Abstract
A comprehensive study of Bacillus subtilis gene expression patterns in response to amino acid availability was performed by means of proteomics and transcriptomics. The methods of two-dimensional protein gel electrophoresis and DNA macroarray technology were combined to analyze cells exponentially grown in minimal medium with and without 0.2% Casamino Acids (CAA). This approach revealed about 120 genes predominantly involved in amino acid biosynthesis, sporulation, and competence, which were downregulated in CAA-containing medium. Determination of sporulation frequencies confirmed the physiological relevance of the expression data.
The soil bacterium Bacillus subtilis is capable of synthesizing all proteinogenic amino acids. Besides their function as building blocks for cellular proteins, amino acids represent precursors in the biosynthesis of nucleotides and other cellular components such as cell wall polymers. Amino acid biosynthetic pathways are regulated on the level of enzyme activity as well as on the level of enzyme synthesis to ensure cellular adaptation to various requirements for amino acids under different growth conditions.
The expression of many amino acid biosynthetic genes in B. subtilis is controlled by transcription antitermination mechanisms: the ilv-leu operon (15, 27), the cysES operon (14), and the proBA operon (3) belong to the T-box family, which includes most of the aminoacyl-tRNA synthetase genes (17, 31). These genes are regulated by tRNA-mediated antitermination in response to starvation for a particular amino acid (16). The genes of the S-box regulon are controlled by a transcription antitermination system in response to methionine availability (18). The S-box-specific leader region elements were identified in 11 transcriptional units in the B. subtilis genome, whereby the majority of the 26 gene products fulfills functions in sulfate assimilation and methionine biosynthesis (18, 26, 29). Regulation of tryptophan biosynthetic genes by transcription attenuation and translation control mechanisms is mediated by the RNA binding protein TRAP as well as by a T-box-dependent regulatory mechanism (reviewed in reference 1). Expression of the lysC gene encoding the lysine feedback-controlled aspartokinase II (30) is regulated by lysine availability via an antitermination system, too (20). In B. subtilis, the arginine biosynthetic operons are repressed by the AhrC regulatory protein, which is activated in the presence arginine (6, 37).
In this study we report on the gene expression profile of B. subtilis exponentially grown in minimal medium with and without 0.2% Casamino Acids (CAA), thereby providing an insight into the response of B. subtilis to different amino acid availabilities. The genes which were differentially expressed under the two growth conditions included those for amino acid biosynthesis, sporulation, and competence development.
Characterization of the proteome under conditions of different amino acid availability.
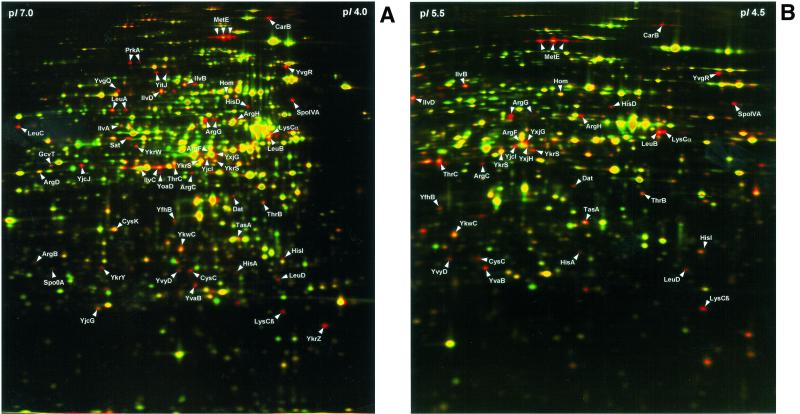
The B. subtilis 168 strain was cultivated aerobically at 37°C in a minimal medium (pH 7.5) containing 50 mM Tris, 8 mM MgSO4, 13 mM KCl, 18 mM NaCl, 0.6 mM KH2PO4, 2 mM CaCl2, 0.001 mM FeSO4, 0.01 mM MnSO4, 10 mM glutamine, 0.2% (wt/vol) glucose, and 0.8 mM tryptophan. Cells were grown in the presence or absence of 0.2% CAA (vitamin free; Difco, Detroit, Mich.) and harvested in the exponential growth phase after reaching an optical density at 500 nm of 0.5. Compared to the cultures in minimal medium (G = 45 min), shorter generation times (G = 25 min) were observed for the cultures in CAA-containing minimal medium (see Fig. 2). Preparation of protein extracts and two-dimensional protein gel electrophoresis were performed as previously described (2). About 65 protein spots present on the control gel in the pH range of 4 to 7 decreased in intensity or were completely absent when the medium was supplemented with CAA (Fig. 1A). In addition, narrow pH gradient gels were utilized in the pH range of 4.5 to 5.5, which allows for a better resolution of the most overcrowded region of the pH 4 to 7 gels (Fig. 1B). Altogether, 58 protein spots representing 50 different proteins downregulated by CAA (Table 1 laser desorption ionization-time of flight mass spectrometry as previously described (2).
FIG. 2.
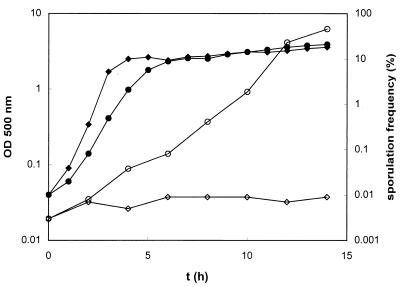
Growth curve (closed symbols) and sporulation frequencies (open symbols) of B. subtilis 168 cultivated in minimal medium (circles) and in minimal medium supplemented with 0.2% CAA (diamonds). The number of spores per milliliter of culture was determined as the number of heat-resistant (80°C for 30 min) CFU on Luria-Bertani plates, and the number of viable cells was determined as the total number of CFU (before heat treatment). Sporulation frequencies were defined as the percentage of heat-resistant CFU. Four independent experiments,which gave comparable results, were carried out.
FIG. 1.
Dual-channel image analysis of two-dimensional protein patterns of B. subtilis 168 exponentially grown in minimal medium and minimal medium supplemented with 0.2% CAA. Cytosolic protein extracts were separated by two-dimensional gel electrophoresis in the pH gradient of 4 to 7 (A) and in the pH gradient of 4.5 to 5.5 (B). Dual-channel images of the silver-stained gels were created by computer-aided transformation of the gel images by using the software DECODON Delta2D (DECODON GmbH, Greifswald, Germany). Red spots represent proteins whose synthesis was decreased in the presence of CAA.
TABLE 1.
Genes with significantly higher expression during exponential growth of B. subtilis in minimal medium without CAA as revealed by transcriptome and proteome analysesa
| Gene and category | Function | Induction (expt 1, expt 2) | Transcriptional organization |
|---|---|---|---|
| Amino acid biosynthesis | |||
| argC | N-acetylglutamate gamma-semialdehyde dehydrogenase | 6.0, 3.8 | argC-argJ-argB-argD-carA-carB-argF |
| argJ | Ornithine acetyltransferase; amino acid acetyltransferase | 20.5, 11.6 | argC-argJ-argB-argD-carA-carB-argF |
| argB | N-acetylglutamate 5-phosphotransferase | 29.3, 34.2 | argC-argJ-argB-argD-carA-carB-argF |
| argD | N-acetylornithine aminotransferase | 4.7, 8.6 | argC-argJ-argB-argD-carA-carB-argF |
| carA | Carbamoyl-phosphate transferase (subunit A) | 30.0, 16.2 | argC-argJ-argB-argD-carA-carB-argF |
| carB | Carbamoyl-phosphate transferase (subunit B) | 4.6, 5.1 | argC-argJ-argB-argD-carA-carB-argF |
| argF | Ornithine carbamyoltransferase | 5.7, 9.6 | argC-argJ-argB-argD-carA-carB-argF |
| argG | Argininosuccinate synthase | 17.3, 23.9 | argG-argH-ytzD |
| argH | Argininosuccinate lyase | 21.4, 27.5 | argG-argH-ytzD |
| ytzD | Unknown function | —, — | argG-argH-ytzD |
| cysH | Phosphoadenosine phosphosulfate sulfotransferase | 3.3, 3.8 | cysH-cysP-sat-cysC-ylnD-ylnE-ylnF |
| cysP | Sulfate permease | 5.5, 3.3 | cysH-cysP-sat-cysC-ylnD-ylnE-ylnF |
| sat | Sulfate adenylyltransferase | 4.7, 4.6 | cysH-cysP-sat-cysC-ylnD-ylnE-ylnF |
| cysC | Adenylylsulfate kinase | 2.7, 4.7 | cysH-cysP-sat-cysC-ylnD-ylnE-ylnF |
| ylnD | Similar to uroporphyrine-III C-methyltransferase | 5.7, 5.2 | cysH-cysP-sat-cysC-ylnD-ylnE-ylnF |
| ylnE | Unknown function | 4.3, 3.1 | cysH-cysP-sat-cysC-ylnD-ylnE-ylnF |
| ylnF | Similar to uroporphyrine-III C-methyltransferase | 4.0, 3.6 | cysH-cysP-sat-cysC-ylnD-ylnE-ylnF |
| cysK | Cysteine synthase A | 2.0, 2.2 | cysK |
| hisZ | Histidyl-tRNA synthetase | —, — | hisZ-hisG-hisD-hisB-hisH-hisA-hisF-hisI |
| hisG | ATP phosphoribosyltransferase | —, 1.1 | hisZ-hisG-hisD-hisB-hisH-hisA-hisF-hisI |
| hisD | Histidinol dehydrogenase | 4.5, 7.2 | hisZ-hisG-hisD-hisB-hisH-hisA-hisF-hisI |
| hisB | Imidazoleglycerol-phosphate dehydratase | 5.7, 5.6 | hisZ-hisG-hisD-hisB-hisH-hisA-hisF-hisI |
| hisH | Amidotransferase | 5.2, 5.1 | hisZ-hisG-hisD-hisB-hisH-hisA-hisF-hisI |
| hisA | Phosphoribosylformimino-5-aminoimidazole carboxamideribotide isomerase | 5.5, 7.3 | hisZ-hisG-hisD-hisB-hisH-hisA-hisF-hisI |
| hisF | Cyclase-like protein (synthesis of imidazole glycerol phosphate) | 4.2, 6.6 | hisZ-hisG-hisD-hisB-hisH-hisA-hisF-hisI |
| hisI | Phosphoribosyl-ATP pyrophosphohydrolase; phosphribosyl-AMP cyclohydrolase | 8.5, 10.4 | hisZ-hisG-hisD-hisB-hisH-hisA-hisF-hisI |
| hom | Homoserine dehydrogenase | 2.2, 2.1 | hom-thrC-thrB |
| thrC | Threonine synthase | 2.0, 2.2 | hom-thrC-thrB |
| thrB | Homoserine kinase | 3.2, 3.5 | hom-thrC-thrB |
| ilyA | Threonine dehydratase | 3.9, 3.6 | ilvA-ypmP |
| ypmP | Unknown function | —, — | ilvA-ypmP |
| ilvB | Acetolactate synthase (large subunit) | 7.1, 7.9 | ilvB-ilvH-ilvC-leuA-leuB-leuC-leuD |
| ilvH | Acetolactate synthase (small subunit) | 11.0, 7.0 | ilvB-ilvH-ilvC-leuA-leuB-leuC-leuD |
| ilvC | Ketol-acid reductoisomerase | 9.9, 10.1 | ilvB-ilvH-ilvC-leuA-leuB-leuC-leuD |
| leuA | 2-Isopropylmalate synthase | 9.5, 9.7 | ilvB-ilvH-ilvC-leuA-leuB-leuC-leuD |
| leuB | 3-Isopropylmalate dehydrogenase | 9.6, 10.6 | ilvB-ilvH-ilvC-leuA-leuB-leuC-leuD |
| leuC | 3-Isopropylmalate dehydratase (large subunit) | 10.0, 10.9 | ilvB-ilvH-ilvC-leuA-leuB-leuC-leuD |
| leuD | 3-Isopropylmalate dehydratase (small subunit) | 7.0, 10.5 | ilvB-ilvH-ilvC-leuA-leuB-leuC-leuD |
| ilvD | Dihydroxyacid dehydratase | 4.6, 4.4 | ilvD |
| lvsC | Aspartokinase II (alpha and beta subunit) | 8.5, 13.4 | lysC |
| metE | Cobalamin-independent methionine synthase | 56.8, 59.9 | metC |
| ybgE | Similar to branched chain amino acid aminotransferase | 3.0, 4.1 | ybgE |
| yitJ | Similar to methionine synthase | 4.7, — | yitJ |
| yjcI | Similar to cystathionine gamma-synthase | 9.9, 6.9 | yjcI-yjcJ |
| yjcJ | Similar to cystathionine beta-lyase | 14.0, 9.9 | yjcI-yjcJ |
| yoaD | Similar to phosphoglycerate dehydrogenase | 3.1, — | yoaD-yoaC-yoaB |
| yoaC | Similar to xylulokinase | 2.5, 3.2 | yoaD-yoaC-yoaB |
| yoaB | Unknown function | 14.5, 9.2 | yoaD-yoaC-yoaB |
| yvgR | Similar to sulfite reductase | 2.1, 2.9 | yvgR-yvgQ |
| yvgQ | Similar to sulfite reductase | 5.0, 8.6 | yvgR-yvgQ |
| yxjG | Similar to methionine synthase | 10.5, 7.6 | yxjG |
| yxjH | Similar to methionine synthase | —, — | yxjH |
| Competence | |||
| comER | Nonessential gene for competence | 3.2, 3.4 | comER |
| comGA | Required for exogenous DNA binding | 2.0, 5.3 | comGA-comGB-comGC-comGD-comGE-comGF-comGG |
| comGB | Required for exogenous DNA binding | 2.2, 4.2 | comGA-comGB-comGC-comGD-comGE-comGF-comGG |
| comGC | Required for exogenous DNA binding | 3.2, 7.1 | comGA-comGB-comGC-comGD-comGE-comGF-comGG |
| comGD | Required for exogenous DNA binding | 3.0, 5.6 | comGA-comGB-comGC-comGD-comGE-comGF-comGG |
| comGE | Required for exogenous DNA binding | —, — | comGA-comGB-comGC-comGD-comGE-comGF-comGG |
| comGF | Required for exogenous DNA binding | 4.2, 4.2 | comGA-comGB-comGC-comGD-comGE-comGF-comGG |
| comGG | Required for exogenous DNA binding | 2.4, 4.3 | comGA-comGB-comGC-comGD-comGE-comGF-comGG |
| comK | Competence transcription factor | 3.1, 4.8 | comK |
| Transition state functions and sporulation | |||
| appD | Oligopeptide ABC transporter (ATP-binding protein) | —, — | appD-appF-appA-appB-appC |
| appF | Oligopeptide ABC transporter (ATP-binding protein) | 0.7, 0.9 | appD-appF-appA-appB-appC |
| appA | Oligopeptide ABC transporter (peptide-binding protein) | 21.9, 22.5 | appD-appF-appA-appB-appC |
| appB | Oligopeptide ABC transporter (permease) | —, 5.7 | appD-appF-appA-appB-appC |
| appC | Oligopeptide ABC transporter (permease) | —, — | appD-appF-appA-appB-appC |
| cotE | Spore coat protein | 13.5, 5.8 | cotE |
| cotV | Spore coat protein | 6.1, — | cotV-cotW-cotX |
| cotW | Spore coat protein | 3.5, 3.5 | cotV-cotW-cotX |
| cotX | Spore coat protein | 7.5, — | cotV-cotW-cotX |
| dacF | Penicillin binding protein; required for spore cortex synthesis | 4.6, 2.4 | dacF-spoIIAA-spoIIAB-sigF |
| spoIIAA | Anti-anti-sigma factor (antagonist of SpoIIAB) | 0.9, 0.9 | dacF-spoIIAA-spoIIAB-sigF |
| spoIIAB | Anti-sigma factor (antagonist of sigma F); serine kinase | —, — | dacF-spoIIAA-spoIIAB-sigF |
| sigF | RNA polymerase sporulation-specific sigma factor | 5.9, 5.3 | dacF-spoIIAA-spoIIAB-sigF |
| gerM | Germination (cortex hydrolysis) and sporulation (putative role in peptidoglycan synthesis) | 3.3, 3.8 | gerM |
| prkA | Serine protein kinase | 2.5, 2.5 | prkA |
| qcrA | Menaquinol:cytochrome c oxidoreductase (iron-sulfur subunit) | 4.1, 4.0 | qcrA-qcrB-qcrC |
| qcrB | Menaquinol:cytochrome c oxidoreductase (cytochrome b subunit) | —, — | qcrA-qcrB-qcrC |
| qcrC | Menaquinol:cytochrome c oxidoreductase (cytochrome c subunit) | 3.1, 5.3 | qcrA-qcrB-qcrC |
| rapA | Aspartyl phosphate phosphatase | 3.2, 3.4 | rapA-phrA |
| phrA | Inhibitor of the activity of phosphatase RapA | 4.1, 1.8 | rapA-phrA |
| rsfA | Probable transcriptional regulator of sigma F-dependent genes | 4.5, 4.2 | rsfA |
| sp0A | Two-component response regulator | 2.9, 2.5 | spo0A |
| spoIIB | Required for endospore development | 6.4, 3.7 | spoIIB |
| spoIIIAG | Mutants block sporulation after engulfment | 5.5, 4.3 | spoIIIAG-spoIIIAH |
| spoIIIAH | Mutants block sporulation after engulfment | 7.8, 4.3 | spoIIIAG-spoIIIAH |
| usd | Required for translation of spoIIID | —, — | usd-spoIIID |
| spoIIID | Transcriptional regulator of sigma E- and sigma K-dependent genes | 10.2, 4.7 | usd-spoIIID |
| spoIVA | Required for proper spore cortex formation and coat assembly | 2.4, 2.5 | spoIVA |
| spoVID | Required for assembly of the spore coat | 3.2, 3.5 | spoVID-ysxE |
| ysxE | Unknown function | —, — | spoVID-ysxE |
| sspE | Small acid-soluble spore protein | 4.3, 2.5 | sspE |
| yjbX | Unknown function; glutamic acid-rich protein | 7.2, 6.2 | yjbX |
| ylaK | Similar to phosphate starvation inducible protein | 6.5, 4.7 | ylaK |
| ylbO | Unknown function | 6.8, 3.0 | ylbO |
| yqxM | Unknown function | 0.9, 1.1 | yqxM-sipW-tasA |
| sipW | Type I signal peptidase | 0.9, 1.3 | yqxM-sipW-tasA |
| tasA | Spore-associated antimicrobial protein required for spore coat assembly | 2.0, 3.1 | yqxM-sipW-tasA |
| ytfI | Unknown function | —, — | ytfI-ytfJ |
| ytfJ | Unknown function | 3.7, 3.8 | ytfI-ytfJ |
| yuiC | Unknown function | 3.1, 3.0 | yuiC |
| ywcI | Unknown function | 10.1, 7.3 | ywcI-sacT |
| sacT | Transcriptional antiterminator (regulation of the sacP operon) | 9.9, 6.4 | ywcI-sacT |
| yvyD | Similar to a sigma 54 modulating factor | 2.4, 3.4 | yvyD |
| Other functions | |||
| dat | Probable d-alanine aminotransferase | 1.5, 1.7 | dat |
| gcvT | Probable aminomethyltransferase | 1.9, 1.9 | gcvT-gcvPA-gcvPB |
| gcvPA | Probable glycine decarboxylase (subunit 1) | 2.8, 2.6 | gcvT-gcvPA-gcvPB |
| gcvPB | Probable glycine decarboxylase (subunit 2) | 2.4, 2.6 | gcvT-gcvPA-gcvPB |
| mpr | Extracellular metalloprotease | 6.6, 3.2 | mpr-ybfJ |
| ybfJ | Unknown function | —, — | mpr-ybfJ |
| yfhK | Similar to cell division inhibitor | 3.2, 6.5 | yfhK |
| yhgB | Unknown function | —, — | yhgB-yhfA-yhaA |
| yhfA | Unknown function | —, — | yhgB-yhfA-yhaA |
| yhaA | Similar to aminoacylase | 4.2, 4.5 | yhgB-yhfA-yhaA |
| ykrT | Unknown function | 1.6, 1.7 | ykrT-ykrS |
| ykrS | Similar to initiation factor eIF-2B (alpha subunit) | 2.5, 3.5 | ykrT-ykrS |
| ykrW | Similar to ribulose-bisphosphate carboxylase | 2.7, 3.3 | ykrW-ykrX-ykrY-ykrZ |
| ykrX | Unknown function | 3.9, 4.9 | ykrW-ykrX-ykrY-ykrZ |
| ykrY | Unknown function | 6.8, 4.8 | ykrW-ykrX-ykrY-ykrZ |
| ykrZ | Unknown function | 3.1, 2.1 | ykrW-ykrX-ykrY-ykrZ |
| ykuN | Similar to flavodoxin | —, — | ykuN-ykuO-ykuP-ykuQ |
| ykuO | Unknown function | 2.8, 2.9 | ykuN-ykuO-ykuP-ykuQ |
| ykuP | Similar to flavodoxin | 4.7, 3.5 | ykuN-ykuO-ykuP-ykuQ |
| ykuQ | Similar to tetrahydrodipicolinate succinylase | 0.9, 1.3 | ykuN-ykuO-ykuP-ykuQ |
| ykwC | Similar to 3-hydroxyisobutyrate dehydrogenase | 1.7, 1.9 | ykwC |
| yodF | Similar to proline permease | 4.5, 4.4 | yodF |
| yojA | Similar to gluconate permease | —, — | yojA-yojB-yojC |
| yojB | Unknown function | 3.8, 3.9 | yojA-yojB-yojC |
| yojC | Unknown function | —, — | yojA-yojB-yojC |
| yqiX | Similar to amino acid ABC transporter (binding protein) | 7.3, 10.3 | yqiX-yqiY-yqiZ |
| yqiY | Similar to amino acid ABC transporter (permease) | 5.3, 7.1 | yqiX-yqiY-yqiZ |
| yqiZ | Similar to amino acid ABC transporter (ATP binding) | 7.1, 16.0 | yqiX-yqiY-yqiZ |
| yuaF | Unknown function | —, — | yuaF-yuaG-yuaI |
| yuaG | Similar to epidermal surface antigen | 3.4, 3.1 | yuaF-yuaG-yuaI |
| yuaI | Unknown function | —, — | yuaF-yuaG-yuaI |
| yvaC | Unknown function | —, — | yvaC-yvaB |
| yvaB | Similar to NAD(P)H dehydrogenase (quinone) | 2.3, 1.7 | yvaC-yvaB |
| ywfH | Similar to 3-oxoacyl-acyl-carrier protein reductase | 3.8, 3.2 | ywfH |
| Unknown functions | |||
| ybdO | Unknown function | 3.2, 7.2 | ybdO |
| yfhB | Unknown function | 3.7, 3.0 | yfhB |
| yfmA | Unknown function | —, — | yfmA-yflT |
| yflT | Unknown function | 4.6, 40.9 | yfmA-yflT |
| yjcE | Unknown function | 3.8, 3.6 | yjcE-yjcD |
| yjcD | Similar to ATP-dependent DNA helicase | 0.7, 1.4 | yjcE-yjcD |
| yjcH | Unknown function | 1.7, 3.1 | yjcH-yjcG-yjcF |
| yjcG | Unknown function | 3.7, 3.6 | yjcH-yjcG-yjcF |
| yjcF | Unknown function | 2.4, 1.8 | yjcH-yjcG-yjcF |
| ykvR | Unknown function | 3.2, 3.3 | ykvR |
| ylaJ | Unknown function | 3.1, 3.0 | ylaJ |
| ylqB | Unknown function | 3.3, 3.8 | ylqB |
| yqgZ | Unknown function | 4.6, 3.4 | yqgZ |
| yvaW | Unknown function | —, — | yvaW-yvaX-yvaY |
| yvaX | Unknown function | —, — | yvaW-yvaX-yvaY |
| yvaY | Unknown function | 10.6, 12.2 | yvaW-yvaX-yvaY |
Significantly regulated genes are given in bold face. Significant regulation was defined as at least threefold changes in the mRNA levels in both macroarray experiments. Genes were also regarded as significantly regulated when at least twofold changes in the mRNA levels were confirmed by the proteome analysis or the operon structure. Underlined gene names indicate higher expression in minimal medium without CAA as revealed by the proteome analysis. The calculated expression level ratios are shown for both independent macroarray experiments in the column “induction,” whereby dashes indicate that specific signals for these genes were below the significance threshold. The putative functions of the y-gene-encoded proteins were obtained from the SubtiList database.
Of these 50 proteins, 35 represented amino acid biosynthetic enzymes involved in the synthesis of lysine, methionine, threonine, arginine, cysteine, histidine, leucine, isoleucine, and valine. Furthermore, the sporulation proteins SpoIVA, SpoOA, and TasA, the serine protein kinase PrkA, the proteins Dat and GcvT involved in metabolism of amino acids, and 9 proteins with still-unknown functions (Y-proteins) were identified to be downregulated by addition of CAA.
Analysis of the transcriptome under conditions of differentamino acid availability.
B. subtilis strain 168 was cultivated in the described minimal medium with and without 0.2% CAA. Total RNA was isolated from exponentially growing cells (optical density at 500 nm of 0.5) and was checked by Northern blot analysis (data not shown). Cell harvesting, preparation of RNA, and macroarray analysis with Panorama B. subtilis gene arrays and specific cDNA labeling primers (Sigma-Genosys, The Woodlands, Tex.) were performed as described by Eymann et al. (10). Two macroarray experiments were carried out by using independently isolated RNA preparations and different array batches. Quantification of hybridization signals, background subtraction, and calculation of normalized intensity values of the individual spots were performed with the ArrayVision software (version 5.1; Imaging Research, St. Catherines, Ontario, Canada) as described by Eymann et al. (10). Expression level ratios of three or more in both independent experiments were considered significant. Final evaluation of the macroarray data included the consideration of putative operons derived from the genome sequence, using the SubtiList database (http://genolist.pasteur.fr/SubtiList/) as well as previously known transcriptional units.
Scatter plots comparing the normalized intensity values revealed that mRNA levels of the majority of genes did not differ significantly between both growth conditions, whereas about 100 genes were expressed at a level more than threefold higher in minimal medium without CAA (data not shown). According to the criteria specified in the footnote to Table 1, altogether 114 genes showed significantly elevated expression levels. Of these genes, most encode proteins with functions in amino acid biosynthesis (42 genes), transition state processes and sporulation (32 genes), and competence (8 genes). The patterns of CAA-regulated genes found by the proteomic and transcriptomic approaches were similar, whereby about 50% of the differentially expressed genes could be detected in the proteome analysis. Interestingly, only three genes (guaC, purK, and yxjA) involved in nucleotide metabolism and transport were expressed at a significantly higher level during growth in CAA-containing medium.
At the mRNA level, 16 transcriptional units involved in amino acid biosynthesis were identified to be significantly downregulated by CAA (Table 1). Previous studies suggested regulation by amino acid availability for the operons argCJBD-carAB-argF, argGH (37), hom-thrCB (40), and ilvBHC-leuABCD (15), the lysC gene (20), and also the S-box-regulated transcriptional units cysHP-sat-cysC-ylnDEF, yjcIJ, yoaDCB, ykrWXYZ, ykrTS, metE, yitJ, and yxjG. In this study, of the 11 transcriptional units potentially belonging to the S box regulon (18) the 8 mentioned above were expressed at a significantly lower level in CAA-containing medium. Of the approximately 50 B. subtilis genes involved in amino acid transport, only the yqiXYZ operon encoding an amino acid ABC transport system (33) and the monocistronic-transcribed yodF gene encoding a putative proline permease showed significantly different expression levels in response to amino acid availability. Like the argCJBD-carAB-argF and argGH operons, the yqiXYZ operon is preceded by an AhrC recognition site (25). As a further result of the transcriptome study, addition of 0.2% CAA did not affect expression of glycine, serine, proline, tyrosine, and phenylalanine biosynthetic genes. Glutamine and tryptophan biosynthesis was not expected to be regulated under the conditions compared in this study because of the presence of these amino acids in both cultivation media.
A second group of genes expressed at a significantly higher level during growth in the absence of CAA encodes proteins with functions in competence development. This group includes the comK gene encoding the competence transcription factor that activates the genes involved in DNA binding and uptake (reviewed in reference 8). Furthermore, nearly the complete comG operon (4) shared the same expression pattern. The srfA operon also involved in competence regulation exhibited an approximately twofold increased mRNA level. These results are in agreement with previous observations that competence is repressed by the addition of CAA during exponential growth of B. subtilis in minimal medium (35).
As shown in Table 1, many B. subtilis genes that encode products with functions in transient-phase adaptation and sporulation exhibited significantly higher mRNA levels in minimal medium without CAA. Among these genes were the Spo0A dependently regulated operons appDFABC (21, 22), qcrABC (41), and ywcI-sacT (11). The phosphorylated response regulator Spo0A activates transcription of many sporulation genes and negatively regulates genes preventing sporulation, such as abrB (19). The abrB gene encodes a repressor of early-stationary-phase and sporulation genes, including the sigH gene. Thus, by repressing abrB transcription Spo0A-P stimulates synthesis of σH and thereby enhances its own transcription. Besides spo0A itself, the σH-dependently transcribed genes tasA (34, 38), spoVG, and yvyD were upregulated in the absence of CAA. Most of the sporulation genes significantly upregulated in the absence of CAA belong to the σE regulon (U. Völker, personal communication), which comprises early-mother-cell-specific genes.
Sporulation frequency in response to amino acid availability.
Sporulation genes were shown to be regulated in response to amino acid availability during exponential growth of B. subtilis. To verify the physiological relevance of the proteome and transcriptome data, sporulation frequencies were determined at several time points during growth of B. subtilis in the described minimal medium with and without CAA. The cultures grown under the two different conditions were inoculated with the same preculture. As shown in Fig. 2, the sporulation frequency of the minimal medium culture without CAA increased continuously up to 14 h after inoculation, whereas addition of CAA almost completely prevented the appearance of spores at least up to 14 h after inoculation. These data confirmed that a significantly lower amount of the population enters the sporulation process during exponential growth in the presence of CAA.
Concluding remarks.
Several adaptive processes are involved in the response of B. subtilis to growth-limiting levels of nutrients. As revealed by this study, expression of sporulation and competence genes is affected by amino acid availability during exponential growth of B. subtilis. Due to the fact that a relatively small portion of the culture enters sporulation in the exponential growth phase, only strongly expressed sporulation genes caused significant signals in the transcriptome analysis.
In agreement with these results, Cosby and Zuber (5) described a negative effect of amino acids on expression of early-stage sporulation genes. They reported that addition of CAA to minimal medium affects sigH expression as well as σH-dependent transcription. The alternative σ factor σH is required for the transcription of early-stationary-phase and sporulation genes and represents the first σ factor in a gene expression cascade resulting in spore formation (23). As reported by Eymann et al. (9), a few σH-dependent genes (spo0A, spoVG, yvyD, and ytxGHI) are induced in response to amino acid starvation in a RelA-dependent manner. It was shown earlier that a relA mutant sporulates less effectively than the wild type after a shift down from CAA-containing medium to medium without CAA (24). The ribosome-bound ppGpp synthetase RelA is activated by uncharged tRNAs or by glucose starvation. Increased ppGpp levels mediate the stringent control which allows adaptation of cell growth to the present nutrient conditions. Cells growing in minimal medium might be partially starved for amino acids, which possibly elevates the basal ppGpp level. In this study, higher expression of the σH-dependent genes yvyD, spo0A, spoVG, and tasA in minimal medium without CAA was shown during exponential growth. It is interesting that almost the same σH-dependent genes which are induced in a RelA-dependent manner in amino-acid-starved cells were expressed at significantly higher levels during growth in minimal medium without CAA. In B. subtilis the CodY regulator mediates amino acid repression of several genes involved in nitrogen metabolism (7, 12, 13, 36, 39) as well as competence development (35), motility (28), and sporulation. Recently, Ratnayake-Lecamwasam et al. (32) reported that CodY represents a GTP-sensing protein and functions as a repressor under conditions of high GTP levels. They suggested that the stringent response might be involved in the inactivation of the CodY regulator by decreasing the cellular GTP pool. As spo0A is repressed by CodY (32), a decrease in the GTP level might result in enhanced sigH transcription. In the present study, expression of the genes spo0A and comK as well as the srfA operon was shown to be downregulated in CAA-containing medium, whereas most of the other known CodY-dependent genes did not share the same regulatory pattern, indicating the multiple regulation of CodY controlled genes.
Acknowledgments
This work was supported from grants of the DFG, the BMBF, and the Fonds der Chemischen Industrie to M.H.
We are indebted to U. Völker for valuable information concerning the sporulation sigma factor regulons.
REFERENCES
- 1.Babitzke, P., and P. Gollnick. 2001. Posttranscription initiation control of tryptophan metabolism in Bacillus subtilis by the trp RNA-binding attenuation protein (TRAP), anti-TRAP, and RNA structure. J. Bacteriol. 183:5795-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Büttner, K., J. Bernhardt, C. Scharf, R. Schmid, U. Mäder, C. Eymann, H. Antelmann, U. Völker, A. Völker, and M. Hecker. 2001. A comprehensive two-dimensional map of cytosolic proteins of Bacillus subtilis. Electrophoresis 22:2908-2935. [DOI] [PubMed] [Google Scholar]
- 3.Chopin, A., V. Biaudet, and S. D. Ehrlich. 1998. Analysis of the Bacillus subtilis genome sequence reveals nine new T-box leaders. Mol. Microbiol. 29:662-664. [DOI] [PubMed] [Google Scholar]
- 4.Chung, Y. S., and D. Dubnau. 1998. All seven comG open reading frames are required for DNA binding during transformation of competent Bacillus subtilis. J. Bacteriol. 180:41-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosby, W. M., and P. Zuber. 1997. Regulation of Bacillus subtilis σH (spo0H) and AbrB in response to changes in external pH. J. Bacteriol. 179:6778-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czaplewski, L. G., A. K. North, M. C. Smith, S. Baumberg, and P. G. Stockley. 1992. Purification and initial characterization of AhrC: the regulator of arginine metabolism genes in Bacillus subtilis. Mol. Microbiol. 6:267-275. [DOI] [PubMed] [Google Scholar]
- 7.Debarbouille, M., R. Gardan, M. Arnaud, and G. Rapoport. 1999. Role of bkdR, a transcriptional activator of the sigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J. Bacteriol. 181:2059-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubnau, D., and K. Turgay. 2000. Regulation of competence in Bacillus subtilis and its relation to stress response, p. 249-260. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 9.Eymann, C., G. Mittenhuber, and M. Hecker. 2001. The stringent response, σH-dependent gene expression and sporulation in Bacillus subtilis. Mol. Gen. Genet. 264:913-923. [DOI] [PubMed] [Google Scholar]
- 10.Eymann, C., G. Homuth, C. Scharf, and M. Hecker. 2002. Bacillus subtilis functional genomics: characterization of the stringent response by proteome and transcriptome analysis. J. Bacteriol. 184:2500-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fawcett, P., P. Eichenberger, R. Losick, and P. Youngman. 2000. The transcriptional profile of early to middle sporulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 97:8063-8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferson, A. E., L. V. Wray, Jr., and S. H. Fisher. 1996. Expression of the Bacillus subtilis gabP gene is regulated independently in response to nitrogen and amino acid availability. Mol. Microbiol. 22:693-701. [DOI] [PubMed] [Google Scholar]
- 13.Fisher, S. H., K. Rohrer, and A. E. Ferson. 1996. Role of CodY in regulation of the Bacillus subtilis hut operon. J. Bacteriol. 178:3779-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon, Y., R., R. Breton, H. Putzer, M. Pelchat, M. Grunberg-Manago, and J. Lapointe. 1994. Clustering and cotranscription of the Bacillus subtilis genes encoding the aminoacyl-tRNA synthetases specific for glutamate and for cysteine and the first enzyme for cysteine biosynthesis. J. Biol. Chem. 269:2473-2482. [PubMed] [Google Scholar]
- 15.Grandoni, J. A., S. A. Zahler, and J. M. Calvo. 1992. Transcriptional regulation of the ilv-leu operon of Bacillus subtilis. J. Bacteriol. 174:3212-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grundy, F. J., and T. M. Henkin. 1993. tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell 74:475-482. [DOI] [PubMed] [Google Scholar]
- 17.Grundy, F. J., and T. M. Henkin. 1994. Conservation of a transcription antitermination mechanism in aminoacyl-tRNA synthetase and amino acid biosynthesis genes in gram-positive bacteria. J. Mol. Biol. 235:798-804. [DOI] [PubMed] [Google Scholar]
- 18.Grundy, F. J., and T. M. Henkin. 1998. The S box regulon: a new global transcription termination control system for methionine and cysteine biosynthesis genes in gram-positive bacteria. Mol. Microbiol. 30:737-749. [DOI] [PubMed] [Google Scholar]
- 19.Hoch, J. 1995. Control of cellular development in sporulating bacteria by the phosphorelay two-component signal transduction system, p. 129-144. In J. Hoch and T. Sihavy (ed.), Two-component signal transduction. ASM Press, Washington, D.C.
- 20.Kochhar, S., and H. Paulus. 1996. Lysine-induced premature transcription termination in the lysC operon of Bacillus subtilis. Microbiology 142:1635-1639. [DOI] [PubMed] [Google Scholar]
- 21.Koide, A., and J. A. Hoch. 1994. Identification of a second oligopeptide transport system in Bacillus subtilis and determination of its role in sporulation. Mol. Microbiol. 13:417-426. [DOI] [PubMed] [Google Scholar]
- 22.Koide, A., M. Perego, and J. A. Hoch. 1999. ScoC regulates peptide transport and sporulation initiation in Bacillus subtilis. J. Bacteriol. 181:4114-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroos, L., and Y. T. Yu. 2000. Regulation of sigma factor activity during Bacillus subtilis development. Curr. Opin. Microbiol. 3:553-560. [DOI] [PubMed] [Google Scholar]
- 24.Lopez, J. M., A. Dromerick, and E. Freese. 1981. Response of guanosine 5′-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J. Bacteriol. 146:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarova, K. S., A. A. Mironov, and M. S. Gelfand. 2001. Conservation of the binding site for the arginine repressor in all bacterial lineages. Genome Biol. 2:0013.1-0013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansilla, M. C., D. Albanesi, and D. de Mendoza. 2000. Transcriptional control of the sulfur-regulated cysH operon, containing genes involved in l-cysteine biosynthesis in Bacillus subtilis. J. Bacteriol. 182:5885-5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marta, P. T., R. D. Ladner, and J. A. Grandoni. 1996. A CUC triplet confers leucine-dependent regulation of the Bacillus subtilis ilv-leu operon. J. Bacteriol. 178:2150-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirel, D. B., W. F. Estacio, M. Mathieu, E. Olmsted, J. Ramirez, and L. M. Marquez-Magana. 2000. Environmental regulation of Bacillus subtilis σD-dependent gene expression. J. Bacteriol. 182:3055-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy, B. A., F. J. Grundy, and T. M. Henkin. 2002. Prediction of gene function in methylthioadenosine recycling from regulatory signals. J. Bacteriol. 184:2314-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulus, H. 1993. Biosynthesis of the aspartate family of amino acids, p. 237-267. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and other gram-positive bacteria: physiology, biochemistry, and Molecular Genetics. ASM Press, Washington, D.C.
- 31.Pelchat, M., and J. Lapointe. 1999. Aminoacyl-tRNA synthetase genes of Bacillus subtilis: organization and regulation. Biochem. Cell Biol. 77:343-347. [PubMed] [Google Scholar]
- 32.Ratnayake-Lecamwasam, M., P. Serror, K. W. Wong, and A. L. Sonenshein. 2001. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 15:1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekowska, A., S. Robin, J. J. Daudin, A. Henaut, and A. Danchin. 2001. Extracting biological information from DNA arrays: an unexpected link between arginine and methionine metabolism in Bacillus subtilis. Genome Biol. 2:0019.1-0019.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serrano, M., R. Zilhao, E. Ricca, A. J. Ozin, C. P. Moran, Jr., and A. O. Henriques. 1999. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J. Bacteriol. 181:3632-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serror, P., and A. L. Sonenshein. 1996. CodY is required for nutritional repression of Bacillus subtilis genetic competence. J. Bacteriol. 178:5910-5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slack, F. J., P. Serror, E. Joyce, and A. L. Sonenshein. 1995. A gene required for nutritional repression of the Bacillus subtilis dipeptide permease operon. Mol. Microbiol. 15:689-702. [DOI] [PubMed] [Google Scholar]
- 37.Smith, M. C., L. Czaplewski, A. K. North, S. Baumberg, and P. G. Stockley. 1989. Nucleotide sequences required for regulation of arginine biosynthesis promoters are conserved between Bacillus subtilis and Escherichia coli. Mol. Microbiol. 3:23-28. [DOI] [PubMed] [Google Scholar]
- 38.Stöver, A. G., and A. Driks. 1999. Regulation of synthesis of the Bacillus subtilis transition-phase, spore-associated antibacterial protein TasA. J. Bacteriol. 181:5476-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray, L. V., Jr., A. E. Ferson, and S. H. Fisher. 1997. Expression of the Bacillus subtilis ureABC operon is controlled by multiple regulatory factors including CodY, GlnR, TnrA, and Spo0H. J. Bacteriol. 179:5494-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeggy, J. P., and D. P. Stahly. 1980. Sporulation and regulation of homoserine dehydrogenase in Bacillus subtilis. Can. J. Microbiol. 26:1386-1391. [DOI] [PubMed] [Google Scholar]
- 41.Yu, J., L. Hederstedt, and P. J. Piggot. 1995. The cytochrome bc complex (menaquinone:cytochrome c reductase) in Bacillus subtilis has a nontraditional subunit organization. J. Bacteriol. 177:6751-6760. [DOI] [PMC free article] [PubMed] [Google Scholar]