Abstract
Bartonella spp. are small hemotropic bacteria infecting mammals. Four Bartonella species have been recently described in cattle and wild ruminants. To date, the biology and possible pathogenic role of Bartonella species isolated from ruminants are poorly understood. Therefore, a dairy herd of 448 cows and heifers was surveyed in order to establish the prevalence of Bartonella bovis and B. chomelii infections, the level of bacteremia, and the relationship between bacteremia and age or pregnancy status. The putative impact of Bartonella infection on production performance (individual milk cell count, milk yield) and reproductive status (success of artificial insemination [AI], placental retention, embryonic death, and abortion) was also assessed. The overall mean prevalence of B. bovis bacteremia was 59%, with the highest prevalence in heifers (92.5%). No B. chomelii was isolated, and 95% (114/120) of the B. bovis strains isolated and tested by PCR-restriction fragment length polymorphism belonged to type I. The level of bacteremia was higher in pregnant cows than in nonpregnant cows (P = 0.05), and the level of bacteremia rose during the last two-thirds of gestation (P < 0.001). There was no correlation between bacteremia and milk yield, individual milk cell count, success of first AI, interval between two calvings, or incidence of abortion and embryonic death. The interval from calving to first AI was shorter and the incidence of placental retention was lower in bacteremic animals than in nonbacteremic ones (P = 0.03 and P = 0.01, respectively).
Bacteria of the genus Bartonella currently comprise 20 species or subspecies, 10 of which are human pathogens. Bartonella quintana and B. bacilliformis are human-specific pathogens (3), while the remaining eight species or subspecies (B. henselae, B. clarridgeiae, B. vinsonii subsp. berkhoffii, B. vinsonii subsp. arupensis, B. grahamii, B. elizabethae, B. washoensis, and B. koehlerae) are zoonotic agents.
Recently, several new species of Bartonella were isolated from ruminants. B. schoenbuchensis and B. capreoli are commonly isolated from European roe deer (Capreolus capreolus) (1, 10). B. bovis is widely distributed among cattle in Europe and cattle and wild ruminants in North America (4, 5, 25) and has been recently isolated from cattle in Africa (17). The fourth species, B. chomelii, has been isolated for the first time from French cattle (24). These four species have not yet been demonstrated to be pathogenic for ruminants, but B. bovis has been recently implicated in a human patient with bacillary angiomatosis (poster 151 at the 4th International Conference on Rickettsiae, Logrono, Spain, June 2005). The biology and main epidemiological characteristics of these Bartonella species are still poorly understood (4).
Bartonellae induce a wide range of symptoms in their accidental hosts but more rarely in their reservoirs. Lymphadenopathy (29), bacillary angiomatosis and peliosis (19), uveitis (18), endocarditis (28, 30), and prolonged fever are the most common clinical manifestations in humans and to a lesser extent in dogs or cats. Natural reservoirs of Bartonella species are usually asymptomatic (3). However, some clinical signs such as fever, uveitis, lymphadenopathy, and endocarditis have been reported in experimentally or naturally infected cats (8, 21). Moreover, reproductive disorders such as repeat breeding, abortion, and fetal resorption have been described for experimentally infected queens (12). By using a heterologous murine model of infection with B. birtlesii isolated from Apodemus sp., a relationship between Bartonella infection and reproductive status in mice has also been demonstrated. Bacteremia was higher in experimentally infected female mice than in male mice and was significantly higher in pregnant mice than in nonpregnant mice. Furthermore, fetal resorption and abortion were significantly higher in bacteremic mice (2).
The objectives of this study were to establish the prevalence of B. bovis and B. chomelii bacteremia in a dairy cattle herd where reproductive disorders were occurring in order to correlate bacteremia with age, parity, and other reproductive parameters in a situation of natural infection.
MATERIALS AND METHODS
Animals.
The 448 sampled animals belonged to a dairy herd located 50 km south of Paris (France). Out of the 448 blood samples collected, 48 were contaminated and could not be further processed. This herd of Holstein cattle was a self-renewed herd. The ages of the 400 animals for which a blood sample could be processed ranged from 1 week to 9 years, including 42 calves (<6 months of age; mean age, 100.7 ± 50.1 days), 103 heifers (36 heifers 6 to 12 months old and 67 heifers 13 to 24 months old), and 255 cows (>2 years of age). At the time of blood collection, 194 inseminated females (156 cows and 38 heifers) were pregnant. This herd was selected because of its poor reproductive efficiency during the year prior to the present study, including a high percentage of pregnancy losses (embryonic death or abortion rate, >20%), long intervals between calving, and successful insemination (>90 days for one-third of the cows). The herd has been vaccinated against bovine viral diarrhea virus and colibacillosis. A treatment against internal and external parasites was administrated twice a year. Animals are fed with corn silage, hay, and concentrate individually adapted to their milk yield.
Blood sampling and culture.
The whole herd (448 animals) was sampled over a 3-day period in the second week of December. However, a subset of 25 randomly chosen animals (9 months to 5.5 years old) was bled more than once, as a first blood drawing was performed on these 25 animals 7 months earlier, during the spring season.
Four milliliters of whole blood was collected from each animal into EDTA tubes and frozen at −70°C until plated.
After blood centrifugation, the supernatants were kept at −20°C until processing for PCR. Blood sample pellets were cultured on brain heart infusion agar (Difco, Detroit, MI) containing 5% rabbit blood and incubated at 35°C in a 5% CO2 atmosphere. Each plate was checked for bacterial growth for up to 5 weeks. The number of CFU was registered. A serum sample (1 ml) was also collected from each animal and frozen at −70°C until used for pregnancy diagnosis.
Identification of isolates.
Presumptive identification of Bartonella isolates was performed by morphological examination of colonies (gray-white colonies that appeared after at least 4 days of incubation) and Gram staining. One or two suspected colonies for each plate were subcultured, and DNA was extracted from pure cultures of each isolate after boiling the isolates for 10 min in sterile water.
DNA extracts were used as templates in PCR amplification of the citrate synthase gene (gltA) with primers BhCs 781 and BhCs 1137 (27). An approximately 380-bp fragment was amplified. Molecular identification of a subset of isolates (120/236) was based on PCR-restriction fragment length polymorphism (RFLP) by digestion of the amplified gltA product with the restriction endonucleases TacI and MseI (5).
Individual parameters.
The following parameters were recorded for each animal at the time of blood collection: age, number of lactations, pregnancy and pregnancy stage, number of artificial inseminations (AIs) per conception, incidence of pregnancy loss, individual milk cell count, milk yield, and cell count. Pregnancy was assessed by measurement of pregnancy-specific protein B (UNCEIA, Maisons-Alfort, France) in serum (for cows more than 100 days after the previous calving) or by ultrasonography done on cows and heifers more than 35 days after AI. Cows were examined around 30 days postpartum to assess uterine involution.
Statistical analysis. (i) Animal husbandry characteristics associated with bacteremia.
Animal husbandry characteristics of bacteremic cows were compared to those of nonbacteremic ones (chi-square test for qualitative variables, the Student t test for quantitative ones). All the variables significantly associated with bacteremia at a P of 0.20 were introduced in a multivariate logistic regression model (analyzed variable, bacteremia taking the value 0 or 1). Explicative variables were removed from the model step by step to keep only the variables significantly associated with bacteremia at a P of 0.05.
The husbandry factors studied in relation to bacteremia were age (by year), pregnancy status (yes versus no), stage of pregnancy (in days and in months), interval from previous calving to blood sample collection, mean milk yield in 305 days, and mean cell count.
To illustrate the relationship between some factors and bacteremia with more accuracy, five semiquantitative groups (hereafter called SEMIQ) were defined based on the level of bacteremia: SEMIQ 0, no CFU/4 ml; SEMIQ 1, 1 to 399 CFU/4 ml; SEMIQ 2, 400 to 1,000 CFU/4 ml; SEMIQ 3, 1,001 to 4,800 CFU/4 ml; and SEMIQ 4, more than 4,800 CFU/4 ml.
(ii) Relationship between bacteremia and reproductive status.
Relationships between reproductive results and bacteremia were analyzed by using multivariate models (general linear models for quantitative reproductive results and logistic regression models for qualitative ones). Bacteremia was always introduced into the models.
Variables of the reproductive results studied were season of calving, placental retention after calving (yes versus no), uterine involution after calving (good versus bad), calving-to-AI interval, season of insemination, conception rate to first AI, pregnancy loss after AI, milk yield, and mean cell count (recorded monthly) near the date of AI.
RESULTS
Bacteriology of the whole herd.
A positive culture was obtained from 236 (59%) of the 400 usable samples. The number of colonies varied from 1 colony for 4 ml of blood to more than 4,800 colonies for 4 ml of blood. As the prevalence of bacteremia in the 1- to 2-year-old animals was more than 90%, these animals were excluded from studies of the correlation between bacteremia and age and breeding parameters. The prevalence of bacteremia differed among the various age groups, the highest prevalence being among the 1- to 2-year-old animals. Similarly, the bacteremia level varied with age (P < 0.01) (Table 1). The highest levels of bacteremia (≥4,800 CFU/4 ml) were detected mainly in the >1-year-old and <2-year-old age group. Only one of the >4-year-old animals had a high level of bacteremia (>1,000 CFU/4 ml).
TABLE 1.
Number and frequency of bacteremic animals by group level (SEMIQ) of bacteremia and by age
| Age | No. (%) of animalsa
|
|||||
|---|---|---|---|---|---|---|
| SEMIQ 0 | SEMIQ 1 | SEMIQ 2 | SEMIQ 3 | SEMIQ 4 | Total | |
| 0-6 mo | 41 (97.6)* | 0 (0) | 0 (0) | 0 (0) | 1 (2.4) | 42 |
| 7-12 mo | 16 (44.4)† | 10 (27.8) | 4 (11.1) | 2 (5.6) | 4 (11.1) | 36 |
| >1-≤2 yr | 5 (7.5)‡ | 21 (31.3) | 11 (16.4) | 13 (19.4) | 17 (25.4) | 67 |
| >2-≤3 yr | 26 (24.3)§ | 49 (45.8) | 17 (15.9) | 8 (7.5) | 7 (6.5) | 107 |
| >3-4 yr | 25 (42.4)†¶ | 24 (40.7) | 6 (10.2) | 3 (5.1) | 1 (1.7) | 59 |
| >4-≤5 yr | 17 (50.0)†¶∥# | 16 (47.1) | 1 (2.9) | 0 (0) | 0 (0) | 34 |
| >5-≤6 yr | 17 (54.8)†¶∥# | 13 (41.9) | 1 (3.3) | 0 (0) | 0 (0) | 31 |
| >6-≤7 yr | 10 (83.3)@ | 2 (16.7) | 0 (0) | 0 (0) | 0 (0) | 12 |
| >7-≤8 yr | 4 (66.7) | 2 (33.3) | 0 (0) | 0 (0) | 0 (0) | 6 |
| >8 yr | 3 (50) | 2 (33.3) | 0 (0) | 1 (16.7) | 0 (0) | 6 |
| Total | 164 | 139 | 40 | 27 | 30 | 400 |
Rows marked with superscript symbols are significantly different (P < 0.05 chi-square test).
In the age group of 0 to 6 months, all 42 animals but 1 (a 5-month-old calf) were culture negative whereas 55.6% (20/36) of the cows in the 7- to 12-month-old age group were bacteremic. However, none of these animals became bacteremic before 8 months of age. By 2 years of age, 92.5% of the heifers (62/67) were bacteremic. Thereafter, bacteremia prevalence regularly decreased to 29.2% (7/24 cows) in cows >6 years old(Table 1). By univariate analysis, the difference in bacteremia prevalence by age group was statistically significant (P<0.001).
Bacteriology of the subset group.
Eight (32%) of the 25 animals previously sampled were bacteremic at the first bleeding during the spring. Seven months later, 5 of the 8 bacteremic animals remained bacteremic whereas 8 of the 17 previously nonbacteremic animals became bacteremic.
Identification of isolates.
A total of 120 (51%) isolates were tested by PCR-RFLP. Two RFLP profiles of B. bovis were observed: 95% (114/120) of the isolates tested corresponded to RFLP profile type I strains, and 5% (6/120) belonged to RFLP type II. No isolate of B. chomelii was identified.
Bacteremia and milk yield.
There was no significant correlation (P > 0.05) between the presence of bacteremia and milk yield or individual milk cell counts.
Bacteremia and reproduction.
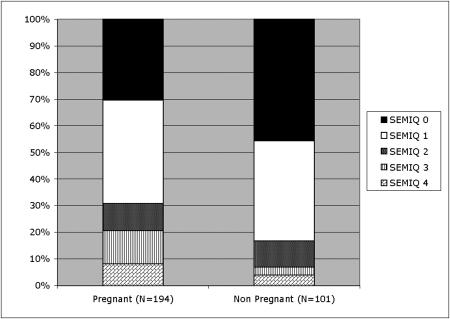
At the time of whole-herd blood sampling, 156 inseminated cows and 38 inseminated heifers were pregnant. The percentage of bacteremic females was significantly higher in pregnant females (135/194; 69.6%) than in nonpregnant females (55/101; 54.5%; P < 0.001) (Fig. 1). Multivariate analysis evidenced a statistically significant association between bacteremia and age and pregnancy (P < 0.0001 and P < 0.05, respectively). The mean level of bacteremia was lower during the first trimester of gestation compared to the second and third trimesters (P < 0.001) (Table 2). Abortion and embryonic death were marginally significant (P = 0.06), and there was no correlation (P > 0.05) between the presence of bacteremia and the percentage of success of AI and the interval between two calvings. On the contrary, there was a positive correlation between bacteremia and absence of placental retention (P = 0.01) (Tables 3 and 4).
FIG. 1.
Qualitative and quantitative aspects of bacteremia and pregnancy.
TABLE 2.
Bacteremia and trimester of pregnancy
| Pregnancy stage | No. (%) of positive females | Mean of SEMIQa ± SD |
|---|---|---|
| 0-89 days | 56/82 (68) | 0.75 ± 0.13 |
| 90-179 days | 33/43 (77) | 1.32 ± 0.17 |
| 180 days-calving | 44/69 (64) | 1.42 ± 0.14 |
The value of bacteremia in each semiquantitative group varied from 0 (SEMIQ 0) to 4 (SEMIQ 4).
TABLE 3.
Relationship and significance of the main parameters recorded and bacteremia (multivariate analysis) in case of calving before blood sampling
| Variable | No. of cowsa | Additional variable | P value |
|---|---|---|---|
| Interval from calving | 99 | Bacteremia | 0.03 |
| to first AI | Season of insemination | 0.04 | |
| Milk yield | 0.02 | ||
| Placental retention | 202 | Bacteremia | 0.01 |
| Age | 0.08 | ||
| Success of first AI | 209 | Bacteremia | 0.55 |
| Age | 0.03 | ||
| Interval from calving to first AI | 0.02 | ||
| Interval from calving | 96 | Bacteremia | 0.77 |
| to successful AI | Interval from calving to first AI | 0.0005 | |
| Success of first AI | 0.0001 | ||
| Milk yield | 0.005 | ||
| Embryonic or fetal | 95 | Bacteremia | 0.06 |
| death | Interval from calving to first AI | 0.006 | |
| Milk yield | 0.002 |
In this model of multivariate analysis.
TABLE 4.
Relationship between bacteremia and breeding performance in case of calving before blood sampling
| Variable | Mean value ± SD (no. of animals)
|
P valuea | |
|---|---|---|---|
| Nonbacteremic animals | Bacteremic animals | ||
| Interval from calving to first AI (days) | 82.1 ± 2.2 (38) | 76.1 ± 1.8 (61) | 0.03 |
| % Placental retention | 33.7 (92) | 17.3 (110) | 0.01 |
| % Success of first AI | 38.1 (97) | 36.1 (112) | 0.55 |
| Interval from calving to successful AI (days) | 130.6 ± 52.5 (36) | 121.2 ± 49.0 (60) | 0.77 |
| % Embryonic or fetal death | 16.7 (36) | 5.1 (59) | 0.06 |
From multivariate analysis.
The interval between calving and the first AI was significantly affected by bacteremia, the season of insemination, and milk yield (production in 305 days); (Table 3). In bacteremic animals, the first AI occurred earlier than in nonbacteremic ones (Table 4).
DISCUSSION
The present study is the first to demonstrate long-term B. bovis infection in a heavily infected dairy herd (overall prevalence, 59%). Bacteremia over a period of at least 7 months was detected in a few animals. Furthermore, it is the first study to establish the dynamics of cattle infection with B. bovis according to the age group of the animals. None of the <8-month-old calves but one were bacteremic, whereas 92.5% of the 1- to 2-year-old animals were bacteremic, indicating that the initial infection of dairy cattle with B. bovis is occurring long before the first calving.
Such high percentages of Bartonella prevalence have been reported for other species, such as roe deer (1), rodents (22), and to a lesser extent stray cats (7, 15). As Bartonella infections are known to be vector borne, the very high level of infection of cattle within the first year of life suggests a high level of exposure to various vectors that are both highly infected and efficient vectors. Among the different possible types of vector transmission, transmission by Bartonella-infected ticks has been suggested (4-6, 13, 32). Ticks collected from roe deer or questing adult ticks were reported to be PCR positive for bartonellae, including B. bovis (6, 32). Similarly, a B. bovis gltA sequence was identified in ticks (Ixodes and Rhipicephalus spp.) collected in the pastures grazed by this herd, but the level of Bartonella infestation in these ticks was very low (<3%) (data not shown). Therefore, the fact that 92.5% of the heifers were bacteremic could not be explained by the unique role of ticks in vector-mediated transmission of B. bovis to cattle. Recent studies of Hippoboscidae have shown a very high prevalence of Bartonella DNA in these biting flies and suggest that bloodsucking insects could be an important vector of bartonellae in ruminants (11, 14). Effective vertical transmission from cow to calf could also explain such a high percentage of infection in heifers. However, the absence of bacteremia in calves <8 months of age despite the lack of maternal antibodies in 19% of the calves (8/42); (data not shown) does not support such a hypothesis.
Bacteremia was also detected 7 months apart in one-fifth of 25 animals. These data can indicate either persistent infection or multiple reinfections, as described in cats for B. henselae or B. clarridgeiae infections (20, 29, 33).
In this study, we failed to isolate B. chomelii, a strain recently discovered in French cattle (24) and which appears to be uncommon in cattle. This Bartonella species has not yet been identified in cattle in North America (4, 5). The repartition of B. bovis strains between RFLP type 1 (95%) and RFLP type 2 (5%) is similar to the percentages observed in cattle in California (5). Because of the low prevalence of RFLP type 2, no associations between RFLP types and age or reproduction status could be assessed. There is no clear explanation for the existence of a predominant type and the respective role of these two types in any pathological effect.
The level of bacteremia (CFU per milliliter) is age dependent and is also associated with the reproductive status and stage of gestation. The level of bacteremia is significantly higher in young heifers but also in pregnant females. This is the first report of such an association in naturally infected animals. In mice experimentally infected (2), pregnancy was correlated with a higher level of bacteremia.
The immunosuppressive effect of pregnancy on specific immunity and its effect on pathogens are well known (23). Hormonal changes during pregnancy could impact the level of Bartonella bacteremia, as suggested by the difference between the Bartonella bacteremia levels of male and female mice (2). Furthermore, the placenta in advanced pregnancy could be a site of intense multiplication of bartonellae, as already demonstrated for other α-2 proteobacteria such as brucellae (31). The last two trimesters of pregnancy seem essential for the relationship between bartonellae and pregnant cows. Firstly, there was no difference in bacteremia prevalence between the nonpregnant and pregnant populations in the first trimester of pregnancy but the level of bacteremia increased in the pregnant animals in the last two trimesters of pregnancy, and secondly, placental postpartum expulsion was easier for bacteremic cows, which could explain the relationship between bacteremia and the interval between calving and the first AI. Davies et al. (9) have demonstrated that bovines are unique among mammals concerning major histocompatibility complex class I antigen expression on trophoblastic cells during pregnancy. In this species, the expression of major histocompatibility complex class I proteins during the second half of gestation triggers an inflammatory reaction that contributes to placental separation at parturition. It is an appropriate supposition that Bartonella infection could also participate in this inflammatory reaction and therefore be associated with the facilitation of placental expulsion.
There is no correlation between bacteremia and milk production parameters. Bartonella infection does not appear to modify milk quality. It is well known that lipopolysaccharides are among the most important modifiers of the cell count of milk (16). Though some Bartonella species possess an active endotoxin (26), we cannot determine the impact of the presence of bartonellae in the mammary gland, as we have not tested the presence of bartonellae in milk.
The lack of effect of Bartonella infection on early events of pregnancy in this natural model of infection is quite surprising, as experimental models of infection have shown that Bartonella primary infection induces embryonic death and/or abortion in cats (12) and mice (2). However, the primary infection of the heifers in this study occurred long (at least 7 months) before their first pregnancy. Furthermore, the effect of primary infection could not be studied in heifers because of the very low percentage of uninfected animals. Therefore, we cannot discard the hypothesis of an effect of Bartonella infection on early events in pregnancy. Further studies of cohorts of infected and noninfected heifers are warranted to investigate the pathological impact of primary Bartonella infection on pregnant heifers. However, a trend was observed for the association between embryonic or fetal death incidence and bacteremia in cows. The lack of significance in the present study might be due to the low number of animals studied. If the data had been generated from more diverse management systems of herds, perhaps this finding would have been statistically significant.
The existence of Bartonella infection in domestic ruminants is now well established. The importance of bacteremia, long-term persistence of infection, and apparent adaptation to the host mimic the already known situation of Bartonella infection in cats and mice with their specific Bartonella species. Differences appear at least in the percentage of the population infected, the pathway of inoculation or transmission (fleas are very uncommon in cattle), and the ambiguous effects on reproductive parameters. The potential role of B. bovis as a zoonotic agent and its infectivity for other animal species, as suggested by isolation of B. bovis (previously B. weissii) from domestic cats (4, 5), underlines the need for further studies of this bacterium in its natural reservoir and its possible accidental hosts.
REFERENCES
- 1.Bermond, D., H. J. Boulouis, R. Heller, G. Van Laere, H. Monteil, B. B. Chomel, A. Sander, C. Dehio, and Y. Piemont. 2002. Bartonella bovis Bermond et al. sp. nov. and Bartonella capreoli sp. nov., isolated from European ruminants. Int. J. Syst. Evol. Microbiol. 52:383-390. [DOI] [PubMed] [Google Scholar]
- 2.Boulouis, H. J., F. Barrat, D. Bermond, F. Bernex, D. Thibault, R. Heller, J. J. Fontaine, Y. Piemont, and B. B. Chomel. 2001. Kinetics of Bartonella birtlesii infection in experimentally infected mice and pathogenic effect on reproductive functions. Infect. Immun. 69:5313-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitschwerdt, E. B., and D. L. Kordick. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 13:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitschwerdt, E. B., S. Sontakke, A. Cannedy, S. I. Hancock, and J. M. Bradley. 2001. Infection with Bartonella weissii and detection of Nanobacterium antigens in a North Carolina beef herd. J. Clin. Microbiol. 39:879-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, C. C., B. B. Chomel, R. W. Kasten, R. M. Heller, H. Ueno, K. Yamamoto, V. C. Bleich, B. M. Pierce, B. J. Gonzales, P. K. Swift, W. M. Boyce, S. S. Jang, H. J. Boulouis, and Y. Piemont. 2000. Bartonella spp. isolated from wild and domestic ruminants in North America. Emerg. Infect. Dis. 6:306-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, C. C., B. B. Chomel, R. W. Kasten, V. Romano, and N. Tietze. 2001. Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J. Clin. Microbiol. 39:1221-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomel, B. B., R. C. Abbott, R. W. Kasten, K. A. Floyd-Hawkins, P. H. Kass, C. A. Glaser, N. C. Pedersen, and J. E. Koehler. 1995. Bartonella henselae prevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J. Clin. Microbiol. 33:2445-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chomel, B. B., A. C. Wey, R. W. Kasten, B. A. Stacy, and P. Labelle. 2003. Fatal case of endocarditis associated with Bartonella henselae type I infection in a domestic cat. J. Clin. Microbiol. 41:5337-5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, C. J., J. R. Hill, J. L. Edwards, F. N. Schrick, P. J. Fisher, J. A. Eldridge, and D. H. Schlafer. 2004. Major histocompatibility antigen expression on the bovine placenta: its relationship to abnormal pregnancies and retained placenta. Anim. Reprod. Sci. 82-83:267-280. [DOI] [PubMed] [Google Scholar]
- 10.Dehio, C., C. Lanz, R. Pohl, P. Behrens, D. Bermond, Y. Piemont, K. Pelz, and A. Sander. 2001. Bartonella schoenbuchii sp. nov., isolated from the blood of wild roe deer. Int. J. Syst. Evol. Microbiol. 51:1557-1565. [DOI] [PubMed] [Google Scholar]
- 11.Dehio, C., U. Sauder, and R. Hiestand. 2004. Isolation of Bartonella schoenbuchensis from Lipoptena cervi, a blood-sucking arthropod causing deer ked dermatitis. J. Clin. Microbiol. 42:5320-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guptill, L., L. N. Slater, C. C. Wu, T. L. Lin, L. T. Glickman, D. F. Welch, J. Tobolski, and H. HogenEsch. 1998. Evidence of reproductive failure and lack of perinatal transmission of Bartonella henselae in experimentally infected cats. Vet. Immunol. Immunopathol. 65:177-189. [DOI] [PubMed] [Google Scholar]
- 13.Halos, L., T. Jamal, R. Maillard, F. Beugnet, A. Le Menach, H. J. Boulouis, and M. Vayssier-Taussat. 2005. Evidence of Bartonella sp. in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet. Res. 36:79-87. [DOI] [PubMed] [Google Scholar]
- 14.Halos, L., T. Jamal, R. Maillard, B. Girard, J. Guillot, B. Chomel, M. Vayssier-Taussat, and H. J. Boulouis. 2004. Role of Hippoboscidae flies as potential vectors of Bartonella spp. infecting wild and domestic ruminants. Appl. Environ. Microbiol. 70:6302-6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller, R., M. Artois, V. Xemar, D. De Briel, H. Gehin, B. Jaulhac, H. Monteil, and Y. Piemont. 1997. Prevalence of Bartonella henselae and Bartonella clarridgeiae in stray cats. J. Clin. Microbiol. 35:1327-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogan, J., and K. L. Smith. 2003. Coliform mastitis. Vet. Res. 34:507-519. [DOI] [PubMed] [Google Scholar]
- 17.Kelly, P. J., B. Davoust, J. Gomez, D. Raoult, and B. La Scola. 2005. Bartonella bovis in cattle in Africa. Vet. Microbiol. 105:155-156. [DOI] [PubMed] [Google Scholar]
- 18.Kerkhoff, F. T., A. M. Bergmans, A. van der Zee, and A. Rothova. 1999. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J. Clin. Microbiol. 37:4034-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koehler, J. E. 1994. Bacillary angiomatosis: investigation of the unusual interactions between Rochalimaea bacilli and endothelial cells. J. Lab. Clin. Med. 124:475-477. [PubMed] [Google Scholar]
- 20.Kordick, D. L., and E. B. Breitschwerdt. 1998. Persistent infection of pets within a household with three Bartonella species. Emerg. Infect. Dis. 4:325-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kordick, D. L., T. T. Brown, K. Shin, and E. B. Breitschwerdt. 1999. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J. Clin. Microbiol. 37:1536-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosoy, M. Y., R. L. Regnery, O. I. Kosaya, D. C. Jones, E. L. Marston, and J. E. Childs. 1998. Isolation of Bartonella spp. from embryos and neonates of naturally infected rodents. J. Wildl. Dis. 34:305-309. [DOI] [PubMed] [Google Scholar]
- 23.Luppi, P. 2003. How immune mechanisms are affected by pregnancy. Vaccine 21:3352-3357. [DOI] [PubMed] [Google Scholar]
- 24.Maillard, R., P. Riegel, F. Barrat, C. Bouillin, D. Thibault, C. Gandoin, L. Halos, C. Demanche, A. Alliot, J. Guillot, Y. Piemont, H. J. Boulouis, and M. Vayssier-Taussat. 2004. Bartonella chomelii sp. nov., isolated from French domestic cattle (Bos taurus). Int. J. Syst. Evol. Microbiol. 54:215-220. [DOI] [PubMed] [Google Scholar]
- 25.Maillard, R., M. Vayssier-Taussat, C. Bouillin, C. Gandoin, L. Halos, B. Chomel, Y. Piemont, and H. J. Boulouis. 2004. Identification of Bartonella strains isolated from wild and domestic ruminants by a single-step PCR analysis of the 16S-23S intergenic spacer region. Vet. Microbiol. 98:63-69. [DOI] [PubMed] [Google Scholar]
- 26.Matera, G., M. C. Liberto, A. Quirino, G. S. Barreca, A. G. Lamberti, M. Iannone, E. Mancuso, E. Palma, F. A. Cufari, D. Rotiroti, and A. Foca. 2003. Bartonella quintana lipopolysaccharide effects on leukocytes, CXC chemokines and apoptosis: a study on the human whole blood and a rat model. Int. Immunopharmacol. 3:853-864. [DOI] [PubMed] [Google Scholar]
- 27.Norman, A. F., R. Regnery, P. Jameson, C. Greene, and D. C. Krause. 1995. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 33:1797-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raoult, D., P. E. Fournier, M. Drancourt, T. J. Marrie, J. Etienne, J. Cosserat, P. Cacoub, Y. Poinsignon, P. Leclercq, and A. M. Sefton. 1996. Diagnosis of 22 new cases of Bartonella endocarditis. Ann. Intern. Med. 125:646-652. [DOI] [PubMed] [Google Scholar]
- 29.Regnery, R., M. Martin, and J. Olson. 1992. Naturally occurring “Rochalimaea henselae” infection in domestic cat. Lancet 340:557-558. [DOI] [PubMed] [Google Scholar]
- 30.Roux, V., S. J. Eykyn, S. Wyllie, and D. Raoult. 2000. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J. Clin. Microbiol. 38:1698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samartino, L. E., and F. M. Enright. 1993. Pathogenesis of abortion of bovine brucellosis. Comp. Immunol. Microbiol. Infect. Dis. 16:95-101. [DOI] [PubMed] [Google Scholar]
- 32.Schouls, L. M., I. Van De Pol, S. G. Rijpkema, and C. S. Schot. 1999. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J. Clin. Microbiol. 37:2215-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto, K., B. B. Chomel, R. W. Kasten, C. M. Hew, D. K. Weber, and W. I. Lee. 2002. Experimental infection of specific pathogen free (SPF) cats with two different strains of Bartonella henselae type I: a comparative study. Vet. Res. 33:669-684. [DOI] [PubMed] [Google Scholar]