C. Protein concentrations were determined with Coomassie protein assay reagent (Pierce) by using bovine serum albumin as a standard.
SDS-PAGE and Western analysis.
Protein expression and purification was monitored by SDS-polyacrylamide gel electrophoresis (PAGE) using the Mini Protean II system (Bio-Rad) and Western blotting conducted as described by Vijgenboom et al. (37), using a 1:5,000 dilution of antibodies. Nonradioactive detection was performed using Western blot chemiluminescence reagent (NEN Life Science Products). The rabbit polyclonal antibodies used were raised against the S. ramocissimus tuf3 gene product expressed in E. coli (anti-EF-Tu3) and against S. ramocissimus EF-Tu1 (anti-EF-Tu1) (37).
In vitro translation assays in the absence and presence of antibiotics.
S30 cell extracts of S. coelicolor LT2 (if necessary harboring pISRT2-1 or pIJ487), from which His6-tagged EF-Tu1 was removed by treatment with Ni2+-NTA-agarose beads, were obtained as described by Olsthoorn-Tieleman et al. (24). The extracts, supplemented with purified EF-Tu species and antibiotics at various concentrations, were incubated in translation buffer (final concentrations: 50 mM Tris-HCl [pH 7.6], 9 mM MgCl2, 60 mM NH4Cl, 1 mM dithiothreitol, 1 mM ATP, 1 mM GTP, 6 mM phosphoenolpyruvate, 50 μg of pyruvate kinase/ml, 0.1 mg of poly[U]/ml, 0.2 mg of E. coli tRNA/ml, and 13.2 μM [14C]Phe [specific activity, 531 mCi/mmol]) at 30°C for 10 min. The total volume was 50 μl. The reaction was stopped by the addition of 15 μl of 1 M NaOH and further incubation at 30°C for 10 min. After precipitation with 5% (wt/vol) trichloroacetic acid and filtration, the incorporation of [14C]Phe was determined by liquid scintillation counting.
Antibiotics kirromycin and pulvomycin were generous gifts from Gist-brocades NV and A. Parmeggiani (Palaiseau, France), respectively. GE2270 A was isolated by V. G. Möhrle (Leiden University, Leiden, The Netherlands) as described by Selva et al. (28).
Nucleotide sequence accession number.
The sequence of the tuf2 downstream region has been deposited in the GenBank under accession no. AY062294.
RESULTS
Number and character of tuf genes in elfamycin-producing actinomycetes.
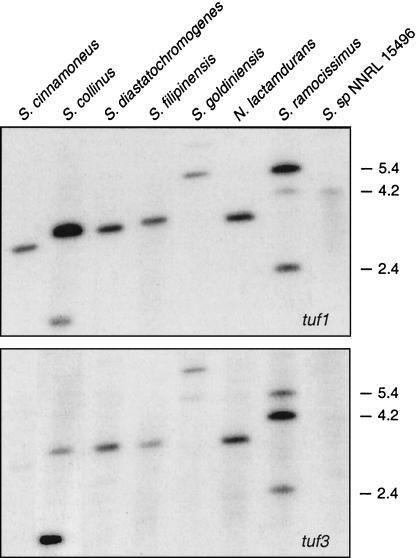
The presence of three tuf genes in S. ramocissimus, in contrast to the two tuf1 and tuf3 homologues in several other streptomycetes (35, 37; L. N. Olsthoorn-Tieleman, unpublished results), raised the question of whether the occurrence of tuf2 is a particular feature of producers of kirromycin-type antibiotics. Therefore, Southern hybridizations employing different digests of chromosomal DNA from several elfamycin producers were carried out using internal fragments of the three S. ramocissimus tuf genes as probes. Hybridization with the tuf1 probe revealed three hybridizing fragments in BamHI- and PstI-digested S. ramocissimus DNA (Fig. 1, top). As described by Vijgenboom et al. (37), the two strong signals of 5.4 and 2.4 kb correspond to tuf1 and tuf2, respectively, while the weaker signal of 4.2 kb indicates the presence of tuf3. In the restriction digests of the other actinomycetes only one strong signal was observed; in some lanes an additional weaker signal was also present. An identical hybridization pattern was observed when the tuf2 probe was used (data not shown), indicating that all actinomycetes investigated possess a tuf1 homologue and that only S. ramocissimus contains the additional tuf2 gene. Hybridization with the internal fragment of S. ramocissimus tuf3 (Fig. 1, bottom) revealed that Streptomyces collinus and Streptomyces goldiniensis contain a close homologue of this divergent tuf gene. The tuf1- and tuf3-hybridizing fragments of Streptomyces filipinensis seemed to have migrated to slightly different positions, and inspection of BglII- and SphI-digested DNA confirmed the presence of two distinct tuf genes (data not shown). The BamHI- and PstI-restricted genomic DNAs of Streptomyces fradiae (not shown in Fig. 1) and S. goldiniensis exhibited the same hybridization patterns with both probes, indicating that the former strain also contains tuf1 and tuf3 homologues. The established number and character of tuf genes in each elfamycin-producing strain were confirmed by similar Southern analyses using other restriction digests.
FIG. 1.
Southern analysis of the number of tuf genes in elfamycin-producing actinomycetes. Chromosomal DNA of the producers of kirromycin-like antibiotics was digested with BamHI and PstI, except for Streptomyces sp. strain NNRL 15496 DNA, which was restricted with BglII and SphI, and probed with internal sequences of S. ramocissimus tuf1 (top) and tuf3 (bottom). The positions and approximate sizes (in kilobases) of the S. ramocissimus fragments that hybridize with the probes are indicated.
Summarizing, the presence of tuf2 seems to be restricted to S. ramnocissimus B7 (Table 1), suggesting a specialized function of the gene for this specific microorganism. Since B7 is a mutant strain that was isolated during selection for increased kirromycin production, the number of tuf genes in the parental strain, CBS 190.6, was also determined by Southern blot experiments (data not shown). Exactly the same hybridization pattern was observed, indicating that the presence of three tuf genes in S. ramocissimus B7 is not an artifact due to the screening procedure.
The genes flanking S. ramocissimus tuf2 are not involved in protein biosynthesis.
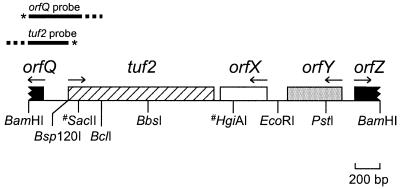
In most bacteria the genes for EF-Tu are found in operons together with genes encoding other components of the translational apparatus, such as ribosomal proteins, EF-G, and tRNAs (17). Close inspection of the DNA sequence preceding S. ramocissimus tuf2 as published by Vijgenboom et al. (37) (GenBank accession no. X67058) revealed the presence of an incomplete open reading frame (ORF) with protein-coding character, designated orfQ, in the orientation opposite to that of tuf2 (Fig. 2). Comparison of the putative gene product with proteins in the databases revealed significant homology (60% amino acid identity in an overlap of 40 amino acids [aa]) to the N-terminal part of a putatively secreted protein of S. coelicolor (GenBank accession no. CAC14349).
FIG. 2.
Organization of the S. ramocissimus tuf2 region. A restriction map of the insert from pUSRT2 is shown. Only the SacII and HgiAI restriction sites relevant to the text are shown (#). The probes used for S1 nuclease mapping of tuf2 and orfQ transcripts (asterisks, 32P-labeled 5′ ends) are shown above the restriction map. Dashed lines, nonhomologous pUC18-derived extensions.
To further study the genetic organization of S. ramocissimus tuf2, the sequence of a 1.3-kb region downstream of tuf2 was determined (GenBank accession no. AY062294; nucleotide [nt] 1 corresponds to nt 1500 of X67058). This revealed two complete ORFs with protein-coding character, as well as the beginning of a third (Fig. 2). The first ORF (orfX; nt 448 to 65) is located 48 nt downstream of and in the opposite orientation to tuf2. orfX encodes a protein of 127 aa with a calculated molecular mass of 13.4 kDa; this protein shows a significant degree of amino acid sequence identity to anti-sigma factor antagonists, the most similar one being from S. coelicolor (GenBank accession no. CAC14346; 67% amino acid sequence identity in an overlap of 116 aa). Intriguingly, the last codon of orfX is the rare TTA leucine codon, potentially subject to bldA-dependent translational control (16). The second ORF, designated orfY, spans from nt 1054 to 617 and is also oriented in the opposite direction to tuf2. Its predicted product of 145 aa (16.3 kDa) shows homology to transcription regulators of the MarR family such as one from S. coelicolor (GenBank accession no. CAC17527), with 32% amino acid sequence identity in an overlap of 125 aa. The third downstream ORF of >226 nt, orfZ, starts at nt 1159 and runs into the same direction as tuf2; its 3′ end appears to be outside of the sequenced region. Searches of the databases revealed the high similarity of the product to aldehyde dehydrogenases (50% amino acid sequence identity in an overlap of 68 aa with Pseudonocardia sp. strain K1 succinate semialdehyde dehydrogenase; GenBank accession no. CAC10505).
These data reveal that in contrast to the operon organization of other tuf genes, no genes for components of the translational apparatus are found in the immediate vicinity of tuf2. The opposite orientations of the flanking genes orfQ and orfX with respect to tuf2 suggest that tuf2 is located in a single transcription unit.
A bidirectional promoter region precedes S. ramocissimus tuf2.
The absence of detectable tuf2 expression in total protein extracts of S. ramocissimus (37) suggests that tuf2 is expressed at very low levels under normal growth conditions or that tuf2 might even be a silent gene. To distinguish between these possibilities, we decided to determine if the tuf2 upstream region displays promoter activity and if tuf2 transcripts could be observed with the sensitive S1 nuclease mapping technique.
To determine the presence and approximate location of the promoter(s) in the tuf2-orfQ intergenic region, we used multicopy promoter-probe vector pIJ4083 (4a), which contains the promoterless xylE as the reporter gene. The tuf2 upstream region was cloned as a 410-bp BamHI/SacII fragment in both orientations in pIJ4083, with the start of tuf2 (pISRT2xylE-1) or of orfQ (pISRT2xylE-1i) proximal to xylE. S. coelicolor M145 transformants containing either pISRT2xylE-1 or pISRT2xylE-1i yielded yellow aerial hyphae when sprayed with catechol after at least 3 days of growth on R5 agar plates, indicative of the presence of at least two divergent promoters in the BamHI/SacII fragment. Control transformants harboring pIJ4083 without an insert displayed no yellow coloring upon being sprayed with catechol.
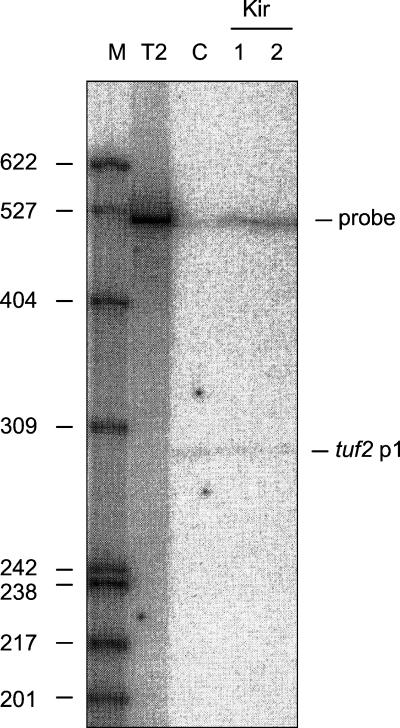
To map the possible transcription start sites of both tuf2 and orfQ, nuclease S1 protection experiments were carried out with RNA isolated from exponentially growing S. ramocissimus (for details see reference 32). The 510-bp Bsp120I/PvuII fragment from pUSRT2-5 and the 600-bp BamHI/PvuII fragment from pUSRT2-U1, uniquely labeled at their 5′ ends, were used as probes to detect tuf2 and orfQ transcripts, respectively; in both cases the nonhomologous pUC18-derived extension allowed discrimination between full-length RNA-protected fragments and the reannealed probe. The tuf2 probe contains no tuf2 coding sequence, because unwanted detection of the abundantly present and almost identical tuf1 transcripts would then likely occur. Only after prolonged exposure of the autoradiograms were tuf2 and orfQ transcripts detected (tuf2 data in Fig. 3, right, lane C; orfQ data not shown). The putative transcriptional initiation sites for tuf2 and orfQ were identified around nt 20 and 290 (tuf2p1 and orfQp1, respectively). Inspection of the DNA sequences preceding these start sites revealed no similarity to the proposed consensus sequence 5′TTGACR-16 to 18 nt-TAGRRT3′, where R is G or A, for promoters recognized by the major RNA polymerase holoenzyme of Streptomyces (31).
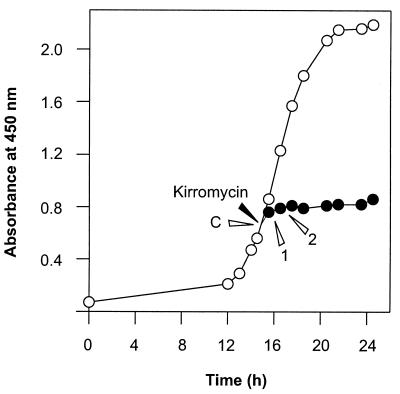
FIG. 3.
Transcription of tuf2 after kirromycin addition. (Left) Growth curve of S. ramocissimus B7 with (•) and without (○) the addition of kirromycin (5 μM) at an OD450 of 0.6. Addition of the antibiotic to a final concentration of 25 μM resulted in a similar growth inhibition. (Right) S1 nuclease protection analysis of tuf2 transcripts in RNA isolated at the time points indicated in the left panel (C, 1, and 2). Probe, reannealed tuf2 probe; tuf2p1, transcripts initiated at tuf2p1. In lane T2, the location of the 510-nt full-length tuf2 probe can be seen. Lane M, end-labeled HpaII-digested pBR322 size markers (sizes are given in nucleotides).
Growth phase-dependent transcription of tuf2 in surface-grown cultures producing kirromycin.
Surface-grown cultures of streptomycetes show complete morphological differentiation. To study tuf2 transcription during the life cycle of S. ramocissimus B7 and to assess the relationship between tuf2 transcription and kirromycin production, S1 nuclease protection experiments were performed with RNA isolated from surface-grown cultures at different stages of growth by using the tuf2 probe as described above. Barely detectable levels of tuf2 transcripts were observed during the formation of vegetative hyphae, with transcription initiating from the same start site (tuf2p1) as found for the liquid culture (data not shown). No transcripts were present during aerial mycelium development and kirromycin production, but tuf2 transcripts reappeared during the sporulation stage. Thus, tuf2 transcription from tuf2p1 shows the same growth phase dependence as S. ramocissimus tuf1 (32), albeit at a much lower level.
Since a role for an EF-Tu-like protein in the kirromycin resistance mechanism of S. ramocissimus is imaginable, we analyzed how tuf2 transcription responded to the addition of kirromycin. The antibiotic (final concentration, 5 μM) was added to exponentially growing S. ramocissimus B7 liquid cultures at an OD450 of 0.6, after which the cultures continued growing (albeit at a slow rate) and stopped growing about 2 h later (Fig. 3, left). S1 nuclease protection experiments with RNA isolated from these cultures 1 and 2 h after addition of the inhibitor revealed that the level of tuf2 transcripts remained unaltered (Fig. 3, right). Thus, tuf2 transcription from tuf2p1 is unaffected by externally added kirromycin, ruling out the possibility that the tuf2 gene product might be directly involved in conferring resistance to kirromycin.
S. ramocissimus tuf2 encodes a functional EF-Tu.
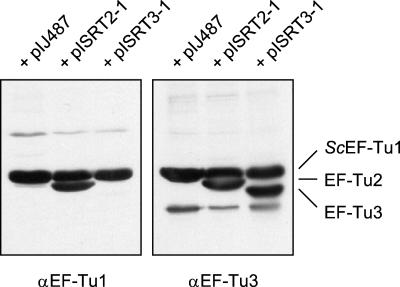
Expression of the tuf2 gene could not be demonstrated in total-protein extracts of S. ramocissimus, and heterologous expression of tuf2 in E. coli yielded a small amount of inactive product, deposited in inclusion bodies (37). To achieve high expression of active EF-Tu2, tuf2 was cloned behind the constitutive ermE promoter in pIJ487, resulting in Streptomyces expression vector pISRT2-1 (for details see Materials and Methods). Since a suitable transformation system for S. ramocissimus was not found, overexpression of tuf2 in S. coelicolor M145, a strain naturally lacking a tuf2 analogue, was studied (35). Constitutive overexpression of the plasmid-borne tuf2 gene affected the host cell, resulting in growth retardation and aberrant production of red pigments. S30 extracts prepared from cultures of pISRT2-1 transformants grown for 40 h in YEME were analyzed by Western blotting. As shown in Fig. 4, high expression of tuf2 was achieved; the overexpressed product was also clearly visible in Coomassie brilliant blue-stained gels (data not shown). Despite its slightly higher calculated molecular mass (44.1 versus 43.6 kDa), EF-Tu2 migrates considerably faster than S. coelicolor EF-Tu1 during SDS-PAGE, consistent with the results of heterologous expression in E. coli (37). Two types of polyclonal antibodies, anti-EF-Tu1 and anti-EF-Tu3, raised against the S. ramocissimus tuf1 and tuf3 gene products, respectively, showed high cross-reactivities to EF-Tu2. Additional minor bands at much higher or lower positions represent nonspecific interactions with S30 extract proteins.
FIG. 4.
Overexpression of the S. ramocissimus tuf2 and tuf3 gene products in S. coelicolor M145. S30 extracts of S. coelicolor M145 transformed with different overexpression plasmids were analyzed by Western blotting using polyclonal antibodies raised against EF-Tu1 (left) or EF-Tu3 (right). Left lane, S. coelicolor(pIJ487); middle lane, S. coelicolor(pISRT2-1); right lane, S. coelicolor(pISRT3-1).
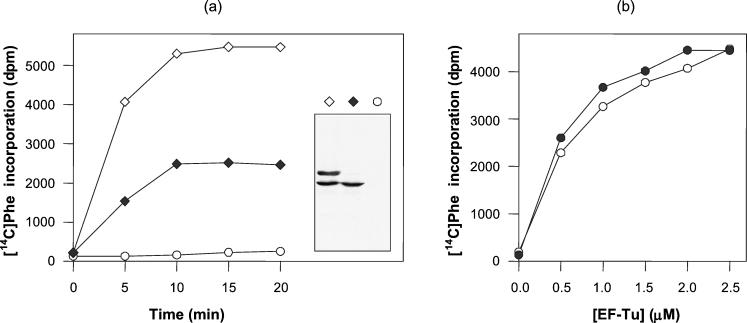
The homology between S. ramocissimus EF-Tu2 and EF-Tu1 (88% amino acid sequence identity) and the conservation of the proposed binding sites for both aminoacyl-tRNA (23) and EF-Ts (13) suggest that EF-Tu2 could also sustain translation in S. ramocissimus. To explore the possible participation of this protein in the elongation cycle, in vitro translation experiments were performed with complete cell S30 extracts from S. coelicolor LT2 harboring EF-Tu2 expression vector pISRT2-1. S. coelicolor LT2 is a J1501 derivative modified in both tuf genes: the tuf3 gene is disrupted and tuf1 is replaced by tuf1His, encoding EF-Tu1His as the only functional EF-Tu (24). As shown in Fig. 5a, translational activity was observed in an extract from LT2 harboring pISRT2-1, even after removal of EF-Tu1His by Ni2+ affinity adsorption, indicating that the overexpressed EF-Tu2 is responsible for protein synthesis in vitro. A control experiment was performed with a Ni2+-NTA-treated extract of LT2 containing pIJ487 as the parental vector without tuf2, and virtually no activity was measured. The predicted presence or absence of the different EF-Tu species in the S30 extracts was confirmed by Western blotting (Fig. 5a).
FIG. 5.
Translational activity of S. ramocissimus EF-Tu2. (a) Translational activity of cell extracts of S. coelicolor LT2 harboring expression vector pISRT2-1 with (⋄) and after removal of (♦) endogenous EF-Tu1His. A Ni2+-NTA-treated cell extract of S. coelicolor LT2 harboring pIJ487, the parental vector without tuf2, was used as a control (○). (Inset) Corresponding Western blot of the three extracts. (b) In vitro translation of an EF-Tu-depleted S. coelicolor cell extract supplemented with S. ramocissimus EF-Tu2 (•) and S. ramocissimus EF-Tu1 (○). The translation of the poly(U) messenger was studied by measuring the incorporation of [14C]Phe at 30°C as a function of time (a) and of the concentrations of the EF-Tu species during a 10-min incubation (b).
To investigate whether EF-Tu2 functions as well as EF-Tu1 in poly(U)-directed poly(Phe) synthesis, the protein was purified from S. coelicolor LT2 harboring pISRT2-1 as described in Materials and Methods. The factors were used to complement a Streptomyces EF-Tu-dependent in vitro translation system (24), and their translational capacities as a function of the EF-Tu concentration were studied. As can be concluded from Fig. 5b, the extents of [14C]Phe incorporation in vitro for the two proteins were comparable.
S. ramocissimus EF-Tu2 is sensitive to kirromycin.
The preceding results indicate that S. ramocissimus EF-Tu2 is perfectly able to sustain the whole elongation process in vitro. This prompted us to investigate whether EF-Tu2 is able to promote translation in the presence of kirromycin and thus is somehow involved in the kirromycin resistance mechanism of S. ramocissimus. Resistance to kirromycin is usually achieved by single amino acid substitutions in highly conserved positions of EF-Tu. These mutations cluster in or near the interface between domains I and III of EF-Tu·GTP (1, 19), a region where the antibiotic was recently found to bind (38). Superposition of the EF-Tu2 amino acid sequence on the crystal structure of Thermus thermophilus EF-Tu·GppNHp (3) revealed that no deviating residues are present at the interface between domains I and III, although kirromycin resistance due to residues elsewhere in the protein cannot be excluded.
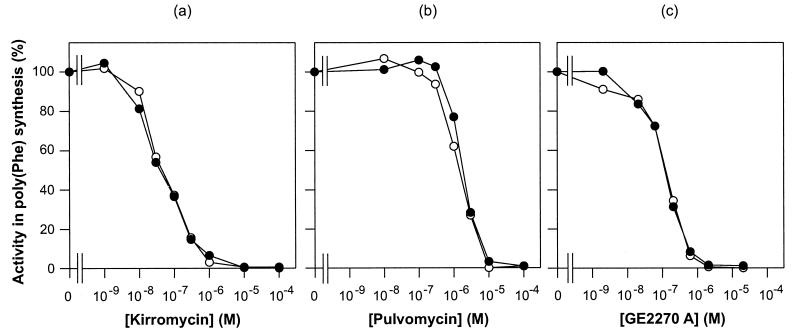
Therefore the kirromycin sensitivities of both S. ramocissimus EF-Tu2 and EF-Tu1 in the Streptomyces in vitro translation system were monitored. As can be concluded from Fig. 6a, EF-Tu2 appeared to be as sensitive to kirromycin as EF-Tu1, with a concentration at which 50% inhibition of poly[U]-directed poly[Phe] synthesis is observed of 0.05 μM. Also no difference in sensitivity to two other EF-Tu-targeted antibiotics, pulvomycin and GE2270 A, between the two EF-Tu proteins was observed, with 50% inhibition occurring at about 1.5 and 0.1 μM, respectively (Fig. 6b and c). Complex formation of EF-Tu2·GDP with these three inhibitors, studied by electrophoresis under nondenaturing conditions, revealed that band shifts for EF-Tu1 were similar to those for EF-Tu2 (data not shown), confirming that the binding sites on EF-Tu2 for these three antibiotics are intact.
FIG. 6.
Translational activities of EF-Tu1 and EF-Tu2 in the presence of antibiotics. Shown is in vitro translation of an EF-Tu-depleted S. coelicolor cell extract supplemented with 1.0 μM S. ramocissimus EF-Tu1 (○) or S. ramocissimus EF-Tu2 (•). The translation of the poly(U) messenger was studied by measuring the incorporation of [14C]Phe at 30°C as a function of the concentrations of kirromycin (a), pulvomycin (b), and GE2270 A (c). For both EF-Tu1 and EF-Tu2 the activity in the absence of the antibiotics was normalized to 100% (the two EF-Tu preparations were about equally active, as shown in Fig. 5b).
These data demonstrate that the minor EF-Tu2 and the major EF-Tu1 of kirromycin producer S. ramocissimus are indistinguishable in their abilities to promote poly(Phe) synthesis in vitro and in their sensitivities to kirromycin and other EF-Tu-targeted antibiotics.
DISCUSSION
Unlike the situation in higher eukaryotes, the occurrence of gene families is not a prominent feature of bacteria, although the presence of duplicate genes encoding EF-Tu (tufA and tufB) in several gram-negative bacteria has been demonstrated (8, 27). Their locations, one tuf gene linked to certain ribosomal protein genes and the other linked to tRNA genes, are also conserved among distantly related bacterial species (14). The maintenance of two active tuf copies, contributing about equally to the total EF-Tu concentration, has been ascribed to evolutionary pressure for an increased amount of protein product and an increased flexibility in expression regulation. The presence of three heterogeneous tuf genes in kirromycin producer S. ramocissimus is intriguing. While streptomycetes generally contain tuf1 and tuf3 homologues, tuf2 has no equal in the actinomycetes studied so far (4, 21, 35, 37; L. N. Olsthoorn-Tieleman, unpublished results). Here we have demonstrated the absence of tuf2 in several producers of kirromycin-type antibiotics. Furthermore, we have gathered information about this unique tuf2 gene and its gene product, EF-Tu2, to determine the role of this additional EF-Tu-like protein in S. ramocissimus.
Unlike other tuf genes, tuf2 is not linked to other genes for components of the translational apparatus but rather seems to be located in a single transcription unit. Low-level transcription from a promoter within the oppositely transcribed orfQ takes place, as demonstrated by promoter-probing experiments and S1 nuclease protection assays. Transcription of tuf2 shows a growth phase dependence similar to that of S. ramocissimus tuf1 (32), although tuf1 and tuf2 transcript levels differ by at least several orders of magnitude during normal growth in either liquid cultures or on agar plates. As a result, EF-Tu2 does not contribute significantly to the total EF-Tu pool in S. ramocissimus. The lack of tuf2 transcripts during kirromycin production and the unresponsiveness of tuf2 transcription to kirromycin induction argue against a role for tuf2 as a kirromycin resistance determinant.
Purified EF-Tu2 was perfectly able to sustain poly(Phe) synthesis in a Streptomyces in vitro translation system and was indistinguishable in this ability from the regular EF-Tu1. Its measured sensitivity to kirromycin eliminates any possibility that this additional elongation factor might be directly involved in conferring resistance to kirromycin. The degree of similarity between EF-Tu1 and EF-Tu2 (88% amino acid sequence identity) is consistent with the notion that the proteins are functionally homologous in vitro but also implies that certain structural and functional differences might exist. It should be noted that S. ramocissimus EF-Tu1 is much more similar (96% amino acid sequence identity) to the EF-Tu1 proteins from S. coelicolor (35), S. collinus (20), and Streptomyces netropsis (L. N. Olsthoorn-Tieleman, unpublished results). Comparison of both S. ramocissimus EF-Tu protein sequences with a defined common eubacterial EF-Tu sequence (24) revealed that EF-Tu2 contains six deviations (I199V, D/E240T, L/I292V, K294R, V/I308A, and K/E390R), while EF-Tu1 differs in only one position (V/I308A) (E. coli EF-Tu numbering is used throughout). Residues 240, 292, and 294 are in an area of domain II that has been implicated in the interaction with the ribosome (29, 34, 39). Answers about the role of tuf2 in vivo might be found by succeeding in tuf gene disruption experiments with S. ramocissimus, which are currently hampered by the presence of an efficient restriction modification system (L. N. Olsthoorn-Tieleman, unpublished results).
The main points to keep in mind for determination of the function of EF-Tu2 are (i) the unique presence of tuf2 in kirromycin producer S. ramocissimus, excluding a general role in Streptomyces spp., (ii) the different genetic environment of tuf2 compared to those of other tuf genes, hinting at some primary function other than acting as a translational EF, and (iii) the extremely low tuf2 expression level in comparison with that of tuf1, ruling out the possibility that EF-Tu2, although capable of sustaining poly(Phe) synthesis, plays a significant role in normal protein biosynthesis. For now, we tentatively conclude that S. ramocissimus EF-Tu2 plays a regulatory role, which requires only trace amounts of protein, rather than having a function as an additional EF-Tu.
Acknowledgments
We thank Manon Gantenbein and Nanna Claij for their experimental contributions.
REFERENCES
- 1.Abdulkarim, F., L. Liljas, and D. Hughes. 1994. Mutations to kirromycin resistance occur in the interface of domains I and III of EF-Tu.GTP. FEBS Lett. 352:118-122. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berchtold, H., L. Reshetnikova, C. O. A. Reiser, N. K. Schirmer, M. Sprinzl, and R. Hilgenfeld. 1993. Crystal structure of active elongation factor Tu reveals major domain rearrangements. Nature 365:126-132. [DOI] [PubMed] [Google Scholar]
- 4.Cappellano, C., F. Monti, M. Sosio, S. Donadio, and E. Sarubbi. 1997. Natural kirromycin resistance of elongation factor Tu from the kirrothricin producer Streptomyces cinnamoneus. Microbiology 143:617-624. [DOI] [PubMed] [Google Scholar]
- 4a.Clayton, T. M., and M. J. Bibb. 1990. Streptomyces promoter-probe plasmids that utilise the xy1E gene of Pseudomonas putida. Nucleic Acids Res. 18:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cundliffe, E. 1989. How antibiotic-producing organisms avoid suicide. Annu. Rev. Microbiol. 43:207-233. [DOI] [PubMed] [Google Scholar]
- 6.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinberg, A. P., and B. Vogelstein. 1983. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 132:6-13. [DOI] [PubMed] [Google Scholar]
- 8.Filer, D., and A. V. Furano. 1981. Duplication of the tuf gene, which encodes peptide chain elongation factor Tu, is widespread in gram-negative bacteria. J. Bacteriol. 148:1006-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glöckner, C., and H. Wolf. 1984. Mechanism of natural resistance to kirromycin-type antibiotics in actinomycetes. FEMS Microbiol. Lett. 25:121-124. [Google Scholar]
- 10.Hobbs, G., C. M. Frazer, D. C. J. Gardner, F. Flett, and S. G. Oliver. 1989. Dispersed growth of Streptomyces in liquid culture. Appl. Microbiol. Biotechnol. 31:272-277. [Google Scholar]
- 11.Hopwood, D. A., M. J. Bibb, K. F. Chater, T. Kieser, C. J. Bruton, H. M. Kieser, D. J. Lydiate, C. P. Smith, J. M. Ward, and H. Schrempf. 1985. Genetic manipulation of Streptomyces: a laboratory manual. John Innes Foundation, Norwich, United Kingdom.
- 12.Hopwood, D. A., K. F. Chater, and M. J. Bibb. 1995. Genetics of antibiotic production in Streptomyces coelicolor A3(2), a model streptomycete. Biotechnology 28:65-102. [DOI] [PubMed]
- 13.Kawashima, T., C. Berthet-Colominas, M. Wulff, S. Cusack, and R. Leberman. 1996. The structure of the Escherichia coli EF-Tu.EF-Ts complex at 2.5 Å resolution. Nature 379:511-518. [DOI] [PubMed] [Google Scholar]
- 14.Keeling, P. J., R. L. Charlebois, and W. F. Doolittle. 1994. Archaebacterial genomes: eubacterial form and eukaryotic content. Curr. Opin. Genet. Dev. 4:816-822. [DOI] [PubMed] [Google Scholar]
- 15.Krab, I. M., and A. Parmeggiani. 1998. EF-Tu, a GTPase odyssey. Biochim. Biophys. Acta 1443:1-22. [DOI] [PubMed] [Google Scholar]
- 16.Leskiw, B. K., E. J. Lawlor, J. M. Fernandez-Abalos, and K. F. Chater. 1991. TTA codons in some genes prevent their expression in a class of developmental, antibiotic-negative, Streptomyces mutants. Proc. Natl. Acad. Sci. USA 88:2461-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindahl, L., and J. M. Zengel. 1986. Ribosomal genes in Escherichia coli. Annu. Rev. Genet. 20:297-326. [DOI] [PubMed] [Google Scholar]
- 18.MacNeil, D. J., K. M. Gewain, C. L. Ruby, G. Dezeny, P. H. Gibbons, and T. MacNeil. 1992. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111:61-68. [DOI] [PubMed] [Google Scholar]
- 19.Mesters, J. R., L. A. H. Zeef, R. Hilgenfeld, J. M. de Graaf, B. Kraal, and L. Bosch. 1994. The structural and functional basis for the kirromycin resistance of mutant EF-Tu species in Escherichia coli. EMBO J. 13:4877-4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikulik, K., and E. Zhulanova. 1995. Sequencing of the tuf gene and the phosphorylation pattern of EF-Tu1 during development and differentiation in Streptomyces collinus producing kirromycin. Biochem. Biophys. Res. Commun. 213:454-461. [DOI] [PubMed] [Google Scholar]
- 21.Möhrle, V. G., L. N. Tieleman, and B. Kraal. 1997. Elongation factor Tu1 of the antibiotic GE2270 A producer Planobispora rosea has an unexpected resistance profile against EF-Tu targeted antibiotics. Biochem. Biophys. Res. Commun. 230:320-326. [DOI] [PubMed] [Google Scholar]
- 22.Murray, M. G. 1986. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal. Biochem. 158:165-170. [DOI] [PubMed] [Google Scholar]
- 23.Nissen, P., M. Kjeldgaard, M. Thirup, G. Polekhina, L. Reshetnikova, B. F. Clark, and J. Nyborg. 1995. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science 270:1464-1472. [DOI] [PubMed] [Google Scholar]
- 24.Olsthoorn-Tieleman, L. N., I. J. Plooster, and B. Kraal. 2001. The variant tuf3 gene of Streptomyces coelicolor A3(2) encodes a real elongation factor Tu, as shown in a novel Streptomyces in vitro translation system. Eur. J. Biochem. 268:3807-3815. [DOI] [PubMed] [Google Scholar]
- 25.Parmeggiani, A., and G. W. M. Swart. 1985. Mechanism of action of kirromycin-like antibiotics. Annu. Rev. Microbiol. 39:557-577. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Sela, S., D. Yoger, S. Razin, and H. Bercovier. 1989. Duplication of the tuf gene: a new insight into the phylogeny of eubacteria. J. Bacteriol. 171:581-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selva, E., G. Beretta, N. Montanini, G. S. Saddler, L. Gastaldo, P. Ferrari, R. Lorenzetti, P. Landini, F. Ripamonti, B. P. Goldstein, L. Montanaro, and M. Denaro. 1991. Antibiotic GE2270 A: a novel inhibitor of bacterial protein synthesis. I. Isolation and characterization. J. Antibiot. 44:693-701. [DOI] [PubMed] [Google Scholar]
- 29.Stark, H., M. V. Rodnina, J. Rinke-Appel, R. Brimacombe, W. Wintermeyer, and M. van Heel. 1997. Visualization of elongation factor Tu on the Escherichia coli ribosome. Nature 389:403-406. [DOI] [PubMed] [Google Scholar]
- 30.Strauch, E., E. Takano, H. A. Baylis, and M. J. Bibb. 1991. The stringent response in Streptomyces coelicolor A3(2). Mol. Microbiol. 5:289-298. [DOI] [PubMed] [Google Scholar]
- 31.Strohl, W. R. 1992. Compilation and analysis of DNA sequences associated with apparent streptomycete promoters. Nucleic Acids Res. 20:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tieleman, L. N., G. P. van Wezel, M. J. Bibb, and B. Kraal. 1997. Growth phase-dependent transcription of the Streptomyces ramocissimus tuf1 gene occurs from two promoters. J. Bacteriol. 179:3619-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tubulekas, I., R. H. Buckingham, and D. Hughes. 1991. Mutant ribosomes can generate dominant kirromycin resistance. J. Bacteriol. 173:3635-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tubulekas, I., and D. Hughes. 1993. A single amino acid substitution in elongation factor Tu disrupts interaction between the ternary complex and the ribosome. J. Bacteriol. 175:240-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Wezel, G. P., L. P. Woudt, R. Vervenne, M. L. A. Verdurmen, E. Vijgenboom, and L. Bosch. 1994. Cloning and sequencing of the tuf genes of Streptomyces coelicolor A3(2). Biochim. Biophys. Acta 1219:543-547. [DOI] [PubMed] [Google Scholar]
- 36.Van Wezel, G. P., E. Takano, E. Vijgenboom, L. Bosch, and M. Bibb. 1995. The tuf3 gene of Streptomyces coelicolor A3(2) encodes an inessential elongation factor Tu that is apparently subject to positive stringent control. Microbiology 141:2519-2528. [DOI] [PubMed] [Google Scholar]
- 37.Vijgenboom, E., L. P. Woudt, P. W. H. Heinstra, K. Rietveld, J. van Haarlem, G. P. van Wezel, S. Shochat, and L. Bosch. 1994. Three tuf-like genes in the kirromycin producer Streptomyces ramocissimus. Microbiology 140:983-998. [DOI] [PubMed] [Google Scholar]
- 38.Vogeley, L., G. J. Palm, J. R. Mesters, and R. Hilgenfeld. 2001. Conformational change of elongation factor Tu (EF-Tu) induced by antibiotic binding. J. Biol. Chem. 276:17149-17155. [DOI] [PubMed] [Google Scholar]
- 39.Vorstenbosch, E., T. Pape, M. V. Rodnina, B. Kraal, and W. Wintermeyer. 1996. The G222D mutation in elongation factor Tu inhibits the codon-induced conformational changes leading to GTPase activation on the ribosome. EMBO J. 15:6766-6774. [PMC free article] [PubMed] [Google Scholar]
- 40.Ward, J. M., G. R. Janssen, T. Kieser, M. J. Bibb, M. J. Buttner, and M. J. Bibb. 1986. Construction and characterisation of a series of multi-copy promoter-probe plasmid vectors for Streptomyces using the aminoglycoside phosphotransferase gene from Tn5 as indicator. Mol. Gen. Genet. 203:468-475. [DOI] [PubMed] [Google Scholar]
- 41.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]