Abstract
Clinical and microbiologic studies of 50 cases of viridans streptococcal bacteremia in cancer patients were performed. The bacteria were identified to species level by sequencing analysis of the 16S rRNA gene. At least nine Streptococcus spp. were found, including S. mitis (25 strains, 50.0% of 50); currently unnamed Streptococcus spp. (11 strains); S. parasanguis (five strains); S. anginosus (three strains); S. salivarius (two strains); and one strain each of S. gordonii, S. sanguis, S. sobrinus, and S. vestibularis. There were no S. oralis strains. Among 11 antibiotics of nine classes tested, no resistance to vancomycin, linezolid, or quinupristin-dalfopristin was seen. Resistance to penicillin (MIC, 4 to 12 μg/ml) was noted only among S. mitis strains (28.0%, 7/25) and not non-S. mitis strains (0/25) (P = 0.004). Significantly more S. mitis strains than non-S. mitis strains were resistant to fluoroquinolones and to ≥3 classes of antibiotics. Isolation of quinolone-resistant organisms was associated with the prior usage of quinolones (P = 0.002). Quantitative blood cultures showed that the strains resistant to levofloxacin or gatifloxacin were associated with higher colony counts than were their corresponding nonresistant strains. The young and elderly patients also had higher levels of bacteremia caused predominantly by S. mitis. Septic shock was present in 17 (34.0% of 50) patients, and 13 of those cases were caused by S. mitis (P = 0.007). These results suggest that S. mitis is the most common cause of viridans streptococcal bacteremia in cancer patients and is more resistant to antibiotics than other species.
Viridans streptococci represent a group of 24 currently described Streptococcus species that are nutritionally fastidious and mainly alpha-hemolytic on sheep blood agar (30). These gram-positive cocci are commensals of the oral cavity, upper airway, and gastrointestinal and genitourinary tracts. Despite the overall low virulence, they may cause infective endocarditis, contribute to polymicrobic abscess, and invade the bloodstream during the state of neutropenia.
The bloodstream infection usually occurs in cancer patients with mucositis and neutropenia due to antineoplastic chemotherapy-related toxicity. In these patients, studies have found that viridans streptococci are among the most common organisms isolated from the cultures of bacteremia samples (17). As cancer care has improved and intensified over recent decades and the patients survive longer, these and other infectious complications become more pronounced. A study from our institution showed that the rate of viridans streptococcal bacteremia increased from 1 per 10,000 admissions to 47 per 10,000 during the 13-year period from 1977 to 1989 (11). These bacteremias cause substantial morbidity, and mortality in these patients is at 6 to 12% (17, 29).
The most common viridans streptococci that cause neutropenic bacteremia have been S. oralis, S. mitis, and S. salivarius (4, 11, 15, 17). Identification of these species, however, was reached through traditional biochemical reactions that may be variable and overlapping among different as well as closely related species. Because of this limitation, most clinical microbiology laboratories rarely attempt to identify viridans streptococci to species level.
Sequencing analysis of the 16S rRNA gene has become an essential part of bacterial taxonomy, forming the backbone of a polyphasic approach for the description of new bacterial species (22). The method has also gained wide application for the identification of various unknown bacteria in research as well as clinical laboratory settings (6, 10, 13, 14). Most (if not all) Streptococcus species have been analyzed for the 16S rRNA gene sequences and their phylogenetic relationships delineated (18, 19, 35), thus establishing the basis for accurate identification of various streptococci by this method. In this study, we used a 16S rRNA gene sequencing method to identify to species level 50 strains of viridans streptococci that were isolated quantitatively from blood cultures of cancer patients. The antibiotic susceptibilities of these species were tested and analyzed. These results were correlated with clinical findings.
MATERIALS AND METHODS
Study setting and cultures.
The cases occurred sporadically from July 2002 to December 2003 at The University of Texas M. D. Anderson Cancer Center in Houston, Texas, a 500-bed comprehensive cancer center. They were identified among the approximately 45,000 blood cultures performed during the period. The bacteria were isolated from blood cultures using the Bactec 9240 automated culturing system (BD Diagnostic Systems, Sparks, MD) and Isolator lysis centrifugation tubes (Wampole Laboratories, Princeton, NJ). When an Isolator tube culture was positive, the number of bacterial colonies was quantitated from the 10 ml of blood cultured (31). Samples from children younger than 15 years of age were cultured using Pedi-Isolator (Wampole) with 1.5 ml blood drawn, and the final colony counts were adjusted to a 10-ml blood volume. All subcultures were plated on blood agar and chocolate agar (BBL, Becton Dickinson Microbiology Systems, Cockeysville, MD) and incubated aerobically at 35°C in 5% CO2.
Identification of organisms.
The organisms were all presumptively identified as viridans streptococci based on alpha-hemolysis, gram-positive reaction, coccus morphology in chains, negative catalase test, and exclusions of pneumococci and enterococci by routine biochemical tests (optochin test, bile solubility, and PYR [N,N-dimethylaminocinnamaldehyde] test). The definitive species identification was achieved through sequence analysis of portions of the 16S rRNA gene described previously (14). Briefly, genomic DNA from pure culture colonies was extracted and subjected to amplification by PCR for a 593-bp fragment of the 16S rRNA gene. A set of universal bacterial primers, 5′-TGCCAGCAGCCGCGGTAATAC-3′ and 5′-CGCTCGTTGCGGGACTTAACC-3′ (positions 515 to 1107 of Escherichia coli J01859 or 517 to 1109 of sequence AF003929 of S. mitis ATCC 49456T, respectively), was used for the amplification. To further differentiate the species between S. mitis and S. oralis (23 strains), the species among the S. salivarius group, and unclassified species (11 strains), a second set of primers was used for the amplification and sequencing of a 352-bp fragment (positions 7 to 358 of S. mitis sequence AF003929) that corresponds to the most variable region for various streptococci. These primers were 5′-GTTTGATCCTGGCTCAGAGCG-3′ and 5′-ACTGCTGCCTCCCGTAGGAG-3′. The amplicon was sequenced by the dye terminator method in an automated ABI sequencer (Applied Biosystems, Foster City, CA), and sequence analysis was performed through a query to the GenBank basic local alignment search tool (BLAST) (3). Species identification was reached by the best sequence match (99.2% to 100%) with the 16S rRNA gene of a type strain in GenBank. Generally, a sequence match of 99.0% or above corresponds to species identification (6, 10, 13); hence, the cutoff of 99.2% chosen here was slightly more stringent for the shorter and more variable sequenced region (18). The reference organisms and sequences used in this study are summarized in Table 1.
TABLE 1.
Reference Streptococcus species and their 16S rRNA gene sequences used in the study
| Streptococcus species | GenBank no. | Strain | Reference |
|---|---|---|---|
| S. anginosus | AF104678 | ATCC 33397T = NCTC10713T | 16 |
| S. gordonii | AF003931 and D38483 | ATCC 10558T = NCTC7865T | Emler et al., 1997, unpublished; 18 |
| S. mitis | AF003929 and D38482 | ATCC 49456T = NCTC12261T | Emler et al., 1997, unpublished; 18 |
| S. oralisa | AF003932 and X58308 | ATCC 35037T = NCTC11427T | Emler et al., 1997, unpublished; 5 |
| S. parasanguis | AF003933 | ATCC 15912T | Emler et al., 1997, unpublished |
| S. salivarius | AY188352 | ATCC 7073T = NCTC8618T | Kiratisin et al., 2003, unpublished |
| S. sanguis | AF003928 | ATCC 10556T | Emler et al., 1997, unpublished |
| S. sobrinus | AY188349 | ATCC 33478T | Kiratisin et al., 2003, unpublished |
| S. vestibularis | AY188353 | ATCC 49124T = NCTC12166T | Kiratisin et al., 2003, unpublished |
| Streptococcus sp. related to S. mitis | AY281086 | ATCC 49296 | Kiratisin et al., 2003, unpublished |
| Streptococcus sp. related to S. mitis | AY005045 | Unspecified | 27 |
| Streptococcus sp. unrelated to S. mitis | AF432134 | Unspecified | 20 |
| Streptococcus sp. unrelated to S. mitis | AY278634 | Unspecified | 25 |
For differentiation from S. mitis only.
Antibiotic susceptibility.
The antibiotic susceptibility tests were performed using Etest (AB Biodisk, Solna, Sweden), which is FDA approved, and the results correlate with results from the microdilution method. Briefly, the organism was plated on blood Mueller-Hinton agar and incubated at 35°C in ambient air (preferred), or with CO2 if required for growth, for 20 to 24 h. Results for MIC (μg/ml) were interpreted according to the breakpoints set by the Clinical and Laboratory Standards Institute (formerly NCCLS) for Streptococcus spp. other than Streptococcus pneumoniae (7). For trimethoprim-sulfamethoxazole and ciprofloxacin, interpretive breakpoints for pneumococcus and enterococci were used, respectively.
Clinical information.
The medical records were reviewed for clinical information, including demographics, underlying diagnosis of cancer, anticancer chemotherapy, dental procedures or periodontitis, status of stem cell transplantation, absolute neutrophil count (ANC; per cubic millimeter), and chemotherapy-induced oral and gastrointestinal toxicity such as mucositis, nausea, vomiting, dysphagia, epigastric pain, and diarrhea. Septic shock was defined as positive blood cultures with associated fever of ≥38.5°C, systolic blood pressure of <90 mm Hg, and heart rate of ≥110/minute (24).
Data analysis.
Categorical data were analyzed for statistical significance using the χ2 test. Quantitative culture data were first transformed by logarithm to normal distribution and then analyzed using the Student t test. Significant P values (≤0.05) or nearly significant ones were given.
RESULTS
Species and quantitative cultures.
The 16S rRNA gene sequencing method allowed accurate species identification of the 50 streptococcal strains, with S. mitis and non-S. mitis each accounting for half (Table 2). The non-S. mitis strains included Streptococcus sp., S. parasanguis (also known as S. parasanguinis), S. salivarius, S. anginosus, S. sanguis (S. sanguinis), S. gordonii, S. sobrinus, and S. vestibularis. The strains of the Streptococcus sp. were diverse: six were closely related to S. mitis (98% to 99.1% matches within the ∼850-bp sequenced regions), and five did not match well with any established species (<98% matches). Phenotypically, these 11 strains were indistinguishable from other established species. There were no S. oralis isolates.
TABLE 2.
Species and quantitative blood cultures of viridans streptococci
| Streptococcus species | Total (%) | Distribution of no. of colonies per culture
|
Single species | |||||
|---|---|---|---|---|---|---|---|---|
| <10 | 10 to 49 | 50 to 99 | 100 to 199 | ≥200 | Meana | |||
| S. mitis | 25 (50.0) | 9 | 4 | 3 | 2 | 7 | 38.8 | 22 |
| All non-S. mitis | 25 (50.0) | 18 | 4 | 2 | 1 | 4.8 | 16 | |
| Streptococcus sp. | 11 (22.0) | 7 | 2 | 2 | ||||
| S. parasanguis | 5 (10.0) | 5 | ||||||
| S. anginosus | 3 (6.0) | 2 | 1 | |||||
| S. salivarius | 2 (4.0) | 1 | 1 | |||||
| S. gordonii | 1 (2.0) | 1 | ||||||
| S. sanguis | 1 (2.0) | 1 | ||||||
| S. sobrinus | 1 (2.0) | 1 | ||||||
| S. vestibularis | 1 (2.0) | 1 | ||||||
| Total or P value | 50 (100) | 27 | 8 | 5 | 3 | 7 | P = 0.001 | P = 0.047 |
Geometric mean.
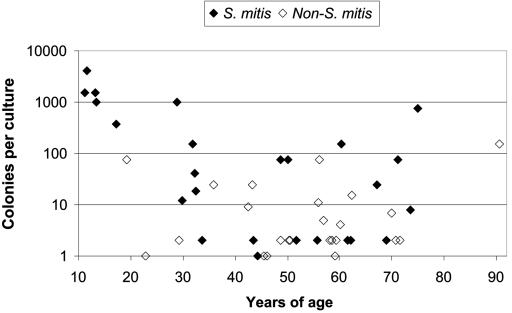
Quantitative cultures showed that the S. mitis strains caused significantly higher levels of bacteremia than the non-S. mitis organisms did (geometric mean, 38.8 colonies per culture versus 4.8; t = 3.45; P = 0.001) (Table 2; Fig. 1). By category, 7 of 25 S. mitis strains had ≥200 colonies per culture in contrast to none of 25 non-S. mitis strains (P = 0.004). More S. mitis strains than non-S. mitis strains caused monomicrobic infection (22/25 versus 16/25, P = 0.047).
FIG. 1.
Analysis of the number of colonies, patient age, and Streptococcus species.
The ages of patients also affected the colony counts. High numbers of colonies were seen mainly in the young and elderly (Fig. 1), particularly children. All five children (11 to 17 years of age) had high-level S. mitis bacteremia (≥375 colonies).
Antibiotic susceptibility.
Eleven antibiotics of nine classes were tested against the organisms (Table 3). During the testing process, most (23 of 25, 92.0%) S. mitis strains grew well in the ambient air without the requirement of CO2, whereas 17 of 25 (68.0%) non-S. mitis strains grew this way (P = 0.034). Thus, S. mitis was less capnophilic.
TABLE 3.
Antibiotic susceptibilities of blood isolates of viridans streptococcia
| Antibiotic | No. of strains
|
|||||||
|---|---|---|---|---|---|---|---|---|
| S. mitis (n = 25) | All non-S. mitis (n = 25) | Streptococcus sp. (n = 11) | S. parasanguis (n = 5) | S. salivarius, S. vestibularis (n = 3) | S. anginosus (n= 3) | S. gordonii, S. sanguis, S. sobrinus (n = 3) | Resistance comparisonb | |
| Vancomycin | S25 | S25 | S11 | S5 | S3 | S3 | S3 | NS |
| Linezolid | S25 | S23/I2 | S11 | S4/I1 | S3 | S2/I1 | S3 | NS |
| Quin-dalfo | S24/I1 | S23/I2 | S11 | S4/I1 | S3 | S2/I1 | S3 | NS |
| Ceftriaxone | S18/I4/R3 | S23/I1/R1 | S9/I1/R1 | S5 | S3 | S3 | S3 | NS |
| TMP-SMZ | S14/I3/R8 | S20/I1/R4 | S9/I1/R1 | S4/R1 | S3 | S2/R1 | S2/R1 | NS |
| Tetracycline | S15/I1/R9 | S15/I2/R8 | S6/I1/R4 | S1/I1/R3 | S3 | S2/R1 | S3 | NS |
| Azithromycin | S9/I3/R13 | S16/R9 | S8/R3 | R5 | S2/R1 | S3 | S3 | NS |
| Penicillin | S11/I7/R7 | S14/I11 | S7/I4 | I5 | S1/I2 | S3 | S3 | P = 0.004 |
| Gatifloxacin | S9/I2/R14 | S16/I1/R8 | S5/R6 | S3/I1/R1 | S2/R1 | S3 | S3 | NSc |
| Levofloxacin | S8/I1/R16 | S16/R9 | S5/R6 | S3/R2 | S2/R1 | S3 | S3 | P = 0.048 |
| Ciprofloxacin | S4/I2/R19 | S9/I3/R13 | I2/R9 | S2/I1/R2 | S2/R1 | S3 | S2/R1 | P = 0.077 |
| R to ≥3 classes | 14 | 3 | 2 | 1 | P = 0.001 | |||
Abbreviations: S, susceptible; I, intermediate; R, resistant; NS, not significant; Quin-dalfo, quinupristin-dalfopristin; TMP-SMZ, trimethoprim-sulfamethoxazole.
S. mitis strains versus all non-S. mitis strains.
P = 0.048 when susceptible strains were compared (9/25 versus 16/25).
All 50 strains were susceptible to vancomycin, and no resistance to linezolid or quinupristin-dalfopristin was seen either. Resistance to other antibiotics varied with species. Seven of the 25 (28.0%) S. mitis strains were resistant to penicillin (MIC, 4 to 12 μg/ml) in contrast to none of the 25 non-S. mitis strains (P = 0.004), making the overall penicillin (high-level) resistance 14.0% (7 of 50). The S. mitis strains also showed higher rates of resistance to each of the three fluoroquinolones tested than did the non-S. mitis strains, but the overall resistance (4 to >32 μg/ml) for all 50 strains was high: 22 strains (44.0%) for gatifloxacin, 25 strains (50.0%) for levofloxacin, and 32 strains (64.0%) for ciprofloxacin. In particular, the resistance to levofloxacin was at a high level for all 25 strains (MIC, ≥32 μg/ml), and the resistant strains were also associated with higher colony counts in blood cultures than the sensitive strains (geometric mean, 27.5 versus 6.8, respectively; t = 2.15; P = 0.037). Similarly, gatifloxacin-resistant strains also had higher colony counts than nonresistant strains (36.3 versus 6.4, respectively; t = 2.73; P = 0.009). For all antibiotics, 14 (56.0%) of the 25 S. mitis strains were resistant to ≥3 classes of antibiotics compared to 3 (12.0%) of 25 non-S. mitis strains (P = 0.001). Therefore, S. mitis was the most resistant viridans streptococcus.
Clinical features of infection.
The clinical features of patients are shown in Table 4. The patients included 21 women and 29 men with a mean age of 48 years. Hematologic cancers were predominant (74.0%, 37 of 50). The majority (82.0%) of patients had undergone anticancer chemotherapy within 3 weeks with frequent (62.0%) occurrence of mucositis and gastrointestinal symptoms. Severe neutropenia (ANC of <500/mm3) was common (66.0%), and it lasted significantly longer and was more pronounced in patients with S. mitis than in those with non-S. mitis strains.
TABLE 4.
Clinical features of viridans streptococcal bacteremia in cancer patients
| Feature | No. of patients
|
|||
|---|---|---|---|---|
| S. mitis, n = 25 | Non-S. mitis, n = 25 | Both, n = 50 (%) | P valueb | |
| Women/men | 10/15 | 11/14 | 21/29 | NS |
| Age <18 yrs | 5 | 0 | 5 (10.0) | 0.018 |
| Hematologic cancers | 21 | 16 | 37 (74.0) | NS |
| Anticancer treatment | 21 | 20 | 41 (82.0) | NS |
| Mucosal/GIa toxicity | 17 | 14 | 31 (62.0) | NS |
| Prior use of quinolone | 20 | 16 | 36 (72.0) | NS |
| Use of corticosteroids | 6 | 8 | 14 (28.0) | NS |
| ANC <500 for >10 days | 18 | 9 | 27 (54.0) | 0.011 |
| ANC <100 | 10 | 3 | 13 (26.0) | 0.024 |
| Fever | 25 | 21 | 46 (92.0) | NS |
| Septic shock | 13 | 4 | 17 (34.0) | 0.007 |
| Cause of death | 1 | 0 | 1 (2.0) | NS |
GI, gastrointestinal.
By t test. NS, nonsignificant.
Prophylactic use of antibiotics was common, particularly fluoroquinolones (mainly levofloxacin) (72.0%, 36 of 50). Among the 25 cases with levofloxacin-resistant streptococci, 23 (92.0%) cases had prophylactic use or treatment of this drug or another quinolone within a week of the positive culture. In contrast, prophylaxis with a quinolone was present only for 13 (52.0%) of the 25 cases that did not have levofloxacin-resistant organisms (P = 0.002). Thus, the emergence or selection of a quinolone-resistant strain was associated with prior usage.
Fever was present in 46 (92.0%) patients. Seventeen (34.0%) patients manifested septic shock, and S. mitis caused 13 of those cases, far more cases than those caused by non-S. mitis organisms (P = 0.007). In addition, all the S. mitis cases were monomicrobic, whereas two of the four non-S. mitis cases also involved methicillin-resistant Staphylococcus aureus and Enterobacter aerogenes, respectively. Ten (58.8%) of the 17 shock cases had colony counts of ≥50 per culture, significantly more than the nonshock cases (15.2%, 5/33) (P = 0.001). One patient died as a consequence of the streptococcal bacteremia; all others responded to specific treatment that usually consisted of vancomycin and another antibiotic(s).
Streptococcus parasanguis.
In view of the recent species status of S. parasanguis and limited clinical experience with it (9, 12, 21, 35), attention was paid to the clinical and microbiologic features of the five S. parasanguis cases. Four patients presented with fever, while the only afebrile one was on steroids. The colony counts were all below 10 per culture. Two cases were monomicrobic with S. parasanguis only. All infections resolved with treatment. Notably, all five strains were resistant to azithromycin, in contrast to 18 of 45 (40.0%) strains of other streptococci (P = 0.011). These strains were also intermediately resistant to penicillin (MIC range, 0.25 to 2 μg/ml).
DISCUSSION
It is well known that, in most centers, isolates of viridans streptococci from blood cultures are more likely to be contaminants than true pathogens (33, 34). In cancer patients with profound neutropenia, however, this dogma probably does not apply, i.e., they are more likely pathogens than contaminants. The cases and strains in this study were consecutive and unselected, yet 92% of patients were febrile and those nonfebrile ones might have had other reasons for being nonfebrile. Various risk factors for viridans streptococcal bacteremia have been identified and reviewed (11, 23, 28, 29, 32), including profound neutropenia, oral mucositis, prophylactic use of a fluoroquinolone or trimethoprim-sulfamethoxazole, exposure to high-dose chemotherapy (particularly cytosine arabinoside), stem cell transplantation, age of <18 years, and others. The importance of such bacteremia among pediatric patients with cancer has also been appreciated (1, 17, 26). The clinical findings from this study are consistent with these general features. The focus of this study, however, is microbiological, i.e., quantitative blood cultures, accurate identification of the streptococcal species, and antibiotic susceptibility.
Of the 50 strains sequenced, at least nine species were identified, more diverse than findings from previous studies that are based on biochemical tests. The new finding of five (10%) cases caused by S. parasanguis contributes to the knowledge about this relatively new species, and they were all intermediately resistant to penicillin and resistant to azithromycin. While the finding of the predominance of S. mitis is consistent with most previous studies, the lack of S. oralis, however, is somewhat surprising. A number of earlier phenotype studies have found that S. oralis was either the predominant species or a significant part, ranging from 32% to 61%, of the bacteremia-causing viridans streptococci (4, 11, 29). Biochemically, S. oralis and S. mitis are closely related, sharing several overlapping reactions. Their 16S rRNA gene sequences, however, differ overall by 13 nucleotides (of 1,453 bp or 99.1% identity) (18), 12 of which are within the beginning ∼300-bp region that was determined in this study. Thus, our differentiation between these two species was confident. Similar to our finding, another sequence-based study of six cases showed that the strains all turned out to be S. mitis (28). Two recent phenotype-based studies of viridans streptococci (2, 23), both from Spain, also found no S. oralis strains, in contrast to S. mitis as the vast majority (77.9 to 81.8%, 60/77 and 72/88, respectively). Therefore, the wide variation in the presence of S. oralis hints at the need for a study to compare the biochemical tests and sequencing method for uniformity.
In addition to the 24 established viridans streptococcal species, several other yet-to-be-described oral streptococci have been noted by culture-independent studies (20, 27). In this study, we found that 11 strains did not match established streptococcal species, some close to S. mitis, some distant. It is possible that these organisms represent novel species. The medical significance of these organisms may warrant further microbiologic studies and extensive sequencing analysis.
Resistance to penicillin among viridans streptococci is commonly seen at present: 13.4 to 23.4% with a MIC of ≥4 μg/ml (high level) (2, 8) and 16.9 to 42.9% with a MIC of 0.25 to 2 μg/ml (intermediate) (2, 8, 23). Our data further showed that high-level resistance was present only among S. mitis strains (28.0%, 7/25), not non-S. mitis strains. The S. mitis strains were also more resistant to fluoroquinolones and to ≥3 classes of antibiotics. This finding is consistent with the results of Doern et al. (8). Selection of quinolone-resistant S. mitis by prophylactic use of these drugs has been reported in a study of six cases (28). Among our 50 cases, statistical analysis showed that isolation of a levofloxacin-resistant organism was linked to prophylactic use of quinolones. In addition, strains resistant to levofloxacin or gatifloxacin were associated with higher colony counts on blood cultures than were their corresponding nonresistant strains.
A recent study has suggested that S. mitis causes more septic shock than non-S. mitis organisms (23). In another report (28), half of the six cases with quinolone-resistant S. mitis also presented with septic shock. Our data are consistent with these findings, and they further show that such septic shock was usually associated with high-level (≥100 colonies) bacteremia on quantitative blood cultures. The S. mitis strains caused higher levels of bacteremia than the non-S. mitis strains (Table 2; Fig. 1), which were seen more often in the young and elderly. Together, these data suggest that S. mitis is a more pathogenic viridans streptococcus.
Acknowledgments
We thank the staff in our clinical microbiology laboratory for the cultures and isolation of these organisms, the staff in our institutional core DNA sequencing facility for nucleotide sequencing, and Audrey Pham for assistance.
This work was supported in part by a University Cancer Foundation grant (to X.Y.H.) from The University of Texas M. D. Anderson Cancer Center and in part by a National Institutes of Health grant (CA16672) for the institutional core DNA sequencing facility.
REFERENCES
- 1.Ahmed, R., T. Hassall, B. Morland, and J. Gray. 2003. Viridans streptococcus bacteremia in children on chemotherapy for cancer: an underestimated problem. Pediatr. Hematol. Oncol. 20:439-444. [PubMed] [Google Scholar]
- 2.Alcaide, F., M. A. Benitez, J. Carratala, F. Guidiol, J. Linares, and R. Martin. 2001. In vitro activities of the new ketolide HMR 3647 (telithromycin) in comparison with those of eight other antibiotics against viridans group streptococci isolated from blood of neutropenic patients with cancer. Antimicrob. Agents Chemother. 45:624-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 4.Beighton, D., A. D. Carr, and B. A. Oppenheim. 1994. Identification of viridans streptococci associated with bacteraemia in neutropenic cancer patients. J. Med. Microbiol. 40:202-204. [DOI] [PubMed] [Google Scholar]
- 5.Bentley, R. W., J. A. Leigh, and M. D. Collins. 1991. Intrageneric structure of Streptococcus based on comparative analysis of small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 41:487-494. [DOI] [PubMed] [Google Scholar]
- 6.Clarridge, J. E., III. 2004. Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17:840-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing; 15th informational supplement. M100-S15, vol. 25(1). Clinical and Laboratory Standards Institute, Wayne, Pa.
- 8.Doern, G. V., M. J. Ferraro, A. B. Brueggemann, and K. L. Ruoff. 1996. Emergence of high rates of antimicrobial resistance among viridans group streptococci in the United States. Antimicrob. Agents Chemother. 40:891-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douglas, C. W., J. Heath, K. K. Hampton, and F. E. Preston. 1993. Identity of viridans streptococci isolated from cases of infective endocarditis. J. Med. Microbiol. 39:179-182. [DOI] [PubMed] [Google Scholar]
- 10.Drancourt, M., C. Bollet, A. Carlioz, R. Martelin, J. P. Gayral, and D. Raoult. 2000. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J. Clin. Microbiol. 38:3623-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elting, L. S., G. P. Bodey, and B. H. Keefe. 1992. Septicemia and shock syndrome due to viridans streptococci: a case-control study of predisposing factors. Clin. Infect. Dis. 14:1201-1207. [DOI] [PubMed] [Google Scholar]
- 12.Gomes, M. F., R. T. Teixeira, G. Plens, M. M. Silva, E. M. Pontes, and J. C. da Rocha. 2004. Naso-orbicular tissue necrosis by Streptococcus parasanguis in a patient with Fanconi anemia: clinical and laboratory aspects. Quintessence Int. 35:572-576. [PubMed] [Google Scholar]
- 13.Han, X. Y. Bacterial identification based on 16S ribosomal RNA gene sequence analysis. In Y.-W. Tang and C. Stratton (ed.), Advanced techniques in diagnostic microbiology, in press. Springer, New York, N.Y.
- 14.Han, X. Y., A. S. Pham, J. J. Tarrand, P. K. Sood, and R. Luthra. 2002. Rapid and accurate identification of mycobacteria by sequencing hypervariable regions of the 16S ribosomal RNA gene. Am. J. Clin. Pathol. 118:796-801. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs, J. A., H. C. Schouten, E. E. Stobberingh, and P. B. Soeters. 1995. Viridans streptococci isolated from the bloodstream. Relevance of species identification. Diagn. Microbiol. Infect. Dis. 22:267-273. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs, J. A., C. S. Schot, and L. M. Schouls. 2000. Haemolytic activity of the Streptococcus milleri group and relationship between haemolysis restricted to human red blood cells and pathogenicity in S. intermedius. J. Med. Microbiol. 49:55-62. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, C. C., and A. R. Tunkel. 2005. Viridans streptococci, groups C and G streptococci, and Gemella morbillorum. p. 2434-2451. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 6th ed. Churchill Livingstone, Inc., Philadelphia, Pa.
- 18.Kawamura, Y., X. G. Hou, F. Sultana, H. Miura, and T. Ezaki. 1995. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int. J. Syst. Bacteriol. 45:406-408. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura, Y., X. G. Hou, Y. Todome, F. Sultana, K. Hirose, S. E. Shu, T. Ezaki, and H. Ohkuni. 1998. Streptococcus peroris sp. nov. and Streptococcus infantis sp. nov., new members of the Streptococcus mitis group, isolated from human clinical specimens. Int. J. Syst. Bacteriol. 48:921-927. [DOI] [PubMed] [Google Scholar]
- 20.Kazor, C. E., P. M. Mitchell, A. M. Lee, L. N. Stokes, W. J. Loesche, F. E. Dewhirst, and B. J. Paster. 2003. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J. Clin. Microbiol. 41:558-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Pardo, F., A. Aguilera, M. Villa, C. Granado, A. Campos, and J. M. Cisneros. 2004. Double-chambered right ventricle associated with mural and pulmonic valve endocarditis: description of a clinical case and review of the literature. Echocardiography 21:171-173. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig, W., and H.-P. Klenk. 2001. Overview: a phylogenetic backbone and taxonomic framework for prokaryotic systematics, p. 49-65. In D. R. Boone, R. W. Castenholz, and G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. I. The archae and the deeply branching and phototrophic bacteria. Springer-Verlag, New York, N.Y. [Google Scholar]
- 23.Marron, A., J. Carratala, E. Gonzalez-Barca, A. Fernandez-Sevilla, F. Alcaide, and F. Gudiol. 2000. Serious complications of bacteremia caused by viridans streptococci in neutropenic patients with cancer. Clin. Infect. Dis. 31:1126-1130. [DOI] [PubMed] [Google Scholar]
- 24.Munford, R. S. 2001. Sepsis and septic shock, p. 799-804. In E. Braunwald, A. S. Fauci, D. L. Kasper, S. L. Hauser, D. L. Longo, and J. L. Jameson (ed.), Harrison's principles of internal medicine, 15th ed. McGraw-Hill, Inc., New York, N.Y.
- 25.Munson, M. A., A. Banerjee, T. F. Watson, and W. G. Wade. 2004. Molecular analysis of the microflora associated with dental caries. J. Clin. Microbiol. 42:3023-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paganini, H., V. Staffolani, P. Zubizarreta, L. Casimir, H. Lopardo, and V. Luppino. 2003. Viridans streptococci bacteraemia in children with fever and neutropenia: a case-control study of predisposing factors. Eur. J. Cancer 39:1284-1289. [DOI] [PubMed] [Google Scholar]
- 27.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Razonable, R. R., M. R. Litzow, Y. Khaliq, K. E. Piper, M. S. Rouse, and R. Patel. 2002. Bacteremia due to viridans group streptococci with diminished susceptibility to levofloxacin among neutropenic patients receiving levofloxacin prophylaxis. Clin. Infect. Dis. 34:1469-1474. [DOI] [PubMed] [Google Scholar]
- 29.Richard, P., D. V. G. Amador, P. Moreau, N. Milpied, M. P. Felice, T. Daeschler, J. L. Harousseau, and H. Richet. 1995. Viridans streptococcal bacteraemia in patients with neutropenia. Lancet 345:1607-1609. [DOI] [PubMed] [Google Scholar]
- 30.Ruoff, K. L., R. A. Whiley, and D. Beighton. 2003. Streptococcus, p. 405-421. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 31.Tarrand, J. J., C. Guillot, M. Wenglar, J. Jackson, J. D. Lajeunesse, and K. V. Rolston. 1991. Clinical comparison of the resin-containing Bactec 26 plus and the Isolator 10 blood culturing systems. J. Clin. Microbiol. 29:2245-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tunkel, A. R., and K. A. Sepkowitz. 2002. Infections caused by viridans streptococci in patients with neutropenia. Clin. Infect. Dis. 34:1524-1529. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein, M. P. 1996. Current blood culture methods and systems: clinical concepts, technology, and interpretation of results. Clin. Infect. Dis. 23:40-46. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein, M. P. 2003. Blood culture contamination: persisting problems and partial progress. J. Clin. Microbiol. 41:2275-2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whiley, R. A., H. Y. Fraser, C. W. Douglas, J. M. Hardie, A. M. Williams, and M. D. Collins. 1990. Streptococcus parasanguis sp. nov., an atypical viridans Streptococcus from human clinical specimens. FEMS Microbiol. Lett. 56:115-121. [DOI] [PubMed] [Google Scholar]