Abstract
Markers of Epstein-Barr virus (EBV) infection include measures of specific serological titers and of viral load (VLo) in peripheral blood mononuclear cells. Few studies have investigated the correlation between these two phenotypes. Here, we found that there was no correlation between VLo and either anti-EBV nuclear antigen type 1 or anti-early antigen immunoglobulin G (IgG) titer but that anti-viral capsid antigen (VCA) IgG titer increased with VLo in peripheral blood mononuclear cells in patients with Hodgkin's lymphoma (P = 3.10−3). A similar pattern was observed in healthy first-degree relatives (parents and siblings) of patients (P = 6.10−4). Our results indicate that anti-VCA IgG titers and EBV VLo are specifically correlated EBV phenotypes.
More than 95% of the human population is infected with Epstein-Barr virus (EBV) (18). Primary infection is usually asymptomatic in childhood but may present as infectious mononucleosis when it occurs later in life (18). The virus infects the oropharynx and then spreads throughout the lymphoid tissues. It persists in the host, remaining latent in memory B cells (18). Although latent EBV infection usually remains asymptomatic, EBV may be associated with malignant lymphoid proliferations such as Burkitt lymphoma (BL) and Hodgkin's lymphoma (HL) (3, 13, 17). In addition, severe immunodeficiency increases the risk of EBV-associated lymphoid malignancies such as AIDS-related lymphomas and posttransplant lymphoproliferative disorders (PTLD).
The EBV antigens classically used for serological testing include EBV nuclear antigen type 1 (EBNA-1), viral capsid antigen (VCA), and early antigen (EA). After primary infection, anti-VCA and anti-EBNA-1 antibodies persist for life. EBNA-1 is expressed during latent infection, whereas VCA and EA, like the recently described EBV transactivator protein (ZEBRA) (4), are expressed during lytic infection. High anti-VCA, anti-EA, and anti-ZEBRA immunoglobulin G (IgG) titers are therefore considered to be markers of EBV reactivation (8). Remarkably, anti-VCA titers are high at the time of diagnosis of HL and BL and also years before and after diagnosis in both diseases (3, 13). A role of EBV reactivation in HL is also suggested by the detection of anti-ZEBRA antibodies in patients at diagnosis of EBV-positive HL (4).
In addition to serological testing, the EBV viral loads (VLo) can be measured in peripheral blood mononuclear cells (PBMCs), serum, or whole blood. In healthy subjects, VLo is frequently detected at low levels in PBMCs, and it remains steady over time in each individual (10). This makes it possible to evaluate the number of circulating EBV-infected memory B cells. As EBV does not replicate in these cells in nonpathological conditions (1), it is not excreted into the serum of healthy subjects. In contrast, high VLos in the PBMCs and/or serum are observed in patients at diagnosis of EBV-related lymphoid malignancies (HL, BL, PTLD) (5, 6, 9, 14). The physiological significance of positive VLo in healthy subjects remains unclear, but high VLo in individuals who have undergone transplants has been shown to be predictive of PTLD (14).
The relationships between anti-EBV serological titers and VLo have rarely been studied. Only one small longitudinal study of healthy subjects has reported that an increase in VLo is frequently followed by an increase in anti-EBV titers (12). In human immunodeficiency virus (HIV)-infected patients without lymphoma, high VLo in whole blood has been found to be associated with high plasma anti-VCA titers (16). In children receiving solid-organ transplants, VLo in blood has also been found to be correlated with anti-EA and anti-VCA IgG titers (15). We recruited HL patients in remission and their families for a genetic study. We report here the significant correlations observed between plasma anti-VCA antibody titers and VLo in the PBMCs of HL patients and their relatives.
MATERIALS AND METHODS
Study population.
HL patients in complete remission were recruited from hematology units at least 1 year after diagnosis. Inclusion criteria were age between 15 and 35 years and HIV-negative serology at diagnosis. First-degree relatives (parents and siblings) of the patients were asked to participate in the study. All participating subjects were interviewed to determine their medical history and gave a blood sample. This study was approved by the appropriate French Consultative Committee Protecting Persons in Biomedical Research. Human experimentation guidelines were followed in the conduct of the research. Informed consent was obtained from adults or from the parents of minors.
EBV measures.
Anti-EBNA-1 IgG, anti-VCA IgM, anti-EA IgG, and anti-VCA IgG titers were determined with enzyme-linked immunosorbent assay kits (DiaSorin, Stillwater, OK) (ImmunoWELL bmd s.a for IgG anti-VCA) and are expressed in units per milliliter. A positive serology for EBV was defined as a positive result for anti-VCA antibody.
PBMCs were isolated from blood samples on Ficoll-Paque (Pharmacia Biotech), and pellets of 106 cells were frozen at −80°C. DNA was extracted from cell pellets by use of a QIAmp blood kit (QIAGEN Inc., Courtaboeuf, France). VLo in PBMCs was determined by real-time quantitative PCR with a fluorogenic probe (2). The PCR primers were selected to amplify the thymidine kinase gene. The forward and reverse primers were 5′-GACAACTCGGCCGTGATGGA-3′ and 5′-TGAAGTTGGAGGCGGACGA-3′, respectively. The fluorogenic TaqMan probe was 5′-6-carboxyfluorescein-TGACCTTTGGCGCGGCCTCCTGC-6-carboxytetramethylrhodamine-3′. For preparation of the standard curve, the 121-bp PCR product was directly inserted into a pcDNA 3.1 vector (Invitrogen, Groningen, The Netherlands) containing one copy of the EBV PCR target region. Tenfold serial dilutions in water (106 to 10 copies) were prepared. The same preparations were used in each test. Real-time quantitative PCR for EBV and albumin DNA quantification were carried out simultaneously to determine the amount of cellular DNA in each sample (2).
Statistical analysis.
We analyzed the data for patients and their relatives separately. Antibody titers were analyzed as a quantitative variable. VLo was considered as a binary variable (virus detectable or undetectable) and as an ordinal variable (undetectable, 10 to 99, 100 to 299, or ≥300 copies/106 PBMCs). Age was considered as a four-class variable (15 to 29, 30 to 44, 45 to 59, or ≥60 years). Statistical analyses were conducted with SAS software (version 8.2; SAS Institute, Cary, NC). Categorical variables (e.g., VLo according to patient status) were compared using chi-square tests (Proc Freq). Antibody titers were compared according to binary and categorical variables by distribution-free analysis of variance (ANOVA) based on ranks (Proc Rank and Proc Anova). We also tested the effect of VLo as an ordinal variable on antibody titers with the nonparametric Jonckheere-Terpstra test for trend (Proc Freq) (7).
RESULTS
HL patients.
We enrolled 75 HL patients. Three patients were excluded, two because serological tests for EBV were negative and one for technical reasons. The mean age (standard error of mean [SEM]) of the remaining 72 patients was 32 (0.9) years. The male-to-female ratio was 1.1. EBV was detected in PBMCs for 29 of 72 (40%) subjects. Mean VLo was 240 (101) copies/106 PBMCs for subjects in whom the virus was detectable. Neither age nor sex had a significant effect on VLo (data not shown).
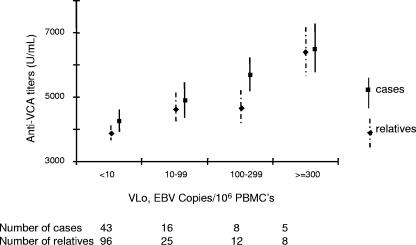
Anti-EBNA-1 and anti-EA IgG titers did not differ between subjects with detectable and undetectable virus (Table 1). In contrast, subjects with detectable virus had significantly higher anti-VCA IgG titers than those in whom the virus was undetectable (P = 0.02). In analysis of VLo as a four-class variable, significant differences in anti-VCA IgG titers were observed between classes (P = 6.10−3). The test for trend confirmed that there was a significant positive correlation between anti-VCA IgG titers and VLo (P = 3.10−3). Mean (SEM) anti-VCA titers increased from 4,278 (344) U/ml for subjects with undetectable virus to 6,520 (752) U/ml for subjects with a VLo of 300 or more copies/106 PBMCs (Fig. 1).
TABLE 1.
Distribution of EBV antibody titersa according to VLo as a binary variable (undetectable versus detectable)
| Subject group and parameter | Total | Viral load
|
Pb | |
|---|---|---|---|---|
| Undetectable | Detectable | |||
| Patients | ||||
| n (%) | 72 (100) | 43 (60) | 29 (40) | |
| IgG anti-EBNA-1 titer (SEM) | 405 (47) | 373 (59) | 452 (78) | 0.49 |
| IgG anti-EA titer (SEM) | 27 (3) | 24 (4) | 31 (6) | 0.36 |
| IgG anti-VCA titer (SEM) | 4,734 (261) | 4,278 (344) | 5,410 (374) | 0.02 |
| Relatives | ||||
| n (%) | 141 (100) | 96 (68) | 45 (32) | |
| IgG anti-EBNA-1 titer (SEM) | 376 (32) | 350 (34) | 433 (70) | 0.51 |
| IgG anti-EA titer (SEM) | 21 (2) | 21 (2) | 22 (3) | 0.99 |
| IgG anti-VCA titer (SEM) | 4,218 (197) | 3,870 (234) | 4,962 (344) | 3.10−3 |
Titers are means (in units per milliliter).
P values were calculated by distribution-free ANOVA based on ranks.
FIG. 1.
Anti-VCA titers distributions according to VLo ordinal classes. Mean titers with standard SEMs are shown for each ordinal class of VLo for patients (squares, full lines) and their relatives (diamonds, dotted lines). Sample sizes for each VLo class are presented in the bottom line.
HL relatives.
There were 160 first-degree relatives of patients (53 mothers, 46 fathers, 19 brothers, and 42 sisters). We excluded 19 of these relatives: 10 because they were seronegative for EBV (three parents and seven siblings) and 9 for technical reasons. The mean (SEM) ages of parents and siblings were 58 (0.8) and 31 (1.1) years, respectively. Relatives had lower anti-VCA IgG titers than HL patients: 4,218 (197) U/ml versus 4,734 (261) U/ml (P = 0.03, after adjustment for age). The proportion of relatives with detectable virus, 45 of 141 (32%), was lower than for patients although not significant (P = 0.23). The proportions of subjects in which virus was detected, in both HL patients and relatives, were similar to that previously reported for healthy subjects (37.5%) (1). Mean (SEM) VLo was 226 (66) copies/106 PBMCs for subjects in whom virus was detected. Neither sex nor age had a significant effect on VLo (data not shown).
There was no significant difference between subjects with and without detectable virus in anti-EBNA-1 or anti-EA IgG titers (Table 1). As seen with HL patients, subjects with detectable virus had significantly higher anti-VCA IgG titers than those in whom the virus was not detected (P = 3.10−3). As anti-VCA IgG titers increased slightly with age in relatives (P = 0.03), we redid the analysis, adjusting for age; similar results were obtained (P = 2.10−3). When VLo was considered as a four-class variable, significant differences in anti-VCA IgG titers between classes were found after adjustment for age (P = 6.10−3). Similar results were obtained when family was included as a covariate in the ANOVA, indicating that the familial relationships did not affect our findings (data not shown). The test for trend, which is not adjusted for age, confirmed that there was a significant positive correlation between VLo and anti-VCA IgG titers (P = 6.10−4) (Fig. 1). Anti-VCA IgG titers increased from 3,870 (234) U/ml for subjects in whom the virus was undetectable to 6,400 (754) for subjects with VLo of 300 or more copies/106 PBMCs.
DISCUSSION
This is the first report combining the analysis of EBV-specific plasma antibody titers and VLo in PBMCs for a large group of immunocompetent subjects. VLo was not correlated with anti-EBNA-1 or anti-EA antibody titers. However, VLo and anti-VCA IgG titers were found to be correlated in both HL patients and their relatives. Anti-VCA IgG titers were significantly higher for subjects with detectable virus than for those in whom the virus was undetectable. When VLo was analyzed as a four-class variable, we observed a significant positive trend, with anti-VCA IgG titers increasing with VLo. This relationship held even for low VLo values (10 to 100 copies/106 PBMCs), although these low VLo are not known to be of clinical relevance (1). Our results indicate that anti-VCA IgG titers and EBV VLo are specifically correlated phenotypes of EBV infection.
The lack of association between VLo in blood and anti-EBNA-1 titers is consistent with previous studies of HIV-infected and posttransplant patients (16). In contrast, anti-EA antibody titers and VLo have been found to be correlated in children followed up after receiving solid-organ transplants (15). This finding contrasts with our results for an adult population of HL patients in remission and healthy subjects. However, in the study of children (15), an increase in anti-EA titers occurred in the context of primary EBV infection, as most children were EBV negative at the time of transplantation. The apparent lack of consistency between the results obtained in these two studies may be accounted for by the known transient nature of anti-EA antibody production in primary infection (3, 13).
A positive correlation between anti-VCA titers and VLo has been reported for HIV-infected subjects. Plasma anti-VCA titers were found to be significantly higher in subjects with a high VLo in blood than in those with a low VLo in blood in a population of 99 HIV-infected subjects (16). Drouet et al. studied patients with EBV-associated HL and also reported that the detection of virus in the serum was correlated with high anti-VCA titers and positive anti-ZEBRA IgG in 30 patients at the time of diagnosis (4). VLo in PBMCs was not determined in this last study. The only study to investigate this relationship in healthy subjects reported the kinetics of serum anti-EBV titers (determined with a mixture of lytic and latent EBV proteins) and VLo in PBMCs for 22 subjects (12). An increase in VLo or the persistence of virus detection was followed by an increase in anti-EBV titers in some subjects (12). Our results showing that there is a strong correlation between anti-VCA IgG titers and VLo in PBMCs are consistent with these previous reports of studies performed in different contexts. Further studies of infectious mononucleosis patients are needed to investigate whether or not the same pattern of correlations is observed in the context of primary EBV infection.
The long-term EBV reservoir consists of EBV-infected memory B cells, which account for a substantial proportion of PBMCs. EBV does not replicate in these cells. Instead, EBV reactivation has been shown to be initiated following the differentiation of EBV-infected memory B cells into plasma cells (11) in the germinal center of lymph nodes or tonsils. The signals driving this differentiation remain to be determined. Virions produced following differentiation may initiate the infection of new memory B cells, replenish the B-cell reservoir, and increase VLo in PBMCs (18). As VCA antigens are expressed during viral replication, virion production is likely to lead to an increase in anti-VCA IgG titers. An increase in viral replication could then lead to increases in both EBV VLo in PBMCs and plasma anti-VCA titers. Our results suggest that anti-VCA IgG titers and VLo may be considered as markers of EBV reactivation.
Acknowledgments
We thank J. L. Casanova, A. Alcaïs, S. Plancoulaine, and all members of the Laboratory of Human Genetics of Infectious Diseases for helpful discussions. We are grateful to P. Flandre (INSERM U472, Villejuif, France) for advice on the statistical analysis of nonparametric data and F. Zatla (Hôpital Tenon, Paris, France) for assistance with management of the biological samples. We also thank the patients and their families for their trust and willingness to participate in this study and R. Delarue and J. Vargaftig (Hopital Necker, Paris, France) for identifying patients. Thanks are also due to J. L. Bresson, A. Mogenet, and all members of the Centre d'Investigation Clinique de l'Hôpital Necker (Paris, France) for the cordial reception of patients and their families.
Financial support was provided by Association pour la Recherche contre le Cancer, Ligue contre le Cancer, Fondation de France, Fondation Schlumberger, and Fondation BNP Paribas. C.B. is supported by Fondation de France.
REFERENCES
- 1.Brengel-Pesce, K., P. Morand, A. Schmuck, M. J. Bourgeat, M. Buisson, G. Bargues, M. Bouzid, and J. M. Seigneurin. 2002. Routine use of real-time quantitative PCR for laboratory diagnosis of Epstein-Barr virus infections. J. Med. Virol. 66:360-369. [DOI] [PubMed] [Google Scholar]
- 2.Dehee, A., C. Asselot, T. Piolot, C. Jacomet, W. Rozenbaum, M. Vidaud, A. Garbarg-Chenon, and J. C. Nicolas. 2001. Quantification of Epstein-Barr virus load in peripheral blood of human immunodeficiency virus-infected patients using real-time PCR. J. Med. Virol. 65:543-552. [PubMed] [Google Scholar]
- 3.de-The, G., A. Geser, N. E. Day, P. M. Tukei, E. H. Williams, D. P. Beri, P. G. Smith, A. G. Dean, G. W. Bronkamm, P. Feorino, and W. Henle. 1978. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature 274:756-761. [DOI] [PubMed] [Google Scholar]
- 4.Drouet, E., P. Brousset, F. Fares, J. Icart, C. Verniol, F. Meggetto, D. Schlaifer, H. Desmorat-Coat, F. Rigal-Huguet, A. Niveleau, and G. Delsol. 1999. High Epstein-Barr virus serum load and elevated titers of anti-ZEBRA antibodies in patients with EBV-harboring tumor cells of Hodgkin's disease. J. Med. Virol. 57:383-389. [DOI] [PubMed] [Google Scholar]
- 5.Fan, H., S. C. Kim, C. O. Chima, B. F. Israel, K. M. Lawless, P. A. Eagan, S. Elmore, D. T. Moore, S. A. Schichman, L. J. Swinnen, and M. L. Gulley. 2005. Epstein-Barr viral load as a marker of lymphoma in AIDS patients. J. Med. Virol. 75:59-69. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher, A., A. A. Armstrong, J. MacKenzie, L. Shield, G. Khan, A. Lake, S. Proctor, P. Taylor, G. B. Clements, and R. F. Jarrett. 1999. Detection of Epstein-Barr virus (EBV) genomes in the serum of patients with EBV-associated Hodgkin's disease. Int. J. Cancer 84:442-448. [DOI] [PubMed] [Google Scholar]
- 7.Hollander, M., and D. A. Wolfe. 1973. Non-parametric statistical methods. John Wiley and Sons, Inc, New York, N.Y.
- 8.Horwitz, C. A., W. Henle, G. Henle, H. Rudnick, and E. Latts. 1985. Long-term serological follow-up of patients for Epstein-Barr virus after recovery from infectious mononucleosis. J. Infect. Dis. 151:1150-1153. [DOI] [PubMed] [Google Scholar]
- 9.Khan, G., A. Lake, L. Shield, J. Freeland, L. Andrew, F. E. Alexander, R. Jackson, P. R. Taylor, E. A. McCruden, and R. F. Jarrett. 2005. Phenotype and frequency of Epstein-Barr virus-infected cells in pretreatment blood samples from patients with Hodgkin lymphoma. Br. J. Haematol. 129:511-519. [DOI] [PubMed] [Google Scholar]
- 10.Khan, G., E. M. Miyashita, B. Yang, G. J. Babcock, and D. A. Thorley-Lawson. 1996. Is EBV persistence in vivo a model for B cell homeostasis? Immunity 5:173-179. [DOI] [PubMed] [Google Scholar]
- 11.Laichalk, L. L., and D. A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurmann, S., L. Fricke, H. J. Wagner, P. Schlenke, H. Hennig, J. Steinhoff, and W. J. Jabs. 2003. Molecular parameters for precise diagnosis of asymptomatic Epstein-Barr virus reactivation in healthy carriers. J. Clin. Microbiol. 41:5419-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mueller, N., A. Evans, N. L. Harris, G. W. Comstock, E. Jellum, K. Magnus, N. Orentreich, B. F. Polk, and J. Vogelman. 1989. Hodgkin's disease and Epstein-Barr virus. Altered antibody pattern before diagnosis. N. Engl. J. Med. 320:689-695. [DOI] [PubMed] [Google Scholar]
- 14.Riddler, S. A., M. C. Breinig, and J. L. McKnight. 1994. Increased levels of circulating Epstein-Barr virus (EBV)-infected lymphocytes and decreased EBV nuclear antigen antibody responses are associated with the development of posttransplant lymphoproliferative disease in solid-organ transplant recipients. Blood 84:972-984. [PubMed] [Google Scholar]
- 15.Rogers, B. B., C. Conlin, C. F. Timmons, D. B. Dawson, K. Krisher, and W. S. Andrews. 1997. Epstein-Barr virus PCR correlated with viral histology and serology in pediatric liver transplant patients. Pediatr. Pathol. Lab. Med. 17:391-400. [PubMed] [Google Scholar]
- 16.Stevens, S. J., B. S. Blank, P. H. Smits, P. L. Meenhorst, and J. M. Middeldorp. 2002. High Epstein-Barr virus (EBV) DNA loads in HIV-infected patients: correlation with antiretroviral therapy and quantitative EBV serology. AIDS 16:993-1001. [DOI] [PubMed] [Google Scholar]
- 17.Wu, T. C., R. B. Mann, P. Charache, S. D. Hayward, S. Staal, B. C. Lambe, and R. F. Ambinder. 1990. Detection of EBV gene expression in Reed-Sternberg cells of Hodgkin's disease. Int. J. Cancer 46:801-804. [DOI] [PubMed] [Google Scholar]
- 18.Young, L. S., and A. B. Rickinson. 2004. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer 4:757-768. [DOI] [PubMed] [Google Scholar]