Until the beginning of the 1990s, it was quite common to learn that many patients with tuberculosis (TB) were treated for several months with a standard regimen including isoniazid (INH) and rifampin (RMP) without the physician knowing whether the causative organism was susceptible to these drugs. The main reason for such an attitude was that the probability of a Mycobacterium tuberculosis complex (MTC) initial isolate being resistant was negligible. Unfortunately, the situation has strikingly changed now. Not only does TB continue to represent one of the most relevant infectious diseases in the world, responsible for 8 million new cases and 2 to 3 million casualties occurring annually, but in low-prevalence countries, the rates of initial-drug- and multidrug-resistant (MDR) TB (e.g., resistant to both INH and RMP) (13) have been climbing, causing a worrying rise in morbidity and mortality. An additional factor is human immunodeficiency virus, which has significantly increased the incidence of TB, particularly in sub-Saharan Africa but also elsewhere. While the long-term solution is likely to come from the development of a better vaccine, for the near future, reliance on chemotherapy will have to be continued. This matter has refocused attention on the importance of MTC drug susceptibility testing (DST) and on the laboratory's key role in providing clinicians with timely, reliable, and comprehensive information (2).
Currently, a number of automated, nonradiometric detection systems (NRS) that can also perform MTC susceptibility testing are commercially available to clinical laboratories (30). Not all the methods presented herewith have been cleared by the Food and Drug Administration. However, we performed a systematic review and meta-analysis to evaluate the performance of these systems in comparison with the radiometric BACTEC 460 TB (B460) system, currently regarded as a reference able to combine timely and reliable results (27, 36).
LITERATURE SEARCH
Literature from January 1998 through December 2004 was investigated with the Medline, Embase, and Cochrane libraries. Studies were included for analysis if (i) they compared DST results obtained by each of the new NRS with the B460, (ii) they reported data on false-positive results (test flagged as susceptible by NRS and resistant by the reference, also reported as very major errors [VME]), (iii) they reported data on false-negative results (test flagged as resistant by NRS and susceptible by the reference, also reported as major errors [ME]), (iv) they reported data on true-positive results (test flagged as susceptible by both opponents), and (v) they reported data on true-negative results (test flagged as resistant by both opponents) separately. Discrepant results were resolved according to data obtained by the agar proportion method (AP), which was set as the “gold standard” (27). When resolution of discrepancies was not performed, the radiometric system was regarded as the reference.
SELECTION OF PAPERS AND DATA ANALYSIS
Of the 20 articles detected, all were reviewed in detail, and data from six of these articles (1, 4, 12, 14, 25, 39) were excluded because they used a standard different from the accepted reference or did not contain all the required information. Diagnostic performance indexes (sensitivity, specificity, predictive values, and diagnostic odds ratio [DOR]) were calculated for each of the primary studies. The DOR measures the discriminatory power of a diagnostic test combining the strengths of sensitivity and specificity as a single indicator of test accuracy. Values higher than 1 indicate an acceptable discriminatory test performance. Since calculated DORs were found to be constant across all the reviewed studies, they were considered a worthy measure of test accuracy. A meta-analysis of diagnostic test accuracy was performed using the summary receiver operating characteristic curve (summary ROC curve) (15), according to the approach proposed by Littenberg, Moses, and Shapiro (23, 26) and as suggested by the Cochrane Methods Group on Systematic Review of Screening and Diagnostic Tests (11). Calculated DORs were then pooled by combining primary studies by the Manthel-Haenszel method. Statistical heterogeneity was evaluated by Cochrane's Q test and I2 test.
Publication bias was assessed using both Begg's test and Egger's test. In order to strengthen the quality of statistical evaluation, sensitivity analysis, which consists of a multiple meta-analysis performed by omitting one study at a time to detect influential studies, was carried out. In addition, we carried out subgroup analysis for each of the drugs evaluated and overall, summarizing outcomes as DOR and 95% confidence interval (CI). Risk of ME or VME was calculated only for the MGIT 960 system versus B460 (by pooling odds ratios for each of the tested drugs and overall) and summarized as the odds ratio plus 95% CI. In some reports dealing with MGIT 960 (6, 38), only low-concentration-resistant strains out of the assayed microorganisms were tested with high concentrations. These results were not considered in our analyses as they produced evidence of selection bias. The procedure described above could not be fully applied to the MB/BacT system because (i) only two studies out of the four retrieved performed resolution of discrepancies by the AP and (ii) due to the point estimates' lack of precision, test outcomes appeared uncertain. Turnaround times (TATs) were described as overall values (expressed as means or medians) and range. Since these values were reported as single points, we carried out comparative evaluations using a descriptive analysis. The Stata 8.2 software (Stata Corporation, College Station, TX) was used to perform statistical analysis. Finally, as the Versa TREK system was evaluated in three studies only (two for the first-line drugs plus one for pyrazinamide [PZA]), we decided to exclude this method from the meta-analysis producing a more appropriate descriptive analysis.
COMPARISON OF NONRADIOMETRIC DST SYSTEMS
BACTEC MGIT 960. (i) Manufacturer.
The manufacturer of the BACTEC MGIT 960 system is Becton Dickinson Microbiology Systems, Sparks, Md.
(ii) Description.
MGIT 960 is a nonradiometric antimicrobial susceptibility system for testing MTC from isolated culture. It has been validated to provide results for streptomycin (SM), INH, RMP, and ethambutol (EMB) (SIRE kit) and PZA (PZA kit) in a time frame close to the BACTEC 460TB system (34). PZA testing requires modification of the general method because the drug is active in vitro at a reduced pH of 5.9 instead of the standard pH of 6.8 (35). The system uses a modified Middlebrook 7H9 broth (7-ml plastic tubes) and a fluorescent technology. The initial concentration of oxygen dissolved in the broth quenches fluorescence, but as growth occurs (mycobacteria and other microorganisms), the oxygen is being consumed, thereby permitting the indicator to fluoresce under a 365-nanometer UV lamp. The MGIT 960 kits perform a qualitative susceptibility testing for the drugs listed above that is completed within 4 to 19 days. The test is based on growth of MTC isolate in a drug-containing tube compared to a drug-free control tube. The MGIT 960 instrument continuously monitors tubes to detect an increase in the fluorescence. Comparison of these records in the test and control tubes is used by the instrument algorithms to determine results, which are automatically interpreted and reported as susceptible or resistant.
(iii) Literature review.
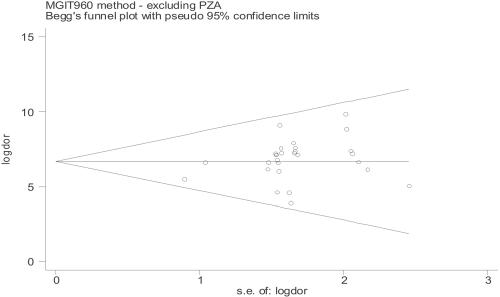
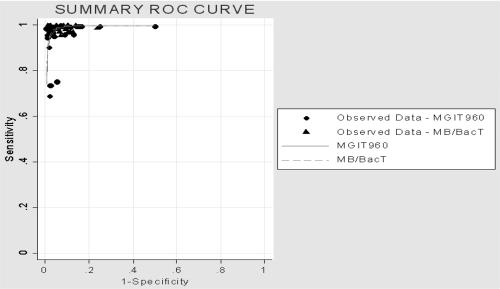
Currently, several studies evaluating the performance of this system, testing both traditional SIRE drugs and PZA, have been published in the literature. When such studies were assessed, no evidence of either publication bias or statistical heterogeneity was found (Fig. 1; see Table 6). High values of sensitivity and specificity and high predictive values were associated with the use of MGIT 960 (Tables 1 and 2) and similar results indicating a good discriminatory performance were obtained when DOR was used as a single indicator of accuracy (range, 5.00 to 9.84). The summary ROC curve, symmetric and very close to the upper left-hand corner of the graph, confirmed the results highlighted by DORs (Fig. 2). False-susceptibility results (VME) represent a serious drawback, as they can result in the failure of anti-TB chemotherapy. When compared with the B460 system, MGIT 960 did not show any evidence of increased VME occurrence, indicating that this system is reliable for detecting true resistance (Tables 3 and 4). False-resistance results (ME), which label as ineffective a drug that can be successfully used, represent a less serious problem. In our analysis, the MGIT 960 system showed an increased risk of ME (with a statistically significant difference in comparison with B460) for SM and INH (P values were 0.017 and 0.025, respectively), while no statistically significant difference was found for EMB (P = 0.065) despite a higher number of ME exhibited by this drug, whose results are well known to be less reproducible regardless of the system used (22, 24). When testing high concentrations (SM, INH, and EMB), the MGIT 960 system did not show any evidence of increased ME risk (Tables 3 and 4). Four issues need to be carefully taken into consideration, as they can lead to inaccurate results: (i) purity of the culture, (ii) homogeneity of mycobacterial suspension, (iii) inoculum size, and (iv) environmental contamination of media during inoculation procedures. Purity check of suspensions (especially those prepared from resistant strains) guarantees that a pure culture of a single mycobacterial species is used for DST inoculation, thereby preventing ME results. Bacterial contamination from the surrounding environment may occur during DST hands-on procedures and sometimes in one tube only. In this context, a high contamination rate in comparison with B460 has been reported by different authors (16, 33, 38), who addressed (i) richness of liquid medium and (ii) replacement of rubber septa with screw caps as being mainly responsible.
FIG. 1.
Funnel plots of reviewed studies on the MGIT 960 system.
TABLE 6.
Meta-analysis main results: point estimates of accuracy and heterogeneity
| Method | Drug | DOR (log) | 95% CI | P | Heterogeneity
|
|
|---|---|---|---|---|---|---|
| Q test (P) | I2 test (%) | |||||
| MGIT 960 | SM | 6.30 | 5.15-7.44 | 0.000 | 0.853 | 0.00 |
| INH | 7.55 | 6.35-8.74 | 0.000 | 0.975 | 0.00 | |
| RMP | 7.88 | 6.21-9.54 | 0.000 | 0.609 | 0.00 | |
| EMB | 6.65 | 5.56-7.74 | 0.000 | 0.999 | 0.00 | |
| PZA | 5.76 | 4.63-6.88 | 0.000 | 0.456 | 0.00 | |
| Overall | 6.68 | 6.14-7.21 | 0.000 | 0.963 | 0.00 | |
| MB/BacT | SM | 6.57 | 5.10-8.03 | 0.000 | 0.586 | 0.00 |
| INH | 7.75 | 6.09-9.42 | 0.000 | 0.652 | 0.00 | |
| RMP | 7.52 | 5.77-9.27 | 0.000 | 0.675 | 0.00 | |
| EMB | 6.03 | 4.69-7.38 | 0.000 | 0.574 | 0.00 | |
| PZA | 7.69 | 5.16-10.22 | 0.000 | 0.520 | 0.00 | |
| Overall | 6.90 | 6.17-7.63 | 0.000 | 0.848 | 0.00 | |
TABLE 1.
Summary of reviewed studies dealing with BACTEC MGIT 960 systema
| Drug | Authors (reference) | Yr | CC (μg/ml) | No. of:
|
Sens. | Spec. | PPV | NPV | DOR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested strains | TP | FN | FP | TN | |||||||||
| SM | Ardito et al. (5) | 2001 | 1.0 | 78 | 74 | 0 | 0 | 4 | 1.00 | 1.00 | 1.00 | 1.00 | 7.20 |
| 4.0 | 78 | 77 | 0 | 0 | 1 | 1.00 | 1.00 | 1.00 | 1.00 | 6.14 | |||
| Bemer et al. (6) | 2002 | 1.0 | 110 | 76 | 8 | 0 | 26 | 0.90 | 1.00 | 1.00 | 0.76 | 6.17 | |
| 4.0 | 34 | 12 | 4 | 0 | 18 | 0.74 | 0.97 | 0.96 | 0.80 | 4.63 | |||
| Tortoli et al. (38) | 2002 | 1.0 | 133 | 120 | 1 | 0 | 12 | 0.99 | 1.00 | 1.00 | 0.92 | 7.61 | |
| Scarparo et al. (33) | 2004 | 1.0 | 100 | 62 | 2 | 4 | 32 | 0.97 | 0.89 | 0.94 | 0.94 | 5.51 | |
| 4.0 | 100 | 75 | 2 | 0 | 23 | 0.97 | 1.00 | 1.00 | 0.92 | 7.26 | |||
| INH | Ardito et al. (5) | 2001 | 0.1 | 78 | 60 | 1 | 0 | 17 | 0.98 | 1.00 | 1.00 | 0.94 | 7.25 |
| 0.4 | 78 | 73 | 0 | 0 | 5 | 1.00 | 1.00 | 1.00 | 1.00 | 7.39 | |||
| Bemer et al. (6) | 2002 | 0.1 | 110 | 81 | 3 | 0 | 26 | 0.96 | 1.00 | 1.00 | 0.90 | 7.12 | |
| Tortoli et al. (38) | 2002 | 0.1 | 133 | 111 | 3 | 0 | 19 | 0.97 | 1.00 | 1.00 | 0.86 | 7.12 | |
| Johansen et al. (16) | 2004 | 0.4 | 222 | 143 | 2 | 0 | 77 | 0.98 | 1.00 | 1.00 | 0.97 | 9.09 | |
| Scarparo et al. (33) | 2004 | 0.1 | 100 | 51 | 2 | 0 | 47 | 0.96 | 1.00 | 1.00 | 0.96 | 7.58 | |
| 0.4 | 100 | 56 | 3 | 0 | 41 | 0.95 | 1.00 | 1.00 | 0.93 | 7.20 | |||
Data are reported by individual drug. DORs are expressed as logarithms. TP, true positive; FN, false negative; FP, false positive; TN, true negative; Sens., sensitivity; Spec., specificity; PPV, positive predictive value; NPV, negative predictive value.
TABLE 2.
Summary of reviewed studies dealing with BACTEC MGIT 960 systema
| Drug | Authors (reference) | Yr | CC (μg/ml) | No. of:
|
Sens. | Spec. | PPV | NPV | DOR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested strains | TP | FN | FP | TN | |||||||||
| RMP | Ardito et al. (5) | 2001 | 1.0 | 78 | 78 | 0 | 0 | 0 | 1.00 | NA | 1.00 | NA | 5.06 |
| Bemer et al. (6) | 2002 | 1.0 | 110 | 92 | 0 | 0 | 18 | 1.00 | 1.00 | 1.00 | 1.00 | 8.83 | |
| Tortoli et al. (38) | 2002 | 1.0 | 132 | 124 | 1 | 0 | 7 | 0.99 | 1.00 | 1.00 | 0.83 | 7.13 | |
| Johansen et al. (16) | 2004 | 1.0 | 222 | 199 | 0 | 0 | 23 | 1.00 | 1.00 | 1.00 | 1.00 | 9.84 | |
| Scarparo et al. (33) | 2004 | 1.0 | 100 | 70 | 0 | 1 | 29 | 1.00 | 0.97 | 0.99 | 1.00 | 7.93 | |
| EMB | Ardito et al. (5) | 2001 | 5.0 | 78 | 64 | 3 | 0 | 11 | 0.96 | 1.00 | 1.00 | 0.79 | 6.05 |
| 7.5 | 78 | 76 | 0 | 0 | 2 | 1.00 | 1.00 | 1.00 | 1.00 | 6.64 | |||
| Bemer et al. (6) | 2002 | 5.0 | 110 | 94 | 3 | 0 | 13 | 0.97 | 1.00 | 1.00 | 0.81 | 6.59 | |
| Tortoli et al. (38) | 2002 | 5.0 | 133 | 125 | 1 | 1 | 6 | 0.99 | 0.86 | 0.99 | 0.86 | 6.62 | |
| Johansen et al. (16) | 2004 | 5.0 | 222 | 203 | 2 | 2 | 15 | 0.99 | 0.88 | 0.99 | 0.88 | 6.63 | |
| Scarparo et al. (33) | 2004 | 5.0 | 100 | 78 | 0 | 3 | 19 | 1.00 | 0.86 | 0.96 | 1.00 | 6.77 | |
| 7.5 | 100 | 86 | 1 | 0 | 13 | 0.99 | 1.00 | 1.00 | 0.93 | 7.35 | |||
| PZA | Pfyffer et al. (29) | 2002 | 100 | 58 | 45 | 2 | 1 | 10 | 0.96 | 0.91 | 0.98 | 0.83 | 5.42b |
| 100 | 58 | 44 | 1 | 0 | 13 | 0.98 | 1.00 | 1.00 | 0.93 | 6.69c | |||
| Kontos et al. (18) | 2003 | 100 | 150 | 144 | 0 | 0 | 6 | 1.00 | 1.00 | 1.00 | 1.00 | 8.23 | |
| Johansen et al. (16) | 2004 | 100 | 57 | 46 | 0 | 0 | 11 | 1.00 | 1.00 | 1.00 | 1.00 | 7.67 | |
| Scarparo et al. (33) | 2004 | 100 | 100 | 66 | 3 | 4 | 27 | 0.96 | 0.87 | 0.94 | 0.90 | 5.00 | |
Data are reported by individual drug. DORs are expressed as logarithms. TP, true positive; FN, false negative; FP, false positive; TN, true negative; Sens., sensitivity; Spec., specificity; PPV, positive predictive value; NPV, negative predictive value; NA, not available.
DST involved inoculation with a primary MGIT tube.
DST involved inoculation with a strain suspension obtained from a solid culture.
FIG. 2.
Summary ROC curves obtained from regression analysis of reviewed studies on MGIT 960 and MB/BacT systems.
TABLE 3.
Summary of ME and VRE according to DST method and individual drug
| Method | Drug (critical concn [μg/ml]) | Tested strains | No. of:
|
|
|---|---|---|---|---|
| ME | VME | |||
| MGIT 960a | SM (1.0) | 421 | 11 | 4 |
| SM (4.0) | 178 | 2 | 0 | |
| INH (0.1) | 421 | 9 | 0 | |
| INH (0.4) | 400 | 5 | 0 | |
| RMP (1.0) | 642 | 1 | 1 | |
| EMB (5.0) | 643 | 9 | 6 | |
| EMB (7.5) | 178 | 1 | 0 | |
| PZA (100) | 423 | 6 | 5 | |
| MB/BacT | SM (0.45) | 166 | 2 | 0 |
| SM (0.9) | 216 | 0 | 7 | |
| SM (1.0) | 233 | 3 | 1 | |
| SM (2.0) | 120 | 0 | 0 | |
| INH (0.09) | 216 | 0 | 1 | |
| INH (1.0) | 233 | 0 | 3 | |
| RMP (0.9) | 216 | 0 | 2 | |
| RMP (1.0) | 233 | 1 | 0 | |
| EMB (2.0) | 233 | 8 | 2 | |
| EMB (3.5) | 166 | 0 | 1 | |
| EMB (7.0) | 82 | 0 | 4 | |
| PZA (200) | 166 | 0 | 0 | |
Only studies with resolution of discrepant results were included.
TABLE 4.
Risk (odds ratios) of ME and VME for MGIT 960 in comparison with the radiometric systema
| Drug (CC) | Major error
|
Very major error
|
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P | Odds ratio | 95% CI | P | |
| SM (1.0) | 6.16 | 1.38-27.55 | 0.017 | 1.00 | 0.32-3.11 | 1 |
| INH (0.1) | 5.62 | 1.24-25.49 | 0.025 | 0.33 | 0.03-3.2 | 0.339 |
| RMP (1.0) | 1.00 | 0.14-7.13 | 1 | 1.00 | 0.14-7.15 | 1 |
| EMB (5.0) | 3.38 | 0.93-12.36 | 0.065 | 2.35 | 0.61-9.13 | 0.217 |
| PZA (100) | 3.58 | 0.74-17.44 | 0.114 | 3.74 | 0.61-22.93 | 0.154 |
| Overall | 3.38 | 1.00-7.56 | 0.0001 | 1.39 | 0.72-2.68 | 0.324 |
| SM (4.0) | 0.66 | 0.11-4.04 | 0.653 | 0.33 | 0.01-8.20 | 0.498 |
| INH (0.4) | 6.11 | 0.73-51.13 | 0.095 | 0.19 | 0.02-1.70 | 0.138 |
| EMB (7.5) | 3.03 | 0.12-75.28 | 0.499 | 0.33 | 0.01-8.20 | 0.499 |
| Overall | 2.13 | 0.69-6.61 | 0.189 | 0.25 | 0.05-1.17 | 0.077 |
Only studies with resolution of discrepant results were included.
Four papers evaluating PZA testing in comparison with B460 were published in the literature: (i and ii) Kontos et al. and Johansen et al. (16, 18) tested 150 and 50 isolates, respectively, but did not report ME or VME values; (iii) Pfyffer et al. (29) tested 58 isolates (by working with inocula prepared from liquid and solid cultures) and observed a tendency of the MGIT 960 to generate ME rather than VME; (iv) Scarparo et al. (33) tested 100 isolates and reported four MVE and three ME. The authors concluded that although the overall agreement of results generated between MGIT 960 and B460 was very high, discrepancies might be related to the uneven distribution of mycobacteria which occurs in DST tubes during inoculation from the primary culture. In fact, the following steps may explain most of the observed ME and VME: (i) DST tubes are seeded using a pipette, which is more likely to collect large mycobacterial clumps than is a fine-needle syringe; (ii) inocula are taken at two different times from the primary tube, instead of at one time only, as in the B460 procedure; and (iii) the PZA control tube is required to be seeded with a 1:5 diluted inoculum. According to the majority of published papers (5, 6, 16, 33), TATs of SIRE drugs (undistinguishable between susceptible and resistant strains) were found to be very similar for MGIT 960 and B460 (Table 5). These results slightly differed from those obtained by Tortoli et al. (38), who reported an average of 2.5-day-earlier results with the B460 system. Similarly, the mean or median times required for MGIT 960 and B460 to test PZA were found to be very close (16, 18, 29). When testing PZA-resistant strains, Scarparo et al. (33) reported shorter TATs for the B460 system, while no difference was found with PZA-susceptible strains. Moreover, according to the manufacturer's instructions, primary MGIT cultures can be used as a DST inoculum on the day after they first become positive on the instrument, while vials flagged positive by the B460 instrument require two or more additional days to get ready for DST. On the basis of previously reported TATs and taking into consideration that the above-mentioned time lag must be added to the mean time to report with B460, the MGIT 960 system appears to be slightly more rapid than the B460 system. Finally, MGIT 960 appears to be the only system for which validation trials on secondary drugs are in progress and preliminary results currently available (32). A major drawback we observed in many published papers was the lack of an adequate number of drug-resistant isolates, thus making it difficult to understand how accurate VME rates could be estimated and valid conclusions drawn. We strongly believe that unless a consistent number of resistant isolates (about 30%) are tested, scientifically sound evaluations and accurate VME rates cannot be determined. In our opinion, this recommendation should be fulfilled as a basic requirement for papers to be considered on international peer-reviewed journals.
TABLE 5.
Turnaround times of DST results: comparison of NRS with BACTEC 460 TB
| Authors (reference) | Yr | Tested drug(s) | Days to report DST resultsa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Min | Max | Median | Min | Max | |||||||
| MGIT 960
|
BACTEC 460
|
|||||||||||
| Ardito et al. (5) | 2001 | SIRE | 7.9 | 4.6 | 13.2 | 7.3 | 6 | 10 | ||||
| Bemer et al. (6) | 2002 | SIRE | 6.5 | 4.6 | 11.7 | 7 | 4 | 10 | ||||
| Pfyffer et al. (29) | 2002 | PZA | 6.8b | 4 | 16.8 | 5.4b | 3 | 21 | ||||
| Tortoli et al. (38) | 2002 | SIRE | 9.4 | 5 | 14 | 6.9 | 5 | 16 | ||||
| Kontos et al. (18) | 2003 | PZA | 6.4b | 5 | 16 | 6.8b | 4 | 18 | ||||
| Johansen et al. (16) | 2004 | SIRE | 5.5 | 4 | 16 | 5 | 5 | 14 | ||||
| PZA | 5 | 4 | 10 | 5 | 5 | 14 | ||||||
| Scarparo et al. (33) | 2004 | SIRE | 8.3 | 5.1 | 12.4 | 8.2 | 4 | 13 | ||||
| PZA | 8.2 | 4.2 | 19 | 7.4 | 4 | 20 | ||||||
| MB/BacT
|
BACTEC 460
|
|||||||||||
| Brunello and Fontana (9) | 2000 | SIRE | 8.5 | 5 | 11 | 6 | 4 | 8 | ||||
| Tortoli et al. (37) | 2000 | SIRE, PZA | 11.6b | 4 | 26 | 7.6b | 4 | 14 | ||||
| Ängeby et al. (3) | 2003 | SIRE | 6.1 | 4.2 | 10 | 6 | 4 | 8 | ||||
| Bemer et al. (7) | 2004 | SIRE | 6.6 | 6 | 9.9 | 5 | 4 | 12 | ||||
| PZA | 7.8 | 5 | 15.2 | 6.7 | 3 | 13 | ||||||
| ESP II
|
BACTEC 460
|
|||||||||||
| Bergmann and Woods (8) | 1988 | SIRE | 7b | 5 | 10 | 7.4b | 6 | 10 | ||||
| Ruiz et al. (31) | 2000 | SIRE | 4.5b | 2 | 8 | 4.8b | 3 | 9 | ||||
Min, minimum; Max, maximum.
Datum reported as mean instead of median.
MB/BacT system. (i) Manufacturer.
The manufacturer of the MB/BacT system is bioMérieux, Durham, N.C.
(ii) Description.
MB/BacT is a nonradiometric antimicrobial susceptibility system for testing MTC isolates cultured from clinical samples. It was developed to provide susceptibility results for SM, INH, RMP, and EMB since 1997, but recently, critical concentrations (CCs) of the drugs listed above were modified and a new acidified vial for standardized PZA testing was introduced. The system consists of a bottle that contains a colorimetric sensor embedded in its bottom. As microorganisms grow and produce carbon dioxide, the sensor changes from dark green to yellow. The change is continuously monitored by a detection unit and promptly reported by the instrument. A primary culture bottle growing MTC is used to inoculate drug-containing bottles and a drug-free control. DST sets are entered into the instrument and continuously monitored until a positive or negative result is obtained. An organism is determined to be susceptible when the antibiotic-containing bottle remains negative or shows a positive detection time greater than drug-free control. In contrast, when the antibiotic-containing bottle becomes positive or has a positive time to detection shorter than the drug-free control, the tested organism is determined to be resistant. No antimycobacterial drugs have been cleared for susceptibility testing with this system by the FDA.
(iii) Literature review.
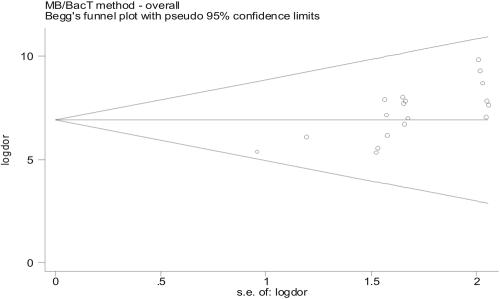
Asymmetric shape of funnel plots (Fig. 3) suggests some evidence of publication bias among assessed papers. In addition, few studies have been published comparing MB/BacT with B460, including both SIRE and PZA drug results, as of this writing. In general, we found high values of sensitivity and specificity and high predictive values, which were confirmed when DOR was used as a single indicator of accuracy (range, 5.34 to 9.83), thus indicating a good discriminatory performance (Table 6). Characteristics of the summary ROC curve strengthened the results highlighted by DORs (Fig. 2). In addition to the paucity of peer-reviewed papers in this area (Table 7), another limitation is represented by the number of different protocols used in comparative evaluations. Main discrepancies may be summarized as follows: (i) choice of CCs with SM (0.45, 0.9, 1, and 2 μg/ml), EMB (2.0 and 3.5 μg/ml), and PZA (50 and 200 μg/ml); (ii) dilution of SIRE and PZA drug-free controls; (iii) PZA medium acidification; (iv) resolution of discrepant results, which was performed in only two studies (7, 9), employing different reference methods; (v) reading and interpretation of test results, especially involving high concentrations (SM, INH, and EMB). As expected (22, 24), ME and VME were more frequently observed for SM (five and eight, respectively) and EMB (eight and seven, respectively), while four VME for INH and two VME for RMP were also reported out of 399 tested strains (Table 3) (3, 7, 9, 37). In our opinion, such a high rate of VME, even for INH and RMP, whose discriminatory power is now accepted as being fully reliable, may be referred to as depending on the wide discrepancy of CCs adopted by different authors. For PZA, Bemer et al. (7), using the commercial kit which provides the acidified ready-to-use PZA medium, did not find any ME or VME at the concentration of 200 μg/ml, while Tortoli et al. (37) acidified the medium environment themselves by adding a 1 M KH2PO4 solution and reported one ME at the concentration of 50 μg/ml. In this paper, however, 7 strains out of 113 (6%) could not be evaluated, as they failed to grow into the modified medium. It is well established that the shorter the incubation time (DST is read when the drug-free control turns positive), the higher the risk of VME results. In this context, the introduction of a newly 1:10 diluted drug-free control instead of the older (1:100 diluted) one may explain many of the observed discrepancies (3). In the paper by Bemer et al. (7), no difference in susceptibility results was detected by adopting two different DST procedures including both diluted and undiluted drug-free controls. According to the majority of published papers (3, 7, 9, 37), TATs of SIRE drugs were found to be very similar for both systems. Means or medians were found to range from 6.1 to 6.6 days for MB/BacT and from 5 to 6 days for B460, respectively (3, 7). More prolonged times to report were observed by Brunello and Fontana (8.5 and 6 days, respectively) (9), while Tortoli et al. (37) reported DST results, on average, 4 days earlier with the B460 (11.6 and 7.6 days, respectively). Mean TATs of the PZA assay were found to be of 7.8 and 6.7 days for MB/BacT system and B460, respectively (7).
FIG. 3.
Funnel plots of reviewed studies on MB/BacT system.
TABLE 7.
Summary of reviewed studies dealing with MB/BacT systema
| Drug | Authors (reference) | Yr | CC (μg/ml) | No. of:
|
Sens. | Spec. | PPV | NPV | DOR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested strains | TP | FN | FP | TN | |||||||||
| SM | Brunello and Fontana (9) | 2000 | 1.0 | 120 | 115 | 0 | 0 | 5 | 1.00 | 1.00 | 1.00 | 1.00 | 7.84 |
| Tortoli et al. (38)b | 2000 | 1.0 | 113 | 95 | 3 | 1 | 14 | 0.97 | 0.93 | 0.99 | 0.82 | 6.09 | |
| Ängeby et al. (3)b | 2003 | 0.9 | 50 | 32 | 4 | 0 | 14 | 1.00 | 0.78 | 0.89 | 1.00 | 5.34 | |
| Bemer et al. (7) | 2004 | 0.45 | 166 | 140 | 2 | 0 | 24 | 0.99 | 1.00 | 1.00 | 0.92 | 7.92 | |
| INH | Brunello and Fontana (9) | 2000 | 1.0 | 120 | 103 | 0 | 2 | 15 | 1.00 | 0.88 | 0.98 | 1.00 | 7.16 |
| Tortoli et al. (38) | 2000 | 1.0 | 113 | 86 | 0 | 1 | 26 | 1.00 | 0.96 | 0.99 | 1.00 | 8.02 | |
| Ängeby et al. (3) | 2003 | 0.09 | 50 | 23 | 0 | 1 | 26 | 1.00 | 0.96 | 0.96 | 1.00 | 6.72 | |
| Bemer et al. (7) | 2004 | 0.09 | 166 | 131 | 0 | 0 | 35 | 1.00 | 1.00 | 1.00 | 1.00 | 9.83 | |
| RMP | Brunello and Fontana (9) | 2000 | 1.0 | 120 | 116 | 0 | 0 | 4 | 1.00 | 1.00 | 1.00 | 1.00 | 7.65 |
| Tortoli et al. (38) | 2000 | 1.0 | 113 | 95 | 1 | 0 | 17 | 0.99 | 1.00 | 1.00 | 0.94 | 7.71 | |
| Ängeby et al. (3) | 2003 | 0.9 | 50 | 25 | 0 | 2 | 23 | 1.00 | 0.92 | 0.93 | 1.00 | 6.17 | |
| Bemer et al. (7) | 2004 | 0.9 | 166 | 148 | 0 | 0 | 18 | 1.00 | 1.00 | 1.00 | 1.00 | 9.30 | |
| EMB | Brunello and Fontana (9) | 2000 | 2.0 | 120 | 109 | 5 | 0 | 6 | 0.96 | 1.00 | 1.00 | 0.55 | 5.56 |
| Tortoli et al. (38) | 2000 | 2.0 | 113 | 94 | 3 | 2 | 14 | 0.97 | 0.88 | 0.98 | 0.82 | 5.39 | |
| Ängeby et al. (3) | 2003 | 3.5 | 50 | 44 | 0 | 0 | 6 | 1.00 | 1.00 | 1.00 | 1.00 | 7.05 | |
| Bemer et al. (7) | 2004 | 3.5 | 166 | 153 | 0 | 1 | 12 | 1.00 | 0.92 | 0.99 | 1.00 | 7.85 | |
| PZA | Tortoli et al. (38) | 2000 | 50 | 106 | 97 | 1 | 0 | 8 | 0.99 | 1.00 | 1.00 | 0.89 | 7.01 |
| Bemer et al. (7) | 2004 | 200 | 166 | 157 | 0 | 0 | 9 | 1.00 | 1.00 | 1.00 | 1.00 | 8.70 | |
Data are reported by individual drug. TP, true positive; FN, false negative; FP, false positive; TN, true negative; Sens., sensitivity; Spec., specificity; PPV, positive predictive value; NPV, negative predictive value. DORs are expressed as logarithms.
Resolution of discrepancies not performed.
MB/BacT exhibited all the advantages of a fully automated, continuously monitoring, hands-off system able to use a primary culture bottle as the inoculum source for the DST. This feature, of course, greatly reduces the time required to generate the final result. Limitations of the system include a more likely occurrence of VME, probably related to the lack of standardized CCs and inoculum preparation. Based on currently available information, it is apparent that further studies are required to fully evaluate the performance of the MB/BacT system.
Versa TREK (formerly ESP Culture System II). (i) Manufacturer.
The manufacturer of the Versa Trek system is Trek Diagnostic Systems, West Lake, Ohio.
(ii) Description.
The Versa TREK system is an automated method that was first developed for blood cultures and later adapted for the recovery and DST of mycobacteria. It has been validated for performing qualitative susceptibility testing with INH, RMP, and EMB with MTC clinical isolates, while SM and PZA have not been cleared by the Food and Drug Administration for testing with this system (20). Since PZA requires an acidic pH to work, the assay utilizes a buffer to rehydrate the drug and is added to the control bottle to provide an acidic environment (21). Growth detection technology relies on the continuous monitoring of gas-related pressure changes resulting from active multiplication of organisms in the liquid medium. In particular, oxygen consumption leads to a reduction of pressure, which is measured in the headspace of the Versa TREK System. A primary culture bottle growing MTC or a standardized suspension of this organism is used to inoculate drug containing bottles and a drug-free control. The bottles are placed into the instrument and monitored until the control bottle signals positive for three consecutive days. Any drug-containing bottle becoming positive within the time given above indicates resistance of the isolate to the tested drug.
(iii) Literature review.
This technique has been evaluated in two studies testing SIRE drugs (8, 31) and in one study testing PZA (19). Although currently available information is inadequate to allow a definitive conclusion to be drawn, relevant differences between performance characteristics of Versa TREK system and B460 system have not been detected. After resolution of discrepancies, Bergmann and Woods (8) reported one VME for SM and two VME for INH out of 20 tested strains. An important weakness in this study was the inclusion of small numbers of strains resistant to INH, SM, and RMP, while the absence of EMB-resistant strains did not permit specificity and negative predictive values for this drug to be estimated (Table 8). In a much sounder study including 389 MTC isolates (31) and after resolution of discrepancies, INH exhibited 44 ME and 2 VME for low and high concentrations, respectively. As regards PZA testing, a comparative evaluation carried out between the Versa TREK system and B460 against 50 MTC strains did not find any ME or VME (19). Versa TREK's mean TATs were found to be very close to those reported for B460 (8, 31), and differences were not significant. The mean time to report for the PZA assay was not reported (19).
TABLE 8.
Summary of reviewed studies dealing with Versa TREK systema
| Drug | Authors (reference) | Yr | CC (μg/ml) | No. of:
|
Sens. | Spec. | PPV | NPV | DOR | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested strains | TP | FN | FP | TN | |||||||||
| SM | Bergmann and Woods (8) | 1998 | 8.0 | 20 | 18 | 0 | 1 | 1 | 1.00 | 0.50 | 0.95 | 1.00 | 3.61 |
| Ruiz et al. (31) | 2000 | 8.0 | 389 | 377 | 0 | 0 | 12 | 1.00 | 1.00 | 1.00 | 1.00 | 9.85 | |
| INH | Bergmann and Woods (8) | 1998 | 0.1 | 20 | 16 | 0 | 2 | 2 | 1.00 | 0.50 | 0.89 | 1.00 | 3.50 |
| 0.4 | 20 | 18 | 0 | 1 | 1 | 1.00 | 0.50 | 0.95 | 1.00 | 3.61 | |||
| Ruiz et al. (31) | 2000 | 0.1 | 389 | 201 | 44 | 0 | 144 | 0.82 | 1.00 | 1.00 | 0.77 | 7.18 | |
| 0.4 | 389 | 241 | 0 | 2 | 146 | 1.00 | 0.99 | 0.99 | 1.00 | 10.25 | |||
| RMP | Bergmann and Woods (8) | 1998 | 1.0 | 20 | 17 | 0 | 0 | 3 | 1.00 | 1.00 | 1.00 | 1.00 | 5.50 |
| Ruiz et al. (31) | 2000 | 1.0 | 389 | 345 | 0 | 0 | 44 | 1.00 | 1.00 | 1.00 | 1.00 | 11.03 | |
| EMB | Bergmann and Woods (8) | 1998 | 8.0 | 20 | 20 | 0 | 0 | 0 | 1.00 | NA | 1.00 | NA | 3.71 |
| Ruiz et al. (31) | 2000 | 8.0 | 389 | 387 | 0 | 0 | 2 | 1.00 | 1.00 | 1.00 | 1.00 | 8.26 | |
| PZA | LaBombardi (19) | 2002 | 300 | 50 | 42 | 0 | 0 | 8 | 1.00 | 1.00 | 1.00 | 1.00 | 7.28 |
Data are reported by individual drug. DORs are expressed as logarithms. TP, true positive; FN, false negative; FP, false positive; TN, true negative; Sens., sensitivity; Spec., specificity; PPV, positive predictive value; NPV, negative predictive value; NA, not available.
As with the previous reported systems, Versa TREK exhibits the decided advantage of being a fully automated, continuously monitoring, hands-off system. Starting from the primary culture bottle, the system is able to perform DST in a considerably short time. Limitations of the system include the lack of FDA clearance for SM and PZA associated with an increased frequency of unrecognized contaminations, which may lead to inappropriate results more frequently reported as false resistance (19). In addition to requiring full FDA clearance, a broader harvest of studies seem to be required to evaluate accuracy and reliability of the Versa TREK system for determining susceptibility of MTC isolates to first-line drugs.
DISCUSSION
The emergence of multidrug-resistant TB points out how important timely identification of MTC strains and their susceptibility testing may be to achieving effective management of the disease and prevention of its spreading. The rationale and principles of management and prevention were established more than 40 years ago and have not changed much since then (10). They have been formulated as having three goals: (i) to guide the choice of drugs for the initial therapy, (ii) to confirm the emergence of a clinically suspected drug resistance and to guide the choice of drugs for further treatment, and (iii) to estimate the prevalence of drug resistance in the community. For each of these purposes, the use of a reliable technique to perform the test is essential. In fact, in vitro results based on the proportion method (which combines a single concentration [critical] of primary antituberculous drugs with a standardized inoculum made from a pure culture of the recovered strain) were shown to correlate well with clinical response to anti-TB chemotherapy (28). Although for several years the recommended method of proportion was to use Löwenstein-Jensen medium, when agar media (Middlebrook 7H10 and 7H11) were developed critical concentration equivalents for these media were introduced. Since then, the method of proportion using agar media became the “gold standard” in the United States. In 1993, facing worrying increases of TB cases and drug resistance through the whole country, the Centers for Disease Control and Prevention (CDC) recommended the performance of DST on all initial MTC isolates from each patient and that testing should be repeated if the patient remained culture positive after 3 months of appropriate therapy or failed to respond clinically to the treatment. Moreover, in order to reduce TATs as much as possible, susceptibility results had to be reported within 4 weeks of specimen receipt (36). As the AP method required longer times to report, new broth-based methods with a shortened incubation time were introduced into clinical laboratories to fulfill CDC recommendations. The B460 system is currently regarded as an excellent method able to provide rapid and reliable results of susceptibility to first-line drugs. This half-automated system, however, lacks computerized data management and presents some well-recognized limitations, among which the growing expense related to the radioactive waste to be disposed of and a potential risk of cross-contamination due to the invasive reading represent the most relevant. Recently, some new liquid medium-based systems have been commercially introduced as alternatives to the radiometric system and are being evaluated for MTC susceptibility testing. Primary antituberculous drugs currently recommended by the Clinical and Laboratory Standards Institute (CLSI) (formerly NCCLS) for liquid systems include INH (two concentrations) and single concentrations of RMP, EMB, and PZA (27). Today, despite a pressing demand for susceptibility testing in order to limit the expansion of drug-resistant TB, a full correlation between results from the newly introduced NRM methods and patients' outcome still remains to be fully demonstrated (17). In fact, studies reported herewith clearly showed that the discriminatory power between resistant and susceptible strains was more reliable for INH and RMP than for other drugs. For this reason, it is recommended to determine in vitro criteria, which could be used to predict clinical resistance and susceptibility with acceptable accuracy, by testing a well-defined and soundly representative number of clinical isolates. In this context, it must be pointed out that most of the discrepant results observed with MGIT 960 and MB/BacT methods are related to strains with a low level of resistance that are difficult to classify as being composed of different mycobacterial subpopulations. A better concordance of results could be achieved by adjusting the drug concentrations according to those used in the conventional AP method, provided that this method can still represent the “gold standard” in the presence of MTC strains showing borderline susceptibility results. In addition, systematic use of double concentrations (low and high) for SM, INH, and EMB seems to be a useful skill to drastically reduce ME. Similarly, procedural test complexity (especially for the MB/BacT system) and poor standardization of inocula need prompt revision as they generate lack of reproducibility and reliability of DST results.
Finally, it is the microbiologist's responsibility to ensure that a sound quality control program be routinely carried out with the aim to evaluate test procedures, reagents' performance, and personnel proficiency. A critical element of quality control is the selection and use of genetically stable reference strains for which susceptibility results are well documented. In this context, the use of the fully susceptible M. tuberculosis H37Rv (ATCC 27294) strain is currently recommended by the CLSI (27). Furthermore, when testing both critical (low) and high drug concentrations, the ideal reference strain would be the one that is able to grow at a low concentration but is susceptible to the high concentration. Unfortunately, such a reference strain for this purpose is not available. Alternatively, in-house isolates with the characteristics listed above may be used, but for safety's sake, use of multiple-drug-resistant strains is not recommended (27). Quality control tests should be performed at least once a week in laboratories that perform tests daily or weekly or whenever a patient isolate is tested, if DST is performed less frequently. Participation in an external proficiency panel including MTC strains featuring low-level resistance to INH and resistance to other first-line drugs is strongly recommended. In addition, with the adoption of a new test method, laboratories should validate test results by performing the current test method and the new method in parallel for a series of patient isolates. Later on, DST results should be checked for several months by testing selected isolates by using another method, if available, or by addressing a reference laboratory. Selected isolates should include both susceptible and resistant MTC strains so as to check for the presence of ME and VME.
In conclusion, although each of the NRS reported above is an acceptable candidate to replace the radiometric system for susceptibility testing of MTC isolates, MGIT 960 presently appears to be the most reliable option.
REFERENCES
- 1.Ådjers-Koskelat, K., and M. L. Katila. 2003. Susceptibility testing with the manual Mycobateria Growth Indicator Tube (MGIT) and the MGIT 960 system provides rapid and reliable verification of multidrug-resistant tuberculosis. J. Clin. Microbiol. 41:1235-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic Society and Centers for Disease Control and Prevention. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376-1395. [DOI] [PubMed] [Google Scholar]
- 3.Ängeby, K. A. K., J. Werngren, J. C. Toro, G. Hedström, B. Petrini, and S. E. Hoffner. 2003. Evaluation of the BacT/ALERT 3D system for the recovery and drug susceptibility testing of Mycobacterium tuberculosis. Clin. Microbiol. Infect. 9:1148-1152. [DOI] [PubMed] [Google Scholar]
- 4.Aono, A., K. Hirano, S. Hamasaki, and C. Abe. 2002. Evaluation of BACTEC MGIT 960 PZA medium for susceptibility testing of Mycobacterium tuberculosis to pyrazinamide (PZA): compared with the results of pyrazinamidase assay and Kyokuto PZA test. Diagn. Microbiol. Infect. Dis. 44:347-352. [DOI] [PubMed] [Google Scholar]
- 5.Ardito, F., B. Posteraro, M. Sanguinetti, S. Zanetti, and G. Fadda. 2001. Evaluation of BACTEC Mycobacteria Growth Indicator Tube (MGIT 960) automated system for drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 39:4440-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bemer, P., F. Palikova, S. Rüsch-Gerdes, H. B. Drugeon, and G. Pfyffer. 2002. Multicenter evaluation of fully automated BACTEC Mycobacteria Growth Indicator Tube 960 system for susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:150-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bemer, P., T. Bodmer, J. Munzinger, M. Perrin, V. Vincent, and H. Drugeon. 2004. Multicenter evaluation of the MB/BacT system for susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 42:1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann, J. S., and G. L. Woods. 1998. Evaluation of the ESP culture system II for testing susceptibilities of Mycobacterium tuberculosis isolates to four primary antituberculous drugs. J. Clin. Microbiol. 36:2940-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunello, F., and R. Fontana. 2000. Reliability of MB/BacT system for testing susceptibility of Mycobacterium tuberculosis complex isolates to antituberculous drugs. J. Clin. Microbiol. 38:872-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canetti, G., W. Fox, A. Khomenko, H. T. Maler, N. K. Menon, D. A. Mitchinson, N. Rist, and N. A. Smeiev. 1969. Advances in techniques of testing mycobacterial in tuberculosis control programmes. Bull. W. H. O. 41:21-43. [PMC free article] [PubMed] [Google Scholar]
- 11.Cochrane Methods Group on Systematic Review of Screening and Diagnostic Tests. 1996. Recommended methods. [Online.] The Cochrane Collaboration, Oxford, United Kingdom. http://www.cochrane.org/docs/sadtdoc1.htm.
- 12.Diaz-Infentes, M. S., M. J. Ruiz-Serrano, L. Martinez-Sanchez, A. Ortega, and E. Bouza. 2000. Evaluation of the MB/BacT Mycobacterium detection system for susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 38:1988-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinal, M. A., A. L. Lazslo, L. Simonsen, F. Boulabhal, S. J. Kim, A. Reniero, S. Hoffner, H. L. Rieder, N. Binkin, C. Dye, R. Williams, and M. Raviglione. 2001. Global trends in resistance to anti-tuberculosis drugs. N. Engl. J. Med. 344:1294-1303. [DOI] [PubMed] [Google Scholar]
- 14.Huang, T., H. Tu, S. S. Lee, W. Huang, and Y. Liu. 2002. Antimicrobial susceptibility testing of Mycobacterium tuberculosis to first-line drugs: comparison of the MGIT 960 and BACTEC 460 systems. Ann. Clin. Lab. Sci. 32:142-147. [PubMed] [Google Scholar]
- 15.Irwig, L., A. N. Tosteson, C. Gatsonis, J. Lau, T. C. Chalmers, and F. Mosteller. 1994. Guidelines for meta-analyses evaluating diagnostic tests. Ann. Intern. Med. 120:667-676. [DOI] [PubMed] [Google Scholar]
- 16.Johansen, I. S., V. Østergaard, M. Mariamäki, A. Sosnovskaja, and B. Lundgren. 2004. Rapid, automated, nonradiometric susceptibility testing of Mycobacterium tuberculosis complex to first-line antituberculous drugs used in standard short-course chemotherapy. Diagn. Microbiol. Infect. Dis. 50:103-107. [DOI] [PubMed] [Google Scholar]
- 17.Kim, S. J. 2005. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur. Respir. J. 25:564-569. [DOI] [PubMed] [Google Scholar]
- 18.Kontos, F., N. Stavroula, C. Kostopulos, Z. Gitti, E. Petinaki, M. Maniati, S. Anagnostu, A. Raftopoulou, P. Papageorgiu, A. Scrioubellou, I. Tselentis, and A. N. Maniatis. 2003. Multicenter evaluation of the fully automated Bactec MGIT 960 system for susceptibility testing of Mycobacterium tuberculosis to pyrazinamide: comparison with the radiometric Bactec 460 system. Diagn. Microbiol. Infect. Dis. 55:331-333. [DOI] [PubMed] [Google Scholar]
- 19.LaBombardi, V. J. 2002. Comparison of the ESP and BACTEC system for testing susceptibilities of Mycobacterium tuberculosis complex isolates to pyrazinamide. J. Clin. Microbiol. 40:2238-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaBombardi, V. J. 2004. Versa TREK (formerly ESP culture system II) indirect susceptibility testing for Mycobacterium tuberculosis, p. 7.8.7.1-7.8.7.4. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook, 2nd ed. American Society for Microbiology, Washington, D.C.
- 21.LaBombardi, V. J. 2004. Versa TREK (formerly ESP culture system II) indirect susceptibility tests with pyrazinamide for Mycobacterium tuberculosis, p. 7.8.8.1-7.8.8.3. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook, 2nd ed. American Society for Microbiology, Washington, D.C.
- 22.Laszlo, A., M. Rahman, M. Espinal, M. Raviglione, et al. 2002. Quality assurance programme for drug susceptibility testing of Mycobacterium tuberculosis in the WHO/IUATLD Supranational Reference Laboratory Network: five rounds of proficiency testing, 1994-1998. Int. J. Tuberc. Lung Dis. 6:748-756. [PubMed] [Google Scholar]
- 23.Littenberg, B., and L. E. Moses. 1993. Estimating diagnostic accuracy from multiple conflicting reports: a new meta-analytic method. Med. Decis. Making 3:313-321. [DOI] [PubMed] [Google Scholar]
- 24.Madison, B., G. I. Robinson-Dunn, W. Gross, H. Lipman, B. Metchock, A. Sloutsky, G. Washabaugh, G. Mazurek, and J. Ridderhof. 2002. Multicenter evaluation of ethambutol susceptibility of Mycobacterium tuberculosis by agar proportion and radiometric method. J. Clin. Microbiol. 40:3976-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marttila, H. J., M. Mariamäki, M. K. Viljanene, and H. Soini. 2003. Performance of BACTEC 960 Mycobacteria Growth Indicator Tube in the susceptibility testing of genetically characterized Mycobacterium tuberculosis isolates. Eur. J. Clin. Microbiol. Infect. Dis. 22:757-759. [DOI] [PubMed] [Google Scholar]
- 26.Moses, L. E., D. Shapiro, and B. Littenberg. 1993. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat. Med. 12:1293-1316. [DOI] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. 2003. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes. Approved standard M24-A. National Committee for Clinical Laboratory Standards, Wayne, Pa. [PubMed]
- 28.Park, M. M., A. L. Davies, N. W. Schluger, et al. 1996. Outcome of MDRTB patients, 1983-1993: prolonged survival with appropriate therapy. Am. J. Respir. Crit. Care Med. 153:317-324. [DOI] [PubMed] [Google Scholar]
- 29.Pfyffer, G., F. Palikova, and S. Rüsch-Gerdes. 2002. Testing of susceptibility of Mycobacterium tuberculosis to pyrazinamide with the nonradiometric BACTEC MGIT 960 system. J. Clin. Microbiol. 40:1670-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts, G. D., L. Hall, and D. M. Wolk. 2002. Mycobacteria, p. 256-273. In A. Truant (ed.), Manual of commercial methods in clinical microbiology. American Society for Microbiology, Washington, D.C.
- 31.Ruiz, P., F. J. Zerolo, and M. J. Casal. 2000. Comparison of susceptibility testing of Mycobacterium tuberculosis using the ESP culture system II with that using the BACTEC method. J. Clin. Microbiol. 38:4663-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders, C. A., R. R. Nieda, and E. P. Desmond. 2004. Validation of the use of Midllebrook 7H10, BACTEC MGIT 960, and BACTEC 460 12B media for testing the susceptibility of Mycobacterium tuberculosis to levofloxacin. J. Clin. Microbiol. 42:5225-5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarparo, C., P. Ricordi, G. Ruggiero, and P. Piccoli. 2004. Evaluation of the fully automated BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide, streptomycin, isoniazid, rifampin and ethambutol and comparison with the radiometric BACTEC 460TB method. J. Clin. Microbiol. 42:1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqi, S. 2004. BACTEC MGIT 960 SIRE. Nonradiometric susceptibility testing for Mycobacterium tuberculosis, p. 7.8.5.1-7.8.5.5. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook, 2nd ed. American Society for Microbiology, Washington, D.C.
- 35.Siddiqi, S. 2004. BACTEC MGIT 960 PZA. Susceptibility testing for Mycobacterium tuberculosis, p. 7.8.6.1-7.8.6.4. In H. D. Isenberg (ed.), Clinical microbiology procedures handbook, 2nd ed. American Society for Microbiology, Washington, D.C.
- 36.Tenover, F. C., J. T. Crawford, R. E. Huebner, L. J. Geiter, C. R. Horsburgh, Jr., and R. C. Good. 1993. The resurgence of tuberculosis: is your laboratory ready? J. Clin. Microbiol. 31:767-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tortoli, E., R. Mattei, A. Savarino, L. Bartolini, and J. Beer. 2000. Comparison of Mycobacterium tuberculosis susceptibility testing performed with BACTEC 460TB (Becton Dickinson) and MB/BacT (Organon Teknika) systems. Diagn. Microbiol. Infect. Dis. 38:83-86. [DOI] [PubMed] [Google Scholar]
- 38.Tortoli, E., M. Benedetti, A. Fontanelli, and M. T. Simonetti. 2002. Evaluation of automated BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to four major antituberculous drugs: comparison with the radiometric BACTEC 460TB method and the agar plate method of proportion. J. Clin. Microbiol. 40:607-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yew, W. W., S. C. W. Tong, K. S. Lui, S. K. F. Leung, C. H. Chau, and E. P. Wang. 2001. Comparison of MB/BacT system and agar proportion method in drug susceptibility testing of Mycobacterium tuberculosis. Diagn. Microbiol. Infect. Dis. 39:229-232. [DOI] [PubMed] [Google Scholar]