Abstract
RsmA (for regulator of secondary metabolism), RsmC, and rsmB RNA, the components of a posttranscriptional regulatory system, control extracellular protein production and pathogenicity in Erwinia carotovora subsp. carotovora. RsmA, an RNA binding protein, acts as a negative regulator by promoting message decay. rsmB RNA, on the other hand, acts as a positive regulator by neutralizing the effect of RsmA. RsmC modulates the levels of RsmA and rsmB RNA by positively regulating rsmA and negatively controlling rsmB. The level of rsmB RNA is substantially higher in RsmA+ bacteria than in RsmA− mutants. We show that rsmB RNA is more stable in the presence of RsmA than in its absence. RsmA does not stimulate the expression of an rsmB-lacZ transcriptional fusion; in fact, the β-galactosidase level is somewhat higher in RsmA− bacteria than in RsmA+ bacteria. We also investigated the basis for increased levels of rsmA and rsmB RNAs in the absence of the quorum-sensing signal, N-[3-oxohexanoyl]-l-homoserine lactone (OHL). The absence of OHL activates transcription of rsmA but not of rsmB. Instead, increased stability of rsmB RNA in the presence of RsmA accounts for the elevated levels of the rsmB RNA in OHL− bacteria. Mutant studies disclosed that while RsmA, OHL, and RsmC control the levels of rsmB RNA, high levels of rsmB RNA occur in the absence of RsmC or OHL only in RsmA+ bacteria, indicating a critical role for RsmA in modulating the levels of rsmB RNA. The findings reported here firmly establish that the quorum-sensing signal is channeled in E. carotovora subsp. carotovora via the rsmA-rsmB posttranscriptional regulatory system.
Rsm (for regulator of secondary metabolism) is a novel type of posttranscriptional regulatory system which has a profound effect on bacterial metabolism and behavior. Rsm of Erwinia carotovora subsp. carotovora consists of three major components: an RNA binding protein, RsmA, which promotes RNA decay (8, 11); an untranslated RNA molecule, rsmB, which neutralizes RsmA action, apparently by sequestering RsmA (29); and RsmC, which positively regulates rsmA and negatively controls rsmB RNA levels (13). It is now apparent that Rsm and Rsm-like systems control diverse phenotypes in many prokaryotic species. In Erwinia, this regulatory system plays a critical role by affecting plant interaction, extracellular enzyme production, extracellular polysaccharide synthesis, swarming motility, secondary metabolite production, and the quorum-sensing system (8, 11, 12, 31, 34, 35). In Escherichia coli, a homologous system comprising CsrA (equivalent to RsmA) and csrB (equivalent to rsmB) RNA, controls glycogen synthesis, metabolism of acetate, motility and flagellum biosynthesis, and biofilm formation (21, 27, 40). There are substantial data indicating the existence of the regulatory system in various enterobacterial species, including human pathogens (2, 3, 5, 9, 14, 40). Moreover, recent studies of several Pseudomonas species have demonstrated that RsmA-rsmB regulatory systems control secondary metabolite production and plant interaction. For example, in Pseudomonas aeruginosa, overexpression of rsmA reduces the levels of protease (Prt), elastase, and staphylolytic (LasA Prt) activity, as well as those of the PA-IL lectin, hydrogen cyanide, and pyocyanin. In Pseudomonas fluorescens, overexpressed prrB, which is structurally similar to rsmB, increases the production of Phl (2,4-diacetylphloroglucinol) and hydrogen cyanide (1, 6, 7, 38). In Pseudomonas syringae pv. tomato strain DC3000, RsmA reduces the production of extracellular proteins, causes attenuation of pathogenicity in Arabidopsis thaliana, and lowers the efficiency of the induction of the hypersensitive reaction in tobacco (A. Chatterjee, Y. Cui, H. Yang, J. Alfano, A. Collmer, and A. K. Chatterjee, Pseudomonas 2001, abstr. PS 8, 2001). Thus, the RsmA-rsmB regulatory system appears to have been conserved in many prokaryotes.
As this system is so critical, it was predicted that rsmA and rsmB expression would be rigorously controlled. Indeed, studies of E. carotovora subsp. carotovora have disclosed that several transcriptional factors control the expression of the rsmA and rsmB genes. Examples include HexA, a LysR-type regulator (17, 36); RsmC, a putative transcriptional adapter (13); and KdgR, an IcIR-type repressor (20, 30). A remarkable feature of these regulators is their dual action: they positively control RsmA production and negatively control the levels of rsmB RNA (13, 20, 30, 36). GacS and GacA, members of a two-component system (19), control rsmB transcription in E. carotovora subsp. carotovora (10) and rsmZ transcription in P. aeruginosa (18).
We have consistently noted that RsmA+ bacteria contain significantly higher levels of rsmB RNA than RsmA− bacteria. Since RsmA acts as a negative regulator by promoting message decay, the occurrence of higher levels of rsmB RNA was deemed unusual, prompting further analysis. While this work was in progress, Gudapaty et al. (16) attributed a similar effect of CsrA on csrB RNA in E. coli to a positive regulation of csrB by CsrA. The CsrA effect is not due to differences in stability of csrB RNA in CsrA+ and CsrA− bacteria. In fact, studies with a csrB-lacZ fusion demonstrated that CsrA, by a yet-undetermined mechanism, controls csrB transcription. Since we have never noted a positive regulatory effect of RsmA in Erwinia, the results with E. coli were quite intriguing and warranted further analysis of the Rsm system.
The quorum-sensing signal, N-[3-oxohexanoyl]-l-homoserine lactone (OHL), is required for extracellular enzyme production, expression of the Hrp regulon, and the pathogenicity of E. carotovora subsp. carotovora (8, 12, 15, 22, 39). The findings (8, 12) that RsmA− mutants of E. carotovora subsp. carotovora in the absence of OHL produce high levels of extracellular enzymes, overexpress the Hrp regulon, and exhibit enhanced virulence have raised the possibility that the OHL response is channeled via the RsmA-rsmB regulatory system. Indeed, a recent report documents the fact that OHL deficiency led to increased production of both rsmA and rsmB RNAs (25). While this finding supports the idea that OHL acts via the RsmA-rsmB pathway, it was not clear (i) how OHL affected rsmB RNA levels and (ii) why OHL deficiency inhibited extracellular enzyme production and negatively affected other traits. Here, we present data that provide answers to those questions.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains and plasmids are described in Table 1. The strains carrying drug markers were maintained on Luria-Bertani (LB) agar containing appropriate antibiotics. The wild-type strains were maintained on LB agar. The recipes for LB medium and minimal-salts medium have been described previously (8, 37). When required, antibiotics were added as follows (in micrograms per milliliter): ampicillin (Ap), 100; chloramphenicol (Cm), 20; kanamycin (Km), 50; spectinomycin (Sp), 50; and tetracycline (Tc), 10. The media were solidified by the addition of 1.5% agar.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| E. carotovora subsp. carotovora | ||
| Ecc71 | Wild type | 44 |
| AC5047 | Nalr derivative of AC5006 | 8 |
| AC5050 | RsmC− derivative of AC5047; Kmr | 13 |
| AC5070 | RsmA− derivative of AC5047; Kmr | 8 |
| AC5071 | RsmA− derivative of Ecc71; Kmr | 35 |
| AC5053 | RsmC− derivative of Ecc71; Kmr | 13 |
| AC5054 | RsmC− derivative of AC5071; Kmr Spr | 13 |
| AC5088 | RsmB− derivative of Ecc71; Cmr | Laboratory collection |
| AC5090 | Ohl− derivative of AC5070; Kmr Spr | 8 |
| AC5091 | Ohl− derivative of AC5047; Kmr | 8 |
| AC5094 | Ohl− derivative of Ecc71; Kmr | 8 |
| E. coli DH5α | φ80lacZΔM15 Δ(lacZYA-argF) U169 hsdR17 recA1 endA1 thi-1 | Gibco BRL |
| Plasmids | ||
| pBluescript SK(+) | Apr | Stratagene |
| pDK7 | Cmr | 24 |
| pMP220 | Tcr; promoter-probe vector | 42 |
| pRK415 | Tcr | 23 |
| pCL1920 | Spr Smr | 26 |
| pAKC783 | Aprpel-1+ DNA in pBluescript SK(+) | 28 |
| pAKC856 | AprohlI DNA in pBluescript SK(+) | 8 |
| pAKC861 | Sprlacp-ohlI in pCL1920 | Laboratory collection |
| pAKC878 | TcrrsmA+ DNA in pRK415 | 29 |
| pAKC975 | SprrsmC+ in pCL1920 | 13 |
| pAKC979 | TcrrsmC+ in pRK415 | 13 |
| pAKC882 | AprrsmA in pT7-7 | 35 |
| pAKC1002 | TcrrsmBEcc-lacZ in pMP220 | 29 |
| pAKC1004 | SprrsmB DNA in pCL1920 | 29 |
| pAKC1047 | TcrrsmBEa-lacZ in pMP220 | 10 |
| pAKC1048 | TcrrsmBEhg-lacZ in pMP220 | 10 |
| pAKC1049 | Sprlacp-rsmB in pCL1920 | 31 |
| pAKC1100 | TcrrsmAEcc-lacZ in pMP220 | This work |
DNA techniques.
Standard procedures were used in the isolation of plasmid and chromosomal DNAs, electroporation, restriction endonuclease digestion, gel electrophoresis, and DNA ligation (41). PCR was performed as described by Liu et al. (29). The Primer-a-Gene DNA labeling system and restriction and modifying enzymes were obtained from Promega (Madison, Wis.).
RNA preparation and Northern hybridization experiments.
Total RNA was isolated from E. carotovora subsp. carotovora constructs grown in minimal-salts medium plus sucrose (0.5% [wt/vol]) with or without drugs at 28°C. To determine the stability of rsmB mRNA in RsmA+ Ohl+ (Ecc71), RsmA− Ohl+ (AC5070 and AC5071), RsmA+ Ohl− (AC5094), and RsmA− Ohl− (AC5090) strains, the bacteria were grown to a Klett value of ca. 150, at which point rifampin (0.2 mg/ml) was added to block further initiation. Culture samples (8 ml each) were then withdrawn at various time points into tubes containing 5 ml of ice-cold water, and total RNA was extracted.
The probes used in this work were the 314-bp EcoRV-KpnI fragment of pel-1 from pAKC783 (28), the 183-bp NdeI-SalI fragment of rsmA from pAKC882 (35), the 304-bp EcoRV-HindIII fragment of rsmC from pAKC975 (13), the 321-bp BamHI-HindIII fragment of rsmB from pAKC1004 (29), and the 386-bp DraI-EcoRI fragment of ohlI from pAKC856 (8). DNA probes (Promega) were labeled with [α-32P]dATP by random priming according to the manufacturer's instructions. Prehybridization (6 h at 65°C) and hybridization (18 h at 65°C) were performed in prehybridization buffer (6× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 2× Denhardt's solution, 0.1% sodium dodecyl sulfate [SDS], 100 μg of denatured salmon sperm DNA/ml). After hybridization, the membranes were washed twice for 20 min at 65°C in 2× SSC-0.5% SDS and then for 30 min at 65°C in 0.5× SSC-0.5% SDS and examined by autoradiography with X-ray film (Kodak, Rochester, N.Y.). The densitometric analysis of autoradiography was performed by using the MetaMorph imaging system (version 4.6r3; Universal Imaging Corp.).
Western blot analysis of RsmA.
E. carotovora subsp. carotovora strains Ecc71 and AC5094 were grown in minimal-salts medium plus sucrose (0.5% [wt/vol]) at 28°C to a Klett value of ca. 150. Total bacterial proteins were precipitated with trichloroacetic acid at a final concentration of 10% (vol/vol), centrifuged, and resuspended in 1× SDS loading buffer (41). Western blot analysis of RsmA was performed according to the method of Mukherjee et al. (34). The anti-RsmA antiserum raised against the synthesized peptide from amino acids 48 to 61 of RsmA in rabbit by Genemed Biotechnologies Inc. (San Francisco, Calif.) was used as the probe.
β-Galactosidase assays.
A DNA segment containing nucleotides −117 to +145 from the transcriptional start site of E. carotovora subsp. carotovora rsmA (rsmAEcc) was amplified by PCR and cloned into the promoter-probe vector pMP220 to produce the rsmAEcc-lacZ fusion, pAKC1100. The rsmBEcc-lacZ (pAKC1002), rsmB gene of Erwinia amylovora (rsmBEa)-lacZ (pAKC1047), and rsmB gene of Erwinia herbicola pv. gypsophilae (rsmBEhg)-lacZ (pAKC1048) fusions used in this experiment have been described previously (10, 29). E. carotovora subsp. carotovora strains carrying these constructs were grown in minimal-salts medium plus sucrose (0.5% [wt/vol]) and tetracycline at 28°C to a Klett value of ca. 200 and harvested for β-galactosidase assays. The β-galactosidase assays were carried out according to the method of Miller (33).
Extracellular-enzyme assays.
The quantitative assay conditions of pectate lyase (Pel) are described in our previous publication (37). For estimation of the levels of extracellular polygalacturonase (Peh), cellulase (Cel), and Prt activities in culture supernatants, we used agarose semiquantitative plate assay procedures (8).
Bioluminescence assay for OHL production.
Ecc71 was grown in minimal-salts medium plus sucrose (0.5% [wt/vol]) at 28°C to Klett values of 150 and 200. Culture supernatants were used for assays by using an E. coli-based bioassay system as described previously (8). There is a linear relationship between the quantity of OHL and the emission of bioluminescence expressed as relative light units (RLU) per minute per milliliter.
The experiments were performed at least two to three times, and the results were reproducible.
RESULTS AND DISCUSSION
RsmA affects the stability of rsmB RNA.
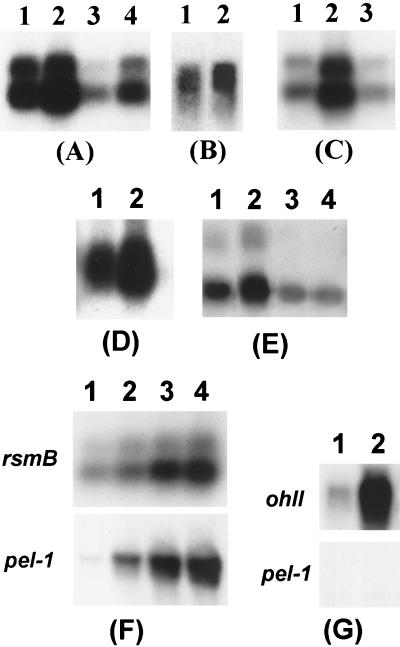
The data in Fig. 1A show that rsmB RNA levels are at least four times higher in the RsmA+ strain (lane 1) than in the RsmA− strain (lane 3). In fact, rsmB transcript levels are low in the absence of RsmA. This contrasts with about three- to fourfold-higher levels of rsmC transcripts in RsmA− bacteria than in RsmA+ bacteria (Fig. 1B).
FIG. 1.
Northern blot analysis of rsmA, rsmB, rsmC, pel-1, and ohlI transcripts in E. carotovora subsp. carotovora strains. (A) rsmB RNA in Ecc71 (wild-type parent; lane 1), AC5053 (RsmA+ RsmC−; lane 2), AC5071 (RsmA− RsmC+; lane 3), and AC5054 (RsmA− RsmC−; lane 4). (B) rsmC RNA in Ecc71 (lane 1) and AC5071 (lane 2). (C) rsmB RNA in AC5054 carrying pRK415 (vector; lane 1), AC5054 carrying pAKC878 (RsmA+; lane 2), and AC5054 carrying pAKC979 (RsmC+; lane 3). (D) rsmA RNA in Ecc71 (lane 1) and AC5094 (Ohl−; lane 2). (E) rsmB RNA in Ecc71 (lane 1), AC5094 (RsmA+ Ohl−; lane 2), AC5071 (RsmA− Ohl+; lane 3), and AC5090 (RsmA− Ohl−; lane 4). (F) rsmB and pel-1 RNAs in AC5094 (Ohl−) carrying pDK7 and pCL1920 (vectors; lanes 1), AC5094 carrying pDK7 and pAKC1049 (plac-rsmB; lanes 2), AC5094 carrying pDK7 and pAKC1049 with 0.1 mM IPTG treatment (lanes 3), and AC5094 carrying pDK7 and pAKC1049 with 1.0 mM IPTG treatment (lanes 4). (G) ohlI and pel-1 RNAs in AC5088 (RsmB−) carrying pCL1920 (vector; lanes 1) or pAKC861 (plac-ohlI; lanes 2). For rsmB, each lane contained 5 μg of total RNA, and for rsmA, rsmC, pel-1, and ohlI, each lane contained 15 μg of total RNA.
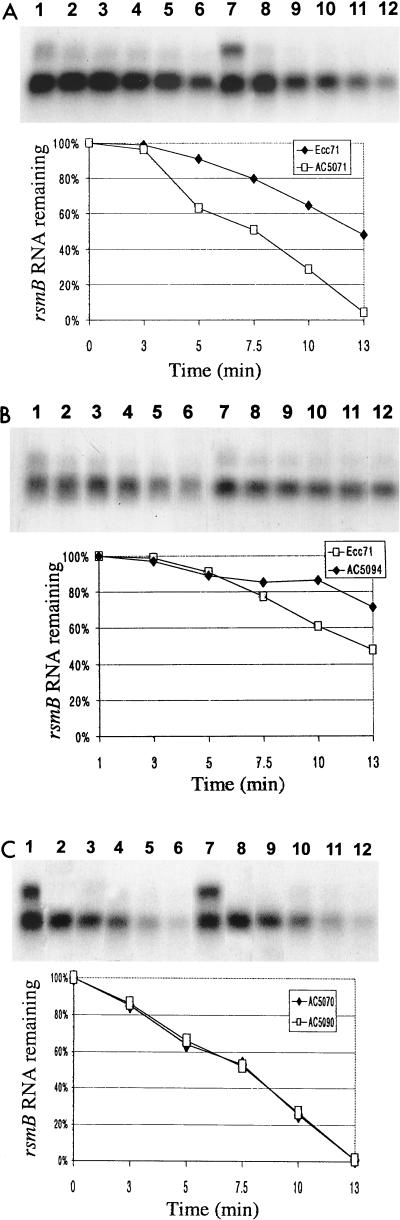
To understand the basis for higher levels of rsmB RNA in RsmA+ bacteria, we compared the stabilities of rsmB RNA in RsmA+ and RsmA− bacteria. The data shown in Fig. 2A clearly establish that in the absence of RsmA, rsmB RNA is quite unstable. The positive effect of RsmA on rsmB RNA stability in E. carotovora subsp. carotovora is very different from the findings in E. coli, as the presence or absence of CsrA had no apparent effect on the stability of csrB RNA (16). The observations with E. coli and E. carotovora subsp. carotovora are hard to reconcile in the present state of our understanding of the Rsm and Csr systems. However, the findings clearly indicate a fundamental difference in the regulation of rsmB and csrB in these bacteria that inhabit different ecosystems.
FIG.2.
Stabilities of rsmB transcript in E. carotovora subsp. carotovora strains. Samples were collected 0, 3, 5, 7.5, 10, and 13 min after the addition of rifampin. The densitometeric scanning results (percentage of remaining mRNA) were plotted against time after rifampin treatment. (A) Ecc71 (lanes 1 to 6; each lane contained 5 μg of total RNA) and AC5071 (RsmA−) (lanes 7 to 12; each lane contained 15 μg of total RNA). The X-ray film was exposed for 5 h at −80°C. (B) Ecc71 (lanes 1 to 6; each lane contained 15 μg of total RNA) and AC5094 (Ohl−) (lanes 7 to 12; each lane contained 7 μg of total RNA). The X-ray film was exposed for 1 h at −80°C. (C) AC5070 (RsmA− Ohl+) (lanes 1 to 6) and AC5090 (RsmA−Ohl−) (lanes 7 to 12). Each lane contained 15 μg of total RNA. The X-ray film was exposed for 5 h at −80°C.
Effect of RsmA on rsmB transcription.
As stated above, the data for E. coli strongly suggest a positive effect of CsrA on csrB transcription. To ascertain if transcription could account at least in part for the differences in RsmA+ and RsmA− bacteria, we examined the expression of an rsmBEcc-lacZ transcriptional fusion (29). In this fusion, 221 bp of rsmBEcc DNA has been cloned in front of lacZ in a low-copy-number promoter-probe vector, pMP220 (42). The lacZ construct or the vector was transferred into RsmA+ strain AC5047, its RsmA− derivative, AC5070, and its RsmC− derivative, AC5050. The data (Table 2) reveal that the expression of rsmBEcc-lacZ is slightly (ca. 1.3 times) higher in RsmA− bacteria than RsmA+ bacteria. These observations are very different from those reported for E. coli, where substantial (ca. 20-fold) stimulation was noted in CsrA+ bacteria compared to CsrA− bacteria. More importantly, our data do not indicate a positive effect of RsmA on rsmB transcription.
TABLE 2.
β-Galactosidase activities of RsmA−, RsmC−, and Ohl− E. carotovora subsp. carotovora strains carrying rsmA-lacZ and rsmB-lacZ fusionsa
| Strain | Relevant phenotype | β-Galactosidase activity (Miller units) |
|---|---|---|
| AC5047/pMP220 | RsmA+ RsmC+ Ohl+/(vector) | 61 |
| AC5070/pMP220 | RsmA− RsmC+ Ohl+/(vector) | 71 |
| AC5047/pAKC1002 | RsmA+ RsmC+ Ohl+/(rsmBEcc-lacZ) | 2,150 |
| AC5070/pAKC1002 | RsmA− RsmC+ Ohl+/(rsmBEcc-lacZ) | 2,900 |
| AC5050/pMP220 | RsmA+ RsmC− Ohl+/(vector) | 72 |
| AC5050/pAKC1002 | RsmA+ RsmC− Ohl+/(rsmBEcc-lacZ) | 3,817 |
| AC5047/pMP220 | RsmA+ RsmC+ Ohl+/(vector) | 88 |
| AC5070/pMP220 | RsmA− RsmC+ Ohl+/(vector) | 96 |
| AC5047/pAKC1047 | RsmA+ RsmC+ Ohl+/(rsmBEa-lacZ) | 1,402 |
| AC5070/pAKC1047 | RsmA− RsmC+ Ohl+/(rsmBEa-lacZ) | 2,712 |
| AC5047/pAKC1048 | RsmA+ RsmC+ Ohl+/(rsmBEhg-lacZ) | 1,530 |
| AC5070/pAKC1048 | RsmA− RsmC+ Ohl+/(rsmBEhg-lacZ) | 2,097 |
| AC5047/pMP220 | RsmA+ Ohl+/(vector) | 49 |
| AC5091/pMP220 | RsmA+ Ohl−/(vector) | 69 |
| AC5047/pAKC1100 | RsmA+ Ohl+/(rsmAEcc-lacZ) | 810 |
| AC5091/pAKC1100 | RsmA+ Ohl−/(rsmAEcc-lacZ) | 1,868 |
| AC5047/pMP220 | RsmA+ Ohl+/(vector) | 57 |
| AC5091/pMP220 | RsmA+ Ohl−/(vector) | 63 |
| AC5047/pAKC1002 | RsmA+ Ohl+/(rsmBEcc-lacZ) | 2,901 |
| AC5091/pAKC1002 | RsmA+ Ohl−/(rsmBEcc-lacZ) | 2,662 |
Bacteria were grown in minimal-salts medium plus sucrose and tetracycline at 28°C to a Klett value of ca. 200 for the assays.
We extended these findings by examining the expression of lacZ transcriptional fusions of rsmBEa and rsmBEhg (see reference 31 for the characteristics of these genes). The fusions were transferred to RsmA+ and RsmA− E. carotovora subsp. carotovora strains and assayed for β-galactosidase activity. The data (Table 2) show that RsmA did not stimulate rsmB transcription. Although somewhat limited in scope, our findings with three different plant-pathogenic Erwinia species and those of Gudapaty et al. (16) with E. coli raise the possibility that rsmB RNA production is differently regulated in plant-pathogenic and non-plant-pathogenic bacteria.
RsmC deficiency in RsmA+ bacteria leads to rsmB RNA overproduction.
We considered the possibility that the RsmA effect on rsmB RNA was exerted via another regulator controlled by RsmA. We considered RsmC as the likely candidate for the following reasons: (i) rsmC RNA levels are higher in RsmA− bacteria than in RsmA+ bacteria (Fig. 1B); (ii) RsmC is known to negatively regulate rsmB RNA production (13; also, Table 2 shows the effect of RsmC deficiency on the expression of an rsmBEcc-lacZ transcriptional fusion); and (iii) RsmA does not affect the levels of GacA, HexA, and KdgR (A. Chatterjee, unpublished data), known to affect RsmA and rsmB RNA production (10, 20, 30, 36). The results (Fig. 1A) with RsmA+ RsmC+ (lane 1), RsmA+ RsmC− (lane 2), RsmA− RsmC+ (lane 3), and RsmA− RsmC− (lane 4) strains reveal that the level of rsmB RNA is highest in RsmA+ RsmC− bacteria, followed by the levels in RsmA+ RsmC+, RsmA− RsmC−, and RsmA− RsmC+ strains. We attribute the presence of the highest level of rsmB RNA in the RsmA+ RsmC− strain to the cumulative effects of the absence of the negative effect of RsmC and the stability of rsmB RNA in the presence of RsmA. By contrast, the marked reduction in the level of rsmB RNA in RsmA− RsmC+ bacteria is due the presence of RsmC and the instability of the RNA in the absence of RsmA.
To assess the relative importance of RsmA and RsmC in rsmB RNA production, we transferred the low-copy-number vector pRK415; the RsmA+ plasmid, pAKC878; or the RsmC+ plasmid, pAKC979, into the RsmA− RsmC− strain and compared the levels of rsmB RNA. The data in Fig. 1C show that while RsmA had a marked effect on the levels of rsmB RNA (lane 2), RsmC in the absence of RsmA had no such effect (lane 3).
Effects of the quorum-sensing signal, OHL, on rsmA and rsmB expression.
A recent report has documented the fact that OHL deficiency results in elevated levels of rsmA and rsmB transcripts in E. carotovora subsp. carotovora strain SCC3193 (25). The data in Fig. 1D and E (lanes 1 and 2) show a similar effect of OHL on Ecc7l. We have extended these observations by examining the expression of an rsmA-lacZ transcriptional fusion and an rsmB-lacZ transcriptional fusion in OHL+ and OHL− bacteria. The data in Table 2 reveal about two- to threefold-higher levels of β-galactosidase in OHL− bacteria carrying the rsmA-lacZ fusion than in OHL+ bacteria carrying the same fusion. On the other hand, the levels of β-galactosidase in OHL+ and OHL− bacteria carrying the rsmB-lacZ transcriptional fusion are comparable. These results suggest that rsmA transcription, but not rsmB transcription, is affected by OHL. Western blot analysis (Fig. 3) also revealed that the RsmA level is higher in Ohl− strains than in the Ohl+ parent.
FIG. 3.
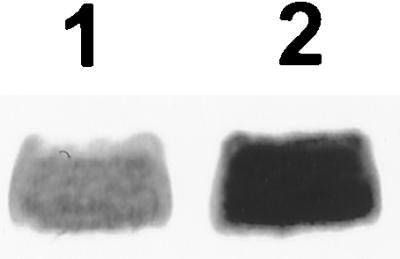
Western blot analysis of RsmA produced by Ecc71 (lane 1) and AC5094 (Ohl−; lane 2). Each lane contained 15 μg of total bacterial protein.
We next considered the possibility that the higher levels of rsmB RNA in OHL− bacteria than in OHL+ bacteria (Fig. 1E, lanes 1 and 2) could be due to increased production of RsmA and consequent RsmA-mediated stability of rsmB RNA. Two lines of evidence support this hypothesis. First, the levels of rsmB RNA are much lower in the OHL− RsmA− double mutant than in its OHL− RsmA+ parent (Fig. 1E, lanes 4 and 2). Second, the results shown in Fig. 2B demonstrate that rsmB RNA is more stable in RsmA+ OHL− bacteria than in RsmA+ OHL+ bacteria. By contrast, the stability of rsmB RNA is not affected in RsmA− OHL+ and RsmA− OHL− strains (Fig. 2C). These observations establish that the OHL effect is abrogated in the absence of RsmA and support the prediction that in OHL− bacteria the pool size of RsmA will be high, which presumably binds every available rsmB RNA, conferring stability.
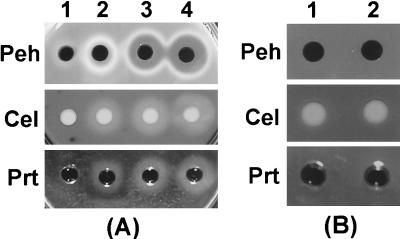
It is now fairly well established that OHL is required for extracellular protein production as well as the expression of various other traits in E. carotovora subsp. carotovora and other soft-rotting Erwinia spp. (8, 12, 22, 39). Since the production of both RsmA and rsmB RNAs is stimulated by OHL deficiency (see above and reference 25) and rsmB RNA is believed to sequester RsmA, at first glance one is inclined to dismiss the possibility that the Rsm regulatory system is responsible for the pleiotropic negative phenotype of OHL− bacteria. Direct evidence for this would require determining the relative levels of free RsmA and RsmA-rsmB RNA complex. While technologies are being developed for such analyses, we have obtained indirect evidence that excess RsmA is indeed responsible for the negative effects of OHL deficiency. We transferred a lacp-rsmB plasmid, pAKC1049, and pDK7 carrying lacIq into the OHL− strain, AC5094, grew the bacteria in minimal-salts medium plus sucrose containing various levels of IPTG (isopropyl-β-d-thiogalactopyranoside), and assayed for Pel, Peh, Cel, and Prt activities. AC5094 carrying the empty vectors served as a control. Table 3 and Fig. 4A show that production of extracellular enzymes did not occur in the absence of pAKC1049 but did occur in AC5094 carrying pAKC1049. Moreover, the data show that increased levels of extracellular enzymes were produced with increasing concentrations of IPTG. The results of Northern analysis indicate a correlation between the levels of rsmB RNA and pel-1 transcripts (Fig. 1F). Since RsmA binds rsmB RNA, we conclude that overproduction of rsmB RNA in the presence of IPTG resulted in titration of most or all available RsmA molecules, thereby relieving the inhibitory effects of RsmA on extracellular enzyme production.
TABLE 3.
Levels of Pel produced by E. carotovora subsp. carotovora constructsa
| Strain | Relevant phenotype | IPTG (mM) | Pel sp act (U/ml/A600 unit) |
|---|---|---|---|
| AC5094/pCL1920/pDK7 | Ohl−/(vectors) | 0 | 0.04 |
| AC5094/pAKC1049/pDK7 | Ohl−/(lacp-rsmBEcc) | 0 | 0.6 |
| AC5094/pAKC1049/pDK7 | Ohl−/(lacp-rsmBEcc) | 0.1 | 1.2 |
| AC5094/pAKC1049/pDK7 | Ohl−/(lacp-rsmBEcc) | 1.0 | 1.4 |
| AC5088/pCL1920 | RsmB−/(vector) | 0 | NDb |
| AC5088/pAKC861 | RsmB−/(lacp-ohlI) | 0 | ND |
Bacteria were grown in minimal-salts medium plus sucrose (0.5% [wt/vol]) plus drugs, with or without IPTG, at 28°C to a Klett value of ca. 200. Culture supernatants were used for enzyme assays.
ND, not detectable.
FIG. 4.
(A) Agarose plate assays of Peh, Cel, and Prt activities of E. carotovora subsp. carotovora strain AC5094 (Ohl−) carrying PDK7 and pCL1920 (vectors; column 1), AC5094 carrying pDK7 and pAKC1049 (lacp-rsmB; column 2), AC5094 carrying pDK7 and pAKC1049 with 0.1 mM IPTG treatment (column 3), and AC5094 carrying pDK7 and pAKC1049 with 1.0 mM IPTG treatment (column 4). (B) Agarose plate assays of Peh, Cel, and Prt activities of E. carotovora subsp. carotovora strain AC5088 (RsmB−) carrying pCL1920 (vector; column 1) and AC5088 carrying pAKC861 (lacp-ohlI; column 2). The bacteria were grown at 28°C in minimal-salts medium plus sucrose and drugs to a Klett value of 200, and the culture supernatants were used for assays. Each well contained 10 μl of culture supernatant.
As discussed above (see the introduction), several lines of evidence suggested that the quorum-sensing signal acts via the posttranscriptional regulatory system. To further test this, we reexamined the properties of an Ohl+ RsmB− mutant. Extracellular enzyme production does not occur in this construct (Chatterjee, unpublished). We transferred the lacp-ohlI plasmid, pAKC861, into the mutant, where the lac promoter drives ohlI expression. The construct was grown in minimal-salts medium plus sucrose. Despite the production of high levels of ohlI RNA, extracellular enzymes and pel-1 transcripts did not occur under these conditions (Fig. 1G and 4B and Table 3). Thus, the OHL effect did not manifest itself in the absence of rsmB RNA. We have previously documented the fact that RsmA− strains do not require OHL for enzyme production and pathogenicity (8). These observations collectively demonstrate that the OHL effect in E. carotovora subsp. carotovora is channeled via the RsmA-rsmB regulatory pathway. To our knowledge, this is the first example of a quorum-sensing signal acting via a posttranscriptional regulatory system. How OHL represses transcription of rsmA is not evident. Several lines of evidence suggest that an OHL receptor, similar to LuxR, does not directly regulate rsmA transcription. First, a mutant of E. carotovora subsp. carotovora strain SCC3193 deficient in a LuxR homolog, ExpR, is not affected in extracellular enzyme production and the expression of rsmA (4, 25). Second, sequences corresponding to the LuxR box are not found upstream of rsmA DNA. Therefore, it is quite likely that the OHL effect in E. carotovora subsp. carotovora is channeled via a novel regulator that controls rsmA transcription. We have initiated a search for such a regulator.
In the experiments described above, the total-RNA and -protein samples were obtained from bacterial cultures grown to a Klett value of 150, and β-galactosidase and extracellular-enzyme assays were performed using cultures grown to a Klett value of 200. To verify that the OHL levels were comparable under these conditions, we have estimated the levels of OHL using an E. coli-based bioluminescence assay system (8). Ecc71 produced 7.3 × 107 RLU at a Klett value of 150 and 7.8 × 107 RLU at a Klett value of 200, demonstrating that OHL levels were generally similar under these growth conditions.
In conclusion, we have established that higher levels of rsmB transcripts in RsmA+ bacteria than in RsmA− bacteria are mostly due to the stability of rsmB RNA conferred by RsmA and not to increased transcription, as is the case with CsrA and csrB in E. coli. In addition, the regulatory effect of RsmA on RsmC may partly contribute to the levels of rsmB RNA. Despite structural and functional similarities between RsmA and CsrA and between rsmB RNA and csrB RNA, there are significant differences in these two systems as well. For example, a homolog of RsmC has not been detected in E. coli. RsmA and CsrA appear to affect different genes in vivo. While CsrA has been found to positively affect the flhCD operon in E. coli (43), no such effect of RsmA was seen in E. carotovora subsp. carotovora (Chatterjee, unpublished). E. coli is known not to produce OHL or its analogs, although E. coli responds to quorum-sensing signals produced by other bacteria (32). Thus, it is clear that OHL or similar molecules, at least in axenic culture, do not affect CsrA production. As documented here, rsmA expression in E. carotovora subsp. carotovora is indeed stimulated by OHL deficiency. These differences probably reflect the fact that the RsmA-rsmB and CsrA-csrB systems target different set of genes in bacteria inhabiting animals and humans and those inhabiting plants.
Acknowledgments
Our work was supported by the National Science Foundation (grants MCB-9728505 and DBI-0077622) and the Food for the 21st Century program of the University of Missouri.
We thank Judy D. Wall for critically reviewing the manuscript.
REFERENCES
- 1.Aarons, S., A. Abbas, C. Adams, A. Fenton, and F. O'Gara. 2000. A regulatory RNA (PrrB RNA) modulates expression of secondary metabolite genes in Pseudomonas fluorescens F113. J. Bacteriol. 182:3913-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altier, C., M. Suyemoto, and S. D. Lawhon. 2000. Regulation of Salmonella enterica serovar typhimurium invasion genes by csrA. Infect. Immun. 68:6790-6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altier, C., M. Suyemoto, A. I. Ruiz, K. D. Burnham, and R. Maurer. 2000. Characterization of two novel regulatory genes affecting Salmonella invasion gene expression. Mol. Microbiol. 35:635-646. [DOI] [PubMed] [Google Scholar]
- 4.Andersson, R. A., A. R. B. Eriksson, R. Heikinheimo, A. Mae, M. Pirhonen, V. Koiv, H. Hyytiainen, A. Tuikkala, and E. T. Palva. 2000. Quorum sensing in the plant pathogen Erwinia carotovora subsp. carotovora: the role of expR (Ecc). Mol. Plant-Microbe Interact. 13:384-393. [DOI] [PubMed] [Google Scholar]
- 5.Ang, S., Y. T. Horng, J. C. Shu, P. C. Soo, J. H. Liu, W. C. Yi, H. C. Lai, K. T. Luh, S. W. Ho, and S. Swift. 2001. The role of RsmA in the regulation of swarming motility in Serratia marcescens. J. Biomed. Sci. 8:160-169. [DOI] [PubMed] [Google Scholar]
- 6.Blumer, C., and D. Haas. 2000. Multicopy suppression of a gacA mutation by the infC operon in Pseudomonas fluorescens CHA0: competition with the global translational regulator RsmA. FEMS Microbiol. Lett. 187:53-58. [DOI] [PubMed] [Google Scholar]
- 7.Blumer, C., S. Heeb, G. Pessi, and D. Haas. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. USA 96:14073-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee, A., Y. Cui, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl. Environ. Microbiol. 61:1959-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatterjee, A. K., C. K. Dumenyo, Y. Liu, and A. Chatterjee. 2000. Erwinia: genetics of pathogenicity factors, p. 236-260. In J. Lederberg (ed.), Encyclopedia of microbiology, 2nd ed., vol. 2. Academic Press, New York, N.Y.
- 10.Cui, Y., A. Chatterjee, and A. K. Chatterjee. 2001. Effect of the two-component system comprising GacA and GacS of Erwinia carotovora subsp. carotovora on the production of global regulatory rsmB RNA, extracellular enzymes, and HarpinEcc. Mol. Plant-Microbe Interact. 14:516-526. [DOI] [PubMed] [Google Scholar]
- 11.Cui, Y., A. Chatterjee, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J. Bacteriol. 177:5108-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui, Y., L. Madi, A. Mukherjee, C. K. Dumenyo, and A. K. Chatterjee. 1996. The RsmA− mutants of Erwinia carotovora subsp. carotovora strain Ecc71 overexpress hrpNEcc and elicit a hypersensitive reaction-like response in tobacco leaves. Mol. Plant-Microbe Interact. 9:565-573. [DOI] [PubMed] [Google Scholar]
- 13.Cui, Y., A. Mukherjee, C. K. Dumenyo, Y. Liu, and A. K. Chatterjee. 1999. rsmC of the soft-rotting bacterium Erwinia carotovora subsp. carotovora negatively controls extracellular enzyme and HarpinEcc production and virulence by modulating the levels of regulatory RNA (rsmB) and RNA binding protein (RsmA). J. Bacteriol. 181:6042-6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fettes, P. S., V. Forsbach-Birk, D. Lynch, and R. Marre. 2001. Overexpresssion of a Legionella pneumophila homologue of the E-coli regulator csrA affects cell size, flagellation, and pigmentation. Int. J. Med. Microbiol. 291:353-360. [DOI] [PubMed] [Google Scholar]
- 15.Fuqua, C., and E. P. Greenberg. 1998. Self perception in bacteria-quorum sensing with acylated homoserine lactones. Curr. Opin. Microbiol. 1:183-189. [DOI] [PubMed] [Google Scholar]
- 16.Gudapaty, S., K. Suzuki, X. Wang, P. Babitzke, and T. Romeo. 2001. Regulatory interactions of Csr components: the RNA binding protein CsrA activates csrB transcription in Escherchia coli. J. Bacteriol. 183:6017-6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, S. J., Y. L. Shih, S. D. Bentley, and G. P. C. Salmond. 1998. The hexA gene of Erwinia carotovora encodes a LysR homologue and regulates motility and the expression of multiple virulence determinants. Mol. Microbiol. 28:705-717. [DOI] [PubMed] [Google Scholar]
- 18.Heeb, S., C. Blumer, and D. Haas. 2002. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 184:1046-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heeb, S., and D. Haas. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other Gram-negative bacteria. Mol. Plant-Microbe Interact. 14:1351-1363. [DOI] [PubMed] [Google Scholar]
- 20.Hyytiainen, H., M. Montesano, and E. T. Palva. 2001. Global regulators ExpA (GacA) and KdgR modulate extracellular enzyme gene expression through the RsmA-rsmB system in Erwinia carotovora subsp. carotovora. Mol. Plant-Microbe Interact. 14:931-938. [DOI] [PubMed] [Google Scholar]
- 21.Jackson, D. W., K. Suzuki, L. Oakford, J. W. Simecka, M. E. Hart, and T. Romeo. 2002. Biofilm formation and dispersal under the influence of the global regulator CsrA of Escherichia coli. J. Bacteriol. 184:290-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, S., B. Yu, N. J. Bainton, M. Birdsall, B. W. Bycroft, S. R. Chhabra, A. J. R. Cox, P. Golby, P. J. Reeves, S. Stephens, M. K. Winson, G. P. C. Salmond, G. S. A. B. Stewart, and P. Williams. 1993. The lux autoinducer regulates the production of exoenzyme virulence determinants in Erwinia carotovora and Pseudomonas aeruginosa. EMBO J. 12:2477-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner, D., W. Paul, and M. J. Merrick. 1988. Construction of multicopy expression vectors for regulated overproduction of proteins in Klebsiella pneumoniae and other enteric bacteria. J. Gen. Microbiol. 134:1779-1784. [DOI] [PubMed] [Google Scholar]
- 25.Koiv, V., and A. Mae. 2001. Quorum sensing controls the synthesis of virulence factors by modulating rsmA gene expression in Erwinia carotovora subsp. carotovora. Mol. Genet. Genomics 265:287-292. [DOI] [PubMed] [Google Scholar]
- 26.Lerner, C. G., and M. Inouye. 1990. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 18:4631.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, M. Y., G. Gui, B. Wei, J. F. Preston III, L. Oakford, Û. Yûksel, D. P. Giedroc, and T. Romeo. 1997. The RNA molecule csrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 272:17502-17510. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Y., A. Chatterjee, and A. K. Chatterjee. 1994. Nucleotide sequence and expression of a novel pectate lyase gene (pel-3) and a closely linked endopolygalacturonase gene (peh-1) of Erwinia carotovora subsp. carotovora 71. Appl. Environ. Microbiol. 60:2545-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Y., Y. Cui, A. Mukherjee, and A. K. Chatterjee. 1998. Characterization of a novel RNA regulator of Erwinia carotovora ssp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol. Microbiol. 29:219-234. [DOI] [PubMed] [Google Scholar]
- 30.Liu, Y., G.-Q. Jiang, Y. Cui, A. Mukherjee, W.-L. Ma, and A. K. Chatterjee. 1999. kdgREcc negatively regulates genes for pectinases, cellulase, protease, harpinEcc, and a global RNA regulator in Erwinia carotovora subsp. carotovora. J. Bacteriol. 181:2411-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, W.-L., Y. Cui, Y. Liu, C. K. Dumenyo, A. Mukherjee, and A. K. Chatterjee. 2001. Molecular characterization of global regulatory RNA species that control pathogenicity factors in Erwinia amylovora and Erwinia herbicola pv. gypsophilae. J. Bacteriol. 183:1870-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michael, B., J. N. Smith, S. Swift, F. Heffron, and B. M. M. Ahmer. 2001. SdiA of Salmonella enterica is a LuxR homolog that detects mixed microbial communities. J. Bacteriol. 183:5733-5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.Mukherjee, A., Y. Cui, Y. Liu, and A. K. Chatterjee. 1997. Molecular characterization and expression of the Erwinia carotovora hrpNEcc gene, which encodes an elicitor of the hypersensitive reaction. Mol. Plant-Microbe Interact. 10:462-471. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee, A., Y. Cui, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1996. Global regulation in Erwinia species by Erwinia carotovora rsmA, a homologue of Escherchia coli csrA: repression of secondary metabolites, pathogenicity and hypersensitive reaction. Microbiology 142:427-434. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee, A., Y. Cui, W.-L. Ma, Y. Liu, and A. K. Chatterjee. 2000. hexA of Erwinia carotovora ssp. carotovora strain Ecc71 negatively regulates production of RpoS and rsmB RNA, a global regulator of extracellular proteins, plant virulence and the quorum sensing signal, N-(3-oxohexanoyl)-L-homoserine lactone. Env. Microbiol. 2:203-215. [DOI] [PubMed] [Google Scholar]
- 37.Murata, H., J. L. McEvoy, A. Chatterjee, A. Collmer, and A. K. Chatterjee. 1991. Molecular cloning of an aepA gene that activates production of extracellular pectolytic, cellulolytic, and proteolytic enzymes in Erwinia carotovora subsp. carotovora. Mol. Plant-Microbe Interact. 4:239-246. [Google Scholar]
- 38.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. G. Holden, M. Camara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirhonen, M., D. Flego, R. Heikinheimo, and E. T. Palva. 1993. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 12:2467-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romeo, T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321-1330. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 42.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 43.Wei, B., A. M. Brun-Zinkernagel, J. W. Simecka, B. M., Pruss, P. Babitzke, and T. Romeo. 2001. Positive regulation of motility and flhDC expression by the RNA-binding protein CsrA of Escherichia coli. Mol. Microbiol. 40:245-256. [DOI] [PubMed] [Google Scholar]
- 44.Zink, R. T., R. J. Kemble, and A. K. Chatterjee. 1984. Transposon Tn5 mutagenesis in Erwinia carotovora subsp. carotovora and Erwinia carotovora subsp. atroseptica. J. Bacteriol. 157:809-814. [DOI] [PMC free article] [PubMed] [Google Scholar]