Abstract
Mycobacterium tuberculosis Beijing strains are prevalent in many parts of the world and often give rise to large institutional outbreaks. Such highly transmissible strains, often associated with multidrug resistance, are likely underrepresented in outbreaks reported from developing countries, mainly due to nonavailability of fast detection methods suitable in epidemiological surveillance studies. We evaluated a PCR assay based on amplification of mycobacterial interspersed repetitive unit locus 26 as a stand-alone method for unambiguous identification of Beijing strains. The method was used on blinded samples from 10 standard strains whose Beijing status was already confirmed by spoligotyping. All 10 strains were accurately identified, and their profiles were corroborated successfully with spoligotypes. The method was also applied to 70 different non-Beijing clinical isolates from different countries to allow discrimination of isolates. Owing to its accuracy, simplicity, and rapidity, the assay can be employed in laboratory-level testing of isolates linked to certain outbreaks. The test can also be adopted for direct application on clinical samples to save time on culturing bacilli for genotyping.
In 1995, a large section of the Mycobacterium tuberculosis patient isolates in Beijing, China, was reported to have shared multicopy IS6110 restriction fragment length polymorphism patterns. These strains were named “Beijing” strains (15). Soon it was realized that they are also present in many other populations irrespective of geographic boundaries (3, 5, 10). M. tuberculosis strains of Beijing family are linked to tuberculosis-related mortality in different parts of the world, sometimes involving clones with multiple drug resistance. Because Beijing strains are a widespread family of strains, often with links to multidrug resistance, there are concerns that these strains might be spreading rapidly due to increased global travel and the human immunodeficiency virus pandemic. Rapid identification of such clones is always an urgent need in case of large outbreaks, particularly those compounded with human immunodeficiency virus infection and/or nosocomial spreads. Such strains have an insertion of IS6110 in the genomic dnaA-dnaN locus (8). All Beijing family strains carry a characteristic spoligotype that appears to be specific for this family (7, 14). Spoligotyping reflects genotypic diversity in the direct repeat region, and “Beijing” spoligotypes only contain the last nine spacers (from 35 to 43). Spoligotyping has so far been the “gold standard” for genotypic identification of Beijing strains. This technique, however, is not very straightforward and is technically complicated and time consuming, besides having some interpretation problems (4). We describe a simple one-step PCR-based rapid detection method for accurate detection of Beijing strains. This PCR method specifically amplifies seven copies of the 51-bp consensus motif at mycobacterial interspersed repetitive unit (MIRU) locus 26 in the M. tuberculosis genome to give a characteristic PCR product of 641 bp.
The MIRU locus 26 coordinate in the M. tuberculosis H37 Rv chromosome is between 2996001 and 2996165 (12). For PCR amplification of this region, flanking primers (5′ TAGGTCTACCGTCGAAATCTGTGAC 3′ and 5′ CATAGGCGACCAGGCGAATAG 3′) were designed essentially as described earlier (13). Genomic DNA from 10 known strains of a confirmed Beijing spoligotype were used to amplify seven alleles at MIRU locus 26. About 70 clinical M. tuberculosis isolates belonging to a non-Beijing spoligotype were also genotyped with MIRU locus 26. A 25-μl PCR mixture contained about 5 ng of genomic DNA; 0.2 U of AmpliTaq DNA polymerase (Applied Biosystems, Foster City, Calif.); 5.0% dimethyl sulfoxide, 10% (10 mg/ml) bovine serum albumin; 0.2 mM (each) dATP, dCTP, dGTP, and dTTP (Amersham Pharmacia Biotech Inc., Little Chalfont, United Kingdom); 1.0× PCR buffer (Applied Biosystems); 0.4 μM (each) primer; and 2.5 μM MgCl2. The thermal cycling reactions were carried out starting with a denaturing step of 15 min at 95°C, followed by 35 cycles of 1 min at 94°C, 1 min at 59°C, and 1.30 min at 72°C. The reactions were terminated by incubation for 10 min at 72°C. Negative controls consisting of PCR mixtures lacking mycobacterial DNA were used. The amplicons were size separated on 2% agarose gel and visualized by ethidium bromide staining. The number of repeats at each locus was calculated as described earlier (13). All the PCR products were confirmed by DNA sequencing of locus 26 to verify the MIRU copy number in each case. To rule out PCR-generated contamination, we retested the samples and routinely included positive and negative controls during extractions, PCR mixture preparation, and electrophoresis. Separate laboratory spaces were dedicated for the processes of DNA extraction, PCR mixture preparation, and cycling to rule out the problem of artifactual contamination.
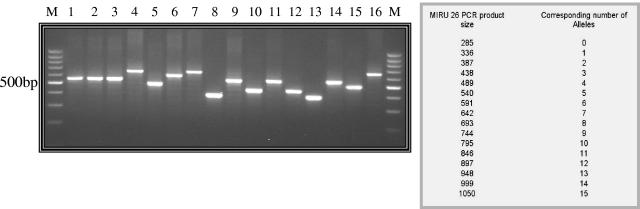
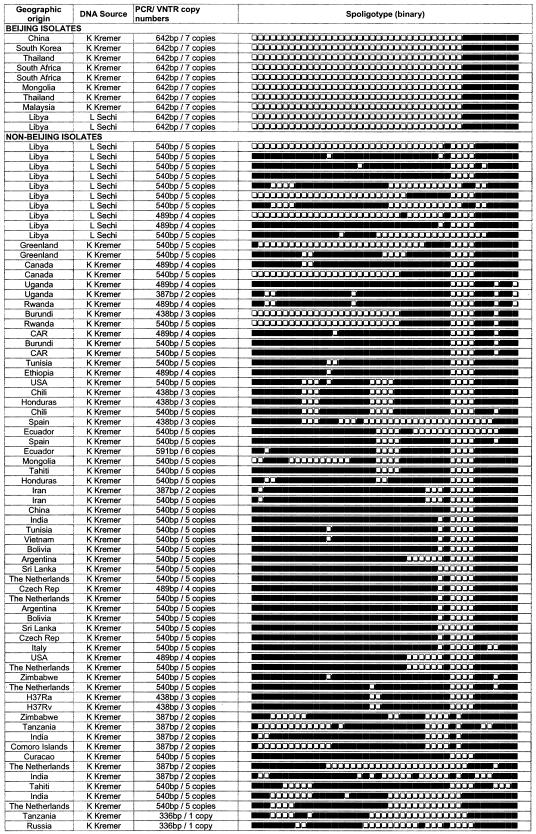
The M. tuberculosis MIRU 26 locus was found to be a highly specific, robust, and reproducible PCR target for epidemiological screening. We have used eight known strains of M. tuberculosis Beijing from the Kremer family of strains (7), and two strains were obtained from Libya. Spoligotypes for these strains were obtained from an online database, AmpliBASE-MT (http://www.cdfd.org.in/amplibase) (9). A blinded set of all these samples, including 70 non-Beijing strains, were tested with MIRU locus 26 PCR, and the results were decoded. All the Beijing genotypes were accurately identified with 100% specificity (Fig. 1). PCR results for all the test samples were extrapolated to spoligotype patterns with 100% concordance (Fig. 2). All 70 non-Beijing control samples could not amplify a product size equivalent to seven copies of MIRU 26. A non-Beijing control strain with a spoligotype nearly similar (but not identical) to those of Beijing strains also failed to amplify a product equal or nearly equal to 642 bp, thus proving the accuracy of our PCR method (Fig. 2).
FIG. 1.
PCR-based rapid identification of M. tuberculosis Beijing strains. Lanes show M. tuberculosis genotyping with the MIRU 26 Beijing isolates and non-Beijing isolates as follows. MIRU 26 Beijing isolates: copy number 7, lanes 4 and 7, Kremer 20 and 44, respectively, and lane 16, Libya LS35. Non-Beijing isolates: copy no. 6, lane 6, Kremer 98; copy no. 5, lanes 1, 2, 3, and 9, Kremer 120, 121, 12, and 95, respectively, and lanes 11 and 14, LS2 and LS7, respectively; copy no. 4, lanes 5 and 15, Kremer 82 and LS14, respectively; copy no. 3, lanes 10 and 12, Kremer 41 and 118, respectively; and copy no. 2, lanes 8 and 13, Kremer 97 and 15, respectively. Lane M, 100-bp marker (MBI Fermentas). The table on the right gives the predicted size of the MIRU 26 PCR product corresponding to the number of alleles. MIRU locus 26 copy numbers are given as per the conventions described in reference 13.
FIG. 2.
Genotypic and other characteristics of M. tuberculosis. Spoligotypes are from the AmpliBASE MT database (K. Kremer and L. Sechi).
Therapeutic regimens for the control of the tuberculosis epidemic will greatly benefit from knowledge gained from the molecular epidemiology, geographic genomics, and transmission dynamics of the pathogen (1, 2, 9, 11). A rapid and categorical method to determine the pathogen genotypes and aggressiveness of the circulating strains will be the key to the success of such programs. Multiple-drug-resistant strains can be highly disseminating, and therefore epidemic control with treatment strategies such as directly observed therapy short course becomes problematic. Strains such as Beijing have a particular propensity for acquiring drug resistance. Recently, the prevalence of the Beijing family of strains in the world has been extensively reviewed (6). However, there are no reports from many African, Latin American, and Asian countries, particularly India, where a lot of Beijing strains are feared to be circulating in cities like Delhi and Hyderabad (N. Ahmed et al., unpublished observations). This is mainly due to the nonavailability of rapid tests for routine screening purposes. Many laboratories in such countries are not sufficiently equipped for spoligotyping or do not have trained personnel. The present protocol, on the basis of its simplicity, specificity, and sensitivity, could provide an alternative genotypic method available to clinical microbiologists in resource-poor countries. Given the technical complexity of the spoligotyping method and obvious disadvantages linked to IS6110 typing (13), the MIRU locus 26 signature-based method appears to be suitable for laboratories involved in diagnostic and epidemiological screening on a routine basis.
The MIRU variable number tandem repeat (VNTR) markers (21 polymorphic loci in the genome) as such are quite robust and resolvable, due to the millions of different allelic combinations they offer for strain discrimination and typing. This PCR method can be used directly on total DNA obtained from patient samples such as peripheral blood, sputa, biopsies, and lavages (rather than waiting for the cultures to grow), thus obviating the need for biological containment laboratories for handling live cultures. While it is tempting to propose clinical laboratories to adapt this method on sputum samples for rapid identification of Beijing genotypes circulating in different geographic areas, further validation and confirmation of this test with a large number of known Beijing strains would be required to ensure that all the Beijing strains stably carry seven copies in MIRU locus 26. Also it will be essential to determine the molecular clock of such loci and ascertain their stability by using serial patient isolates obtained over the years. Nonetheless, this rapid diagnosis method may be used preliminarily to initiate any special precautionary treatments until spoligotyping can be performed.
Acknowledgments
We thank K. Kremer and D. van Soolingen for the use of DNA samples and spoligotype information from the Kremer family of strains.
This work was supported by a core grant to the Centre for DNA Fingerprinting and Diagnostics from the Department of Biotechnology, Government of India.
REFERENCES
- 1.Ahmed, N., M. Alam, K. R. Rao, F. Kauser, N. A. Kumar, N. N. Qazi, V. Sangal, V. D. Sharma, R. Das, V. M. Katoch, K. J. R. Murthy, S. Suneetha, S. K. Sharma, L. A. Sechi, R. H. Gilman, and S. E. Hasnain. 2004. Molecular genotyping of a large, multicentric collection of tubercle bacilli indicates geographical partitioning of strain variation and has implications for global epidemiology of Mycobacterium tuberculosis. J. Clin. Microbiol. 42:3240-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, N., L. Caviedes, M. Alam, K. R. Rao, V. Sangal, P. Sheen, R. H. Gilman, and S. H. Hasnain. 2003. Distinctiveness of Mycobacterium tuberculosis genotypes from human immunodeficiency virus type 1-seropositive and seronegative patients in Lima, Peru. J. Clin. Microbiol. 41:1712-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caminero, J. A., M. J. Pena, M. I. Campos-Herrero, J. C. Rodriguez, I. Garcia, P. Cabrera et al. 2001. Epidemiological evidence of the spread of a Mycobacterium tuberculosis strain of the Beijing genotype on Gran Canaria Island. Am. J. Respir. Crit. Care Med. 164:1165-1170. [DOI] [PubMed] [Google Scholar]
- 4.Cousins, D., S. Williams, E. Liébana, A. Aranaz, A. Bunschoten, J. van Embden., and T. Ellis. 1998. Evaluation of four DNA typing techniques in epidemiological investigation of bovine tuberculosis. J. Clin. Microbiol. 36:168-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doroudchi, M., K. Kremer, E. A. Basiri, M. R. Kadivar, D. Van Soolingen, and A. A. Ghaderi. 2000. IS6110-RFLP and spoligotyping of Mycobacterium tuberculosis isolates in Iran. Scand. J. Infect. Dis. 32:663-668. [DOI] [PubMed] [Google Scholar]
- 6.Glynn, J. R., J. Whiteley, P. J. Bifani, K. Kremer, and D. van Soolingen. 2000. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg. Infect. Dis. 8:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kremer, K., D. van Soolingen, R. Frothingham, W. H. Haas, P. W. M. Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley, M. A. Yakrus, J. M. Musser, and J. D. A. van Embden. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurepina, N. E., S. Sreevatsan, B. B. Plikaytis, P. J. Bifani, N. D. Connell, R. J. Donnelly et al. 1998. Characterization of the phylogenetic distribution and chromosomal insertion sites of five IS6110 elements in Mycobacterium tuberculosis: non-random integration in the dnaA-dnaN region. Tuber. Lung Dis. 79:31-42. [DOI] [PubMed] [Google Scholar]
- 9.Majeed, A. A., N. Ahmed, K. R. Rao, S. Ghousunnissa, F. Kauser, B. Bose, H. A. Nagarajaram, V. M. Katoch, D. V. Cousins, L. A. Sechi, R. H. Gilman, and S. E. Hasnain. 2004. AmpliBASE MT: a Mycobacterium tuberculosis diversity knowledge base. Bioinformatics 20:989-992. [DOI] [PubMed] [Google Scholar]
- 10.Niang, M. N., Y. G. de la Salmoniere, A. Samb, A. A. Hane, M. F. Cisse, B. Gicquel et al. 1999. Characterization of M. tuberculosis strains from west African patients by spoligotyping. Microbes Infect. 1:1189-1192. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqi, N., M. Shamim, A. Amin, D. S. Chauhan, R. Das, K. Srivastava, D. Singh, V. D. Sharma, V. M. Katoch, S. K. Sharma, M. Hanief, and S. E. Hasnain. 2001. Typing of drug resistant isolates of Mycobacterium tuberculosis from India using the IS6110 element reveals substantive polymorphism. Infect. Genet. Evol. 1:109-116. [DOI] [PubMed] [Google Scholar]
- 12.Supply, P., E. Mazars, S. Lesjean, V. Vincent, B. Gicquel, and C. Locht. 2000. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol. Microbiol. 36:762-771. [DOI] [PubMed] [Google Scholar]
- 13.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Crevel, R., R. H. H. Nelwan, W. de Lenne, Y. Veeraragu, A. G. van der Zanden, Z. Amin et al. 2001. Mycobacterium tuberculosis Beijing genotype strains associated with febrile response to treatment. Emerg. Infect. Dis. 7:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Soolingen, D., L. Qian, P. E. W. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. A. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]