Abstract
We report the first documented case of endocarditis in a man infected with Bartonella alsatica, which causes bacteremia in healthy wild rabbits. B. alsatica was identified by serology and culture and by PCR of an aortic valve specimen. B. alsatica should be added to the list of zoonotic agents of blood culture-negative endocarditis.
CASE REPORT
A 74-year-old man was hospitalized for a 3-week history of fever in August 2005. He had had a bioprosthetic aortic valve since 1997 and parotid cancer diagnosed and treated in 2002. The current episode began with remittent fever, hemoptoic cough, and transient aphasia. On examination, he presented with fever, a cardiac murmur, a splenomegaly, and edema of the lower limbs. Echocardiography revealed an aortic vegetation (20 by 5 mm) and an abscess surrounding the valve ring. His blood cell count showed leucopenia (white blood cell count, 3.5 × 109/liter, with 2.3 × 109/liter polymorphonuclear), anemia (98 g/liter of hemoglobin), and thrombocytopenia (94 × 109/liter). Regular blood cultures were negative. He was treated with amoxicillin (12 g daily intravenously) and gentamicin (320 mg daily intravenously). The treatment was changed to doxycycline (200 mg daily) for 6 weeks and ceftriaxone (2 g daily). He became apyretic, and the valve was replaced after 15 days of treatment. On follow-up in April 2005, he was fine and apparently cured. He was reinterviewed retrospectively and revealed that he had been in charge of feeding rabbits in August 2004 and that he had butchered a rabbit in June 2004.
Bartonella quintana subsp. Oklahoma, B. henselae subsp. Houston (ATCC 49882), B. vinsonii subsp. berkhoffi (URBVAIE25), B. vinsonii subsp. arupensis (ATCC 700727), and B. alsatica (CIP 105477 T) strains were used for immunofluorescence and Western blot assays (6). The serum showed an immunoglobulin G titer of 1:800 for B. alsatica and an immunoglobulin G titer of 1:400 for the other Bartonella isolates as determined using an immunofluorescence assay. We performed Western blotting using Bartonella sp. antigens (6), and after adsorption, only B. alsatica antigens retained all antibodies. The cardiac valve and the blood samples were inoculated into human endothelial cells in a shell vial assay and onto Columbia 5% sheep blood agar plates and incubated at 37°C in a 5% CO2 atmosphere (8). Detection of a Bartonella sp. in the shell vial by immunofluorescence was positive after 2 months of culture, and identification was confirmed by PCR-based methods. DNA was extracted from blood and valve specimens as well as from inoculated cells by use of a QIAamp tissue kit (QIAGEN, Hilden, Germany). The DNA was used as a template in two previously described PCR assays targeting a portion of the Bartonella internal transcribed spacer (ITS) region (4, 8) and the ftsZ gene (10) and in a new Bartonella genus Lightcycler assay using primers and a TaqMan probe targeting the ITS gene (forward primer, 5′-GGGGCCGTAGCTCAGCTG-3′; reverse primer, 5′-TGAATATATCTTCTCTTCACAATTTC-3′; probe, 6-carboxyfluorescein-CGATCCCGTCCGGCTCCACCA-6-carboxytetramethylrhodamine).
All three PCR results for the cardiac valve and the cell culture were positive, and amplification products of the expected size were obtained from this extract; however, there were no positive results either from the blood sample or from any concurrently processed negative-control materials (one of every two specimens). The sequences obtained shared 100% similarity with the corresponding ITS (GenBank accession number AF312506) and ftsZ fragment (GenBank accession number AF467763) of B. alsatica. This strain of Bartonella had never been cultivated or amplified in this laboratory before this experiment, and the reference strain of B. alsatica (CIP 105477 T) was cultured only after this amplification for the retrospective serological analysis.
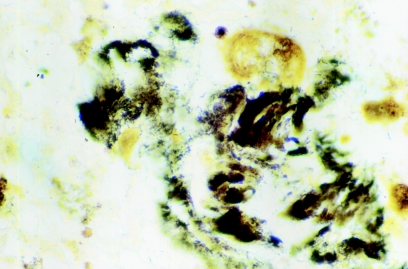
Formalin-fixed, paraffin-embedded valve tissue specimens were cut to 3 μm in thickness and stained with hematoxylin-eosin-saffron. Microscopic examination of valve tissue samples showed typical histological features related to infective endocarditis, with vegetations, inflammatory infiltrates, and destruction of the prosthetic valve tissues. Warthin-Starry staining revealed multitudes of bacillary organisms organized in clusters (Fig. 1).
FIG. 1.
Resected valve with Bartonella alsatica infection showing darkly stained bacilli consistent with Bartonella infections. Numerous clusters of argyrophilic bacteria are present in the fibrin deposits (Warthin-Starry staining; original magnification, ×1,000).
B. alsatica is a recently identified agent that causes bacteremia in healthy wild rabbits in Alsace, France (2, 5). In several cases, Bartonella spp. specifically adapted to rodents have been found in patients with blood-culture-negative endocarditis (BCNE) (8). In this report we identified B. alsatica in a patient for the first time, by culture, PCR, and serology. The bacteria of the genus Bartonella are relatively host specific and cause chronic bacteremia (7). Two species are the primary human bacteremic agents: B. bacilliformis in South America and B. quintana, mainly associated with body lice, worldwide. The Bartonella spp. associated with other mammals could be incidental pathogens for patients. The most common is B. henselae, which causes cat scratch disease. A Bartonella sp. also causes BCNE (8). Sporadic cases have been associated with other Bartonella spp. such as B. koehlerae (1), B. vinsonii subsp. berkoffii (9), B. vinsonii subsp. arupensis (4), and B. elizabethae (3). The current serologic method using an immunofluorescence assay does not distinguish among species, and only Western blot analysis and cross-adsorption allow serological identification of the species (6). PCR and culture are critical when a Bartonella species is identified for the first time as a human pathogen, as in this case. The current case reinforces the hypothesis that any Bartonella may cause BCNE in patients with valve lesions and exposure to an infected animal. B. alsatica should be added to the lists of human pathogens and of etiologic agents of BCNE.
REFERENCES
- 1.Avidor, B., M. Graidy, G. Efrat, C. Leibowitz, G. Shapira, A. Schattner, O. Zimhony, and M. Giladi. 2004. Bartonella koehlerae, a new cat-associated agent of culture-negative human endocarditis. J. Clin. Microbiol. 42:3462-3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulouis, H. J., C. C. Chang, J. B. Henn, R. W. Kasten, and B. B. Chomel. 2005. Factors associated with the rapid emergence of zoonotic Bartonella infections. Vet. Res. 36:383-410. [DOI] [PubMed] [Google Scholar]
- 3.Daly, J. S., M. G. Worthington, D. J. Brenner, W. C. Moss, D. G. Hollis, R. S. Weyant, A. G. Steigerwalt, R. E. Weaver, M. I. Daneshvar, and S. P. O'Connor. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fenollar, F., S. Sire, and D. Raoult. 2005. Bartonella vinsonii subsp. arupensis as an agent of blood culture-negative endocarditis in a human. J. Clin. Microbiol. 43:945-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heller, R., M. Kubina, P. Mariet, P. Riegel, G. Delacour, C. Dehio, F. Lamarque, R. Kasten, H. J. Boulouis, H. Monteil, B. Chomel, and Y. Piemont. 1999. Bartonella alsatica sp. nov., a new Bartonella species isolated from the blood of wild rabbits. Int. J. Syst. Bacteriol. 49:283-288. [DOI] [PubMed] [Google Scholar]
- 6.Houpikian, P., and D. Raoult. 2003. Western immunoblotting for Bartonella endocarditis. Clin. Diagn. Lab. Immunol. 10:95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacomo, V., P. J. Kelly, and D. Raoult. 2002. Natural history of Bartonella infections (an exception to Koch's postulate). Clin. Diagn. Lab. Immunol. 9:8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raoult, D., P. E. Fournier, F. Vandenesch, J. L. Mainardi, S. J. Eykyn, J. Nash, E. James, C. Benoit-Lemercier, and T. J. Marrie. 2003. Outcome and treatment of bartonella endocarditis. Arch. Intern. Med. 163:226-230. [DOI] [PubMed] [Google Scholar]
- 9.Roux, V., S. J. Eykyn, S. Wyllie, and D. Raoult. 2000. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J. Clin. Microbiol. 38:1698-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeaiter, Z., Z. Liang, and D. Raoult. 2002. Genetic classification and differentiation of Bartonella species based on comparison of partial ftsZ gene sequences. J. Clin. Microbiol. 40:3641-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]