Abstract
Objective To determine the impact of a community based Helicobacter pylori screening and eradication programme on the incidence of dyspepsia, resource use, and quality of life, including a cost consequences analysis.
Design H pylori screening programme followed by randomised placebo controlled trial of eradication.
Setting Seven general practices in southwest England.
Participants 10 537 unselected people aged 20-59 years were screened for H pylori infection (13C urea breath test); 1558 of the 1636 participants who tested positive were randomised to H pylori eradication treatment or placebo, and 1539 (99%) were followed up for two years.
Intervention Ranitidine bismuth citrate 400 mg and clarithromycin 500 mg twice daily for two weeks or placebo.
Main outcome measures Primary care consultation rates for dyspepsia (defined as epigastric pain) two years after randomisation, with secondary outcomes of dyspepsia symptoms, resource use, NHS costs, and quality of life.
Results In the eradication group, 35% fewer participants consulted for dyspepsia over two years compared with the placebo group (55/787 v 78/771; odds ratio 0.65, 95% confidence interval 0.46 to 0.94; P = 0.021; number needed to treat 30) and 29% fewer participants had regular symptoms (odds ratio 0.71, 0.56 to 0.90; P = 0.05). NHS costs were £84.70 (£74.90 to £93.91) greater per participant in the eradication group over two years, of which £83.40 ($146; €121) was the cost of eradication treatment. No difference in quality of life existed between the two groups.
Conclusions Community screening and eradication of H pylori is feasible in the general population and led to significant reductions in the number of people who consulted for dyspepsia and had symptoms two years after treatment. These benefits have to be balanced against the costs of eradication treatment, so a targeted eradication strategy in dyspeptic patients may be preferable.
Introduction
Dyspepsia is a common recurrent condition that causes “pain or discomfort centered on the upper abdomen”1 and affects up to 40% of the UK population.2 Dyspepsia accounts for about 4% of all consultations in primary care,3 with annual costs to the NHS of around £1.1 billion ($1.9bn; €1.6bn).4 Most dyspeptic patients are managed in primary care (uninvestigated dyspepsia); a minority of patients, particularly those with “alarm” symptoms such as dysphagia or weight loss, are referred for an endoscopy. Common causes of dyspepsia diagnosed at endoscopy are peptic ulcer disease (mostly due to Helicobacter pylori infection), gastro-oesophageal reflux disease, and “functional” dyspepsia (that is, no identifiable underlying cause).1
Two previous trials of community screening and H pylori eradication in Leeds and in Odense, Denmark, showed modest reductions in self reported dyspepsia symptoms.5,6 However, the Danish study included many uninfected people, thus diluting any effects of the eradication treatment, and the Leeds study included only people aged 40-49 years and eradication therapy failed in a quarter of those assigned to active treatment. A Cochrane review of management of dyspepsia in primary care concluded that H pylori eradication may benefit some dyspeptic patients,7 and the CADET-Hp trial of “test and treat” in uninvestigated dyspeptic patients in Canada showed clinical and economic benefits.8 Two meta-analyses have, however, differed as to whether endoscopically diagnosed functional dyspepsia improves after H pylori eradication,9,10 in part as a result of different studies being included in the reviews.11
The Bristol helicobacter project was established as a large community based randomised controlled trial to assess the impact of H pylori eradication on the outcomes of dyspepsia, quality of life, health resource use, and NHS costs over two years of follow-up among patients detected through screening. The study randomised H pylori positive patients, used a well tolerated and highly effective H pylori eradication regimen, and assessed consultation rates for dyspepsia in primary care. An economic evaluation was done alongside the randomised trial.
Methods
Protocol
The Bristol helicobacter project was a community based screening study followed by a randomised controlled trial of H pylori eradication. The study design and methods have been published elsewhere.12,13 Briefly, unselected patients aged 20-59 years and registered at seven general practices in southwest England were invited by letter to attend for screening. Research nurses used the 13C urea breath test to screen attenders for H pylori infection, and infected participants were randomised to H pylori eradication treatment or placebo.12 Participants gave written informed consent.
Assignment and masking
H pylori infected participants were randomised to receive either ranitidine bismuth citrate (400 mg) and clarithromycin (500 mg) twice daily for 14 days or matching placebo. Staff independent of the study prepared the randomisation sequence with a block size of 10. Randomisation was stratified by sex and age into four 10 year age bands (20-9 to 50-9). Pharmacists who had no contact with participants prepared drug packs, and research nurses who were blind to the treatment allocation dispensed them. Eradication was assessed by a 13C urea breath test six months later, and the results were withheld from participants and the staff conducting follow-up. At two years participants reported what sort of tablets they thought they had received or that they were unsure. General practitioners were requested not to prescribe H pylori eradication treatment during follow-up, except in patients with endoscopically confirmed peptic ulcer disease.
Outcomes and follow-up
The primary outcome was the consultation rate for dyspepsia (epigastric pain) in primary care over two years. Trained research nurses blinded to the treatment allocation examined the primary care records two years after randomisation. They recorded consultations related to dyspepsia (epigastric pain) or to heartburn, reflux, or dysmotility-type symptoms, as well as prescribed dyspepsia treatments and referrals to secondary care for dyspepsia. A gastroenterologist (RFH) who was blind to the treatment allocation verified a sample of the reviews carried out by the nurses against primary care records for accuracy and completeness.
Secondary outcomes were the frequency and type of symptoms, impact on quality of life, resource use, and costs to the NHS two years after randomisation. We measured frequency of symptoms within a three month period with five point Likert-type scales in participant completed questionnaires.14,15 Participants rated the frequency of dyspepsia (epigastric pain) symptoms, as well as heartburn and reflux, belching (wind), nausea, and bloating, from 1 (none) to 5 (daily). We defined regular or frequent symptoms as occurring on two or more occasions over each of the previous three months (as defined by the developers of the questionnaire).14 We used the SF-36 questionnaire to assess generic health status (quality of life)—0 indicated poor health and 100 indicated good health.16
We did the economic evaluation from the NHS viewpoint. The most appropriate form of economic evaluation was a cost consequences analysis to ensure the inclusion of multiple important secondary outcomes alongside the cost data, rather than a cost effectiveness analysis.17 Resource use for each participant for the two years after randomisation came from the note reviews. We analysed dyspepsia (epigastric pain) related general practitioner consultations, secondary care referrals, and drugs from the British National Formulary gastrointestinal systems (section 1.1-1.3 anti-secretory medication).18 We applied unit costs at 2002 prices (including value added tax) from UK sources and discounted costs in the second year at the UK Treasury discount rate of 3.5%.
Sample size calculations and statistical analyses
Before the study started, we predicted H pylori prevalence to be 15%. We assumed conservatively that the successful eradication of H pylori would reduce dyspepsia only in those participants with undiagnosed peptic ulcer disease. Survey results from southern England and elsewhere suggested that in a six month period 8.6% of 20-59 year olds would consult their general practitioner because of dyspepsia, that 20-25% of these patients would have peptic ulcers, and that at least 80% of peptic ulcers would occur in those with H pylori infection.2,19 A sample size of 1550 participants could detect a reduction in the consultation rate from 8.5% to 4.25% in the eradication group, with 90% power at a two sided significance level of 5%.12
We used Stata version 7 to do intention to treat analyses of all randomised participants with note review for the primary analysis and all participants with completed follow-up questionnaires for the self reported secondary outcomes. We used logistic regression with adjustment for the stratification variables for all the parametric analyses; results are presented as odds ratios with 95% confidence intervals. We used Mann-Whitney U tests to analyse quality of life data, because of the non-Gaussian distribution.
The economic evaluation included all participants with complete resource use information. We used t tests to assess cost differences. We used multiple ordinary least squares regression to adjust for the stratification variables. We also estimated bias corrected confidence intervals by bootstrap methods to investigate the underlying data distribution (the confidence intervals were almost identical by the two methods).20
Results
Study population flow, H pylori prevalence, and eradication
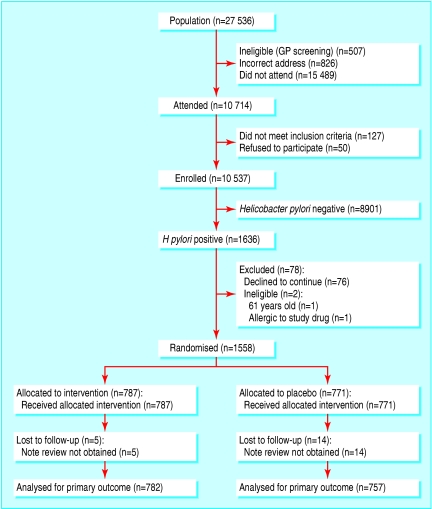
The figure shows the flow of the 27 536 study population. Of the 26 203 people who received an invitation letter, 10 714 (41%) attended (a further 507 were deemed ineligible by their general practitioner, and the letter was returned undelivered for 826). In all, 10 537 participants entered the study (127 people were ineligible, and 50 declined to participate); 1636 (15.5%) participants were infected with H pylori, and 1558 (95%) of these agreed to be randomised. Eradication of H pylori was 91%. Proton pump inhibitors (which can cause false negative urea breath test results) were being taken by only 3.4% of eradication group participants at the second breath test, so any potential impact on the eradication rate was small. Table 1 shows the baseline characteristics of randomised participants. Recruitment took place between 1996 and 1999 and follow-up between 1998 and 2001. We saw two serious unexpected adverse events in the intervention group—one participant had rectal bleeding, and a second had severe vomiting. Two hundred and sixty six participants in the eradication group guessed that they had received active treatment at two years compared with 241 expected by chance (χ2 = 1.90, P = 0.1683). Follow-up was 99% (1539) for the primary outcome and 92% (1438) for the secondary outcomes, based on questionnaire data.
Figure 1.
Trial profile
Table 1.
Characteristics of randomised participants. Values are numbers (percentages) unless stated otherwise
| Characteristic | Eradication group | Placebo group |
|---|---|---|
| Sex | ||
| (n=787) | (n=771) | |
| Men | 385 (49.0) | 378 (49.0) |
| Women | 402 (51.1) | 393 (51.0) |
| Mean (SD) age (years) | 48.4 (8.0) | 48.6 (7.9) |
| Socioeconomic group* | ||
| (n=775) | (n=760) | |
| I and II | 233 (30.0) | 233 (30.7) |
| III | 387 (50.0) | 363 (47.8) |
| IV and V | 155 (20.0) | 164 (21.5) |
| Ethnic origin | ||
| (n=781) | (n=764) | |
| White | 758 (97.1) | 748 (97.9) |
| Non-white† | 23 (2.9) | 16 (2.1) |
| Smoking status | ||
| (n=767) | (n=764) | |
| Never smoked | 405 (52.8) | 389 (50.9) |
| Past smoker | 179 (23.3) | 190 (24.9) |
| Current smoker | 183 (23.9) | 185 (24.2) |
| Alcohol consumption | ||
| (n=743) | (n=722) | |
| None | 145 (19.5) | 123 (17.0) |
| Low alcohol intake | 541 (72.8) | 534 (74.0) |
| High alcohol intake‡ | 57 (7.7) | 65 (9.0) |
| NSAID usage | ||
| (n=729) | (n=723) | |
| None | 558 (76.5) | 526 (72.7) |
| Less than daily | 132 (18.1) | 154 (21.3) |
| Daily | 39 (6.0) | 43 (6.0) |
| (n=787) | (n=771) | |
| Dyspepsia symptoms§ | 185 (24.3) | 194 (26.6) |
NSAID=non-steroidal anti-inflammatory drug.
I and II=professional; III=skilled occupation; IV and V=partly skilled or unskilled occupations.
Non-white participants grouped owing to low numbers enrolled.
Greater than 20 units/week for men and 13 units/week for women.
Epigastric pain symptoms on two or more occasions in each of previous three months.
Health resource use and costs of dyspepsia
The number of people consulting for dyspepsia (epigastric pain) in primary care was reduced by 35% (55 v 78) over two years in the eradication group compared with the placebo group (odds ratio 0.65, 95% confidence interval 0.46 to 0.94; P = 0.021). Thirty people with H pylori would have to be treated to prevent one person consulting their doctor for dyspepsia. We found no difference in the numbers consulting for dyspepsia by sex (odds ratio for men 0.69, 0.39 to 1.19; for women 0.63, 0.63 to 1.01; interaction test χ2 = 0.07, P = 0.79).
The total number of general practice consultations for dyspepsia over two years was also reduced in the eradication group, but all other resource use was very similar (table 2). No significant differences existed between the two groups in the costs of general practice consultations, prescription drugs, or secondary care procedures, including endoscopies for dyspepsia (table 3). However, the cost of the H pylori eradication treatment (£83.40 ($146; €121)) resulted in significantly greater NHS costs per participant in the eradication group (difference = £84.70, 95% confidence interval £74.90 to £93.91).
Table 2.
Health resource use for dyspepsia over two years and unit costs
|
Resource use related to dyspepsia
|
Resource use events; mean (SD) per participant*
|
Unit cost or range (£) and source
|
|
|---|---|---|---|
| Eradication group (n=775) | Placebo group (n=750) | ||
| General practitioner consultations | 88; 0.11 (0.50) | 120; 0.16 (0.52) | 20† |
| General practitioner home visits | 10; 0.001 (0.04) | 10; 0.001 (0.04) | 61† |
| Drug prescriptions | 139; 0.18 (0.65) | 127; 0.17 (0.56) | Variable‡ |
| Endoscopies | 25; 0.03 (0.20) | 19; 0.03 (0.16) | 65§ |
| Secondary care procedures¶ | 21; 0.03 (0.20) | 25; 0.03 (0.20) | 65-226§ |
Table 3.
Health service costs for dyspepsia over two years
|
Costs related to dyspepsia
|
Health service cost (£); mean (SD) per participant*
|
Difference†(95% CI) in mean adjusted costs (£)
|
|
|---|---|---|---|
| Eradication group (n=775) | Placebo group (n=750) | ||
| General practitioner consultations | 1 785; 2.30 (10.06) | 2 417; 3.22 (10.47) | −0.93 (−1.87 to 0.18) |
| Drug prescriptions | 14 363; 18.53 (82.63) | 12 362; 16.48 (97.74) | 2.16 (−7.08 to 10.52) |
| Helicobacter pylori eradication drugs | 64 636; 83.40 (0) | 0 | 83.40 (83.40 to 83.40) |
| Endoscopies | 1 596; 2.06 (12.62) | 1 204; 1.61 (9.97) | 0.47 (−0.71 to 1.53) |
| Secondary care procedures‡ | 1 878; 2.42 (18.73) | 2 119; 2.83 (16.95) | −0.40 (−2.11 to 1.43) |
| Total healthcare costs§ | 84 259; 108.72 (95.14) | 18 101; 24.13 (101.39) | 84.70 (74.90 to 93.91) |
Rounded to two decimal places.
Eradication group minus placebo group, adjusted for the stratification variables.
Non-endoscopic secondary care procedures such as imaging combined, as few occurred and no inpatient stays occurred.
Totals not exact due to rounding up by £0.01.
Cost consequences: dyspepsia symptoms and quality of life
Regular symptoms of dyspepsia (epigastric pain) were reported by 29% fewer participants two years after H pylori eradication treatment than after placebo (odds ratio 0.71, 0.56 to 0.90). In a subgroup analysis, we found no difference in the effect of H pylori eradication on dyspepsia symptoms by sex (odds ratio for men 0.70, 0.49 to 0.98; for women 0.72, 0.52 to 1.01; interaction test χ2 = 0.01, P = 0.90). No differences existed between the two groups in any of the quality of life dimensions at two years (table 4). All participants reported good health “most of the time” except for the vitality dimension, which was equivalent to reduced vitality “a good bit of the time.”
Table 4.
Health related quality of life at two years. Values are mean (SD) scores unless stated otherwise
| Dimension | Eradication group (n=787) | Placebo group (n=771) | P value* |
|---|---|---|---|
| Physical functioning | 87 (19.8) | 87 (19.5) | 0.64 |
| Social functioning | 86 (18.4) | 85 (19.0) | 0.37 |
| Role limitation—physical | 85 (31.0) | 83 (32.5) | 0.20 |
| Role limitation—emotional | 86 (29.1) | 86 (30.8) | 0.95 |
| Pain | 76 (23.8) | 75 (24.7) | 0.34 |
| Mental health | 75 (16.7) | 74 (17.7) | 0.53 |
| Vitality | 67 (18.8) | 67 (20.4) | 0.72 |
| General health perception | 71 (20.6) | 71 (20.5) | 0.64 |
Eradication group minus placebo group.
Discussion
Study validity and generalisability
The number of people who consulted for dyspepsia (epigastric pain) in primary care and had symptoms was reduced by about 30% two years after H pylori eradication. Large numbers of participants across a wide age range were recruited, with few exclusions, thus increasing the study's generalisability. The high rates of H pylori eradication and follow-up, with blinded assessment of the primary outcome, enhanced the internal validity of the study, and the breath test (the non-invasive “gold standard” detection method) minimised misclassification biases compared with serology (as initially used in the Danish screening study)6 and probably facilitated recruitment, as no blood test was necessary. The clinically important primary outcome of dyspepsia consultations in primary care contrasts with the use of self reported dyspepsia symptoms as the primary outcome in the two other community based H pylori studies.5,6
Forty one per cent of the target population participated in this study, which was more than in the other UK based H pylori eradication study (25%) but less than in the Danish study (63%).5,6 Data protection regulations (UK Data Protection Act 1998) prevented access to the primary care notes of non-responders to ascertain if the population recruited was representative of the dyspepsia burden in primary care, but both previous trials found lower healthcare resource use for dyspepsia in non-respondents.5,6 Previously reported analyses from this study showed that non-responders were more likely to be male, younger, and from the lower socioeconomic groups, findings consistent with other population based studies.12 Few non-white patients participated in our trial, so the results of the screening component may not be generalisable to more ethnically diverse populations. H pylori prevalence at 15% was comparable to the Danish study (17.5%) but was lower than in the Leeds study (28%), and prevalence is decreasing to less than 15% in the Western world.1 We chose an efficacious (but relatively expensive) H pylori eradication regimen to achieve a high eradication rate, achieving similar results to another community based trial.21 This regimen does not affect the study's generalisability, however, as the objective was to examine the impact of H pylori eradication on dyspepsia.
Comparison with other studies
Similar reductions in dyspepsia symptoms were observed in all three population based H pylori eradication trials.5,6 Results after one year showed a 4% absolute reduction in dyspepsia symptoms in the intervention arm of the Danish study, which included participants uninfected with H pylori and infected participants who had received eradication treatment. However, participants could have ascribed changes in dyspepsia symptoms to their knowledge of H pylori eradication, as the Danish study was not a placebo controlled trial. The Leeds study reported an overall reduction in dyspepsia symptoms of 5% in people aged 40-49 years, with a 74% H pylori eradication rate and 76% follow-up at two years. The lack of response to treatment in women observed in the Leeds study was not replicated in either this or the Danish study.5,6 Heartburn or acid reflux symptoms were similarly prevalent (28%) to dyspepsia symptoms (25%) in our study, but they were unaltered after H pylori eradication in our study and the Leeds study, whereas the Danish study observed a small reduction after eradication.5,6,13
Economic analyses
Reductions in the numbers of people consulting for dyspepsia were reported in all three H pylori eradication population based trials. None, however, observed significant reductions in endoscopies or prescribed dyspepsia drugs after eradication, and no change in dyspepsia prescriptions was observed after H pylori eradication treatment in a general practice record review of 470 000 people in Denmark.22
At £83.40 per participant, H pylori eradication treatment dominated costs in the economic evaluation using trial data; however, current treatment (Heliclear) costs £37.65, so halving the costs of screening and eradication to the NHS.18 The Leeds study reported non-significantly lower NHS costs (£11.42) over two years in the eradication group, although perhaps the H pylori eradication treatment costs were not included, which would have reversed the difference between the two groups.23 Leeds data were incorporated into a Markov model that explored the impact of H pylori screening and eradication on future peptic ulcer disease and gastric cancer in 45 year olds, with effectiveness measured in life years saved. The model indicated that H pylori screening and treatment would cost less than a non-intervention strategy and save more lives, provided that savings of at least £2.16 a year in men were made after eradication. Our study does not support that conclusion because we found no cost savings after eradication. In contrast, a “test and treat” strategy in uninvestigated dyspeptic patients in Canada showed a cost effectiveness ratio of $C387 (≈£188) in favour of H pylori eradication, showing that a targeted approach was a more cost effective policy.8,24
What is already known on this topic
Dyspepsia is common and is usually managed in primary care
Helicobacter pylori infection is a major cause of peptic ulcer disease, but its role in dyspepsia is less certain
Dyspepsia symptoms were reduced after H pylori eradication in people aged 40-49 years in a community based trial
What this study adds
In the general practice population, the number of people who consulted for dyspepsia (epigastric pain) and who had symptoms decreased by 30% after H pylori screening and eradication
H pylori eradication in patients with dyspepsia offers long term relief from symptoms but with increased cost due to the eradication treatment
A recent systematic review of the effects of H pylori eradication on quality of life in patients with functional dyspepsia noted that most evidence was drawn from secondary care patients.25 No major changes in quality of life were found after H pylori eradication in any of the three eradication trials based in the community.5,6 The scores for the individual dimensions of the quality of life obtained in our study were comparable to normative UK data.16
Policy implications and conclusions
The reduction in the numbers of people consulting for dyspepsia and reporting symptoms—alongside the potential future prevention of peptic ulcers and gastric cancer26—has to be balanced against the lack of benefit to people with heartburn or reflux symptoms after eradication previously reported by this study13 and the NHS costs. Concerns also exist about the widespread use of antibiotics in healthy people as part of the H pylori eradication regimen.27 A targeted H pylori test and treat strategy focusing on uninvestigated dyspeptic patients was highly effective,8 and our study potentially supports that approach, as many participants gained long term relief from dyspepsia symptoms after eradication. Nevertheless, both test and treat and population screening strategies are likely to be more cost effective in areas of higher H pylori prevalence, as fewer people have to be tested for one to gain benefit from treatment.
We thank all participants; the general practice staff; the nursing team of Lynne Bradshaw, Julie Watson, Tina Critchley, Jo Lee, Carol Everson-Coombe, Penny Nettlefield, and Joanne Smith; Judy Millward, Helen Davies, Amy Hawkins, and Sarah Pike for secretarial support; Erwin Brown, Phil Hedges, and Nick Pope of the microbiology department and Pete Spurr, Martin Bullock, and Fiona Greenwood of the pharmacy department, Frenchay Hospital for help with the breath tests and the study drugs; and Chris Metcalfe of the department of social medicine for statistical advice.
Contributors: JAL ran the project from day to day and did most of the analyses for the paper with advice from ME. LJM, IMH, JLD, and RFH initiated, planned, and obtained funding for the project. PN helped to set up the project. SN did the economic analysis and was responsible for this aspect of the paper. JAL wrote the initial draft of the paper, and all authors contributed to the final version. JAL is the guarantor.
Funding: This study was jointly funded by the South and West NHS Research and Development Directorate and GlaxoSmithKline. The department of social medicine of the University of Bristol is the lead centre of the Medical Research Council Health Services Research Collaboration. The randomisation sequence was generated by one of the funders (GlaxoSmithKline), but the sponsors had no further role in the study design, data collection, analyses, or writing up of reports and publications.
Competing interests: JAL and RFH were funded by GlaxoSmithKline to attend the AGA meeting in 2000.
Ethical approval: The local research ethics committee approved the study.
References
- 1.Talley NJ. Dyspepsia: how to manage and how to treat? Aliment Pharmacol Ther 2005;16: 95-104. [DOI] [PubMed] [Google Scholar]
- 2.Jones R, Lydeard S. Prevalence of symptoms of dyspepsia in the community. BMJ 1989;298: 30-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosengren H, Polson RJ. The role of screening for Helicobacter pylori in patients with duodenal ulceration in primary health care. Br J Gen Pract 1996;46: 177-9. [PMC free article] [PubMed] [Google Scholar]
- 4.Asante MA, Lord J, Mendall M, Northfield T. Endoscopy for Helicobacter pylori sero-negative young dyspeptic patients: an economic evaluation based on a randomised trial. Eur J Gastroenterol Hepatol 1999;11: 851-6. [DOI] [PubMed] [Google Scholar]
- 5.Moayyedi P, Feltbower R, Brown J, Mason S, Mason J, Nathan J, et al. Effect of population screening and treatment for Helicobacter pylori on dyspepsia and quality of life in the community: a randomised controlled trial. Lancet 2000;355: 1665-9. [DOI] [PubMed] [Google Scholar]
- 6.Wildner-Christensen M, Moller Hansen J, Schaffalitzky De Muckadell O. Rates of dyspepsia one year after Helicobacter pylori screening and eradication in a Danish population. Gastroenterology 2003;125: 372-9. [DOI] [PubMed] [Google Scholar]
- 7.Delaney BC, Moayyedi P, Forman D. Initial management strategies for dyspepsia. Cochrane Database Syst Rev 2003;(2): CD001961. [DOI] [PubMed]
- 8.Chiba N, Veldhuyzen van Zanten SJ, Sinclair P, Ferguson RA, Escobedo S. Treating Helicobacter pylori infection in primary care patients with uninvestigated dyspepsia: the Canadian adult dyspepsia empiric treatment—Helicobacter pylori positive (CADET-Hp) randomised controlled trial. BMJ 2002;324: 1012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moayyedi P, Soo S, Deeks JJ, Forman D, Mason J, Innes M, et al. Systematic review and economic evaluation of Helicobacter pylori eradication treatment for non-ulcer dyspepsia. BMJ 2000;321: 659-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laine L, Schoenfeld P, Fennerty MB. Therapy for Helicobacter pylori in patients with non-ulcer dyspepsia: a meta-analysis of randomized, controlled trials. Ann Intern Med 2001;134: 361-9. [DOI] [PubMed] [Google Scholar]
- 11.Moayyedi P, Deeks JJ, Talley NJ, Delaney BC, Forman D. An update of the Cochrane systematic review of Helicobacter pylori eradication therapy in nonulcer dyspepsia: resolving the discrepancy between the systematic reviews. Am J Gastroenterol 2003;98: 2621-6. [DOI] [PubMed] [Google Scholar]
- 12.Lane JA, Harvey RF, Murray LJ, Harvey IM, Donovan JL, Nair P, et al. A placebo-controlled randomised trial of eradication of Helicobacter pylori in the general population: study design and response rates in the Bristol helicobacter project. Control Clin Trials 2002;23: 321-32. [DOI] [PubMed] [Google Scholar]
- 13.Harvey RF, Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P. Randomised controlled trial of effects of Helicobacter pylori infection and its effects on heartburn and gastro-oesphageal reflux disease: Bristol helicobacter project. BMJ 2004;328: 1417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy T, Jones R. Development of a postal health status questionnaire to identify people with dyspepsia in the general population. Scand J Prim Health Care 1995;13: 243-9. [DOI] [PubMed] [Google Scholar]
- 15.Hobbs FD, Delaney BC, Rowsby M, Kenkre JE. Effect of Helicobacter pylori eradication therapy on dyspeptic symptoms in primary care. Fam Pract 1996;13: 225-8. [DOI] [PubMed] [Google Scholar]
- 16.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care [see comments]. BMJ 1992;305: 160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coast J. Is economic evaluation in touch with society's health values? BMJ 2004;329: 1233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.British Medical Association, Royal Pharmaceutical Society of Great Britain. British National Formulary. London: BMA, RPS, 2004. (No 48.)
- 19.Heatley RV, Rathbone BJ. Dyspepsia: a dilemma for doctors? Lancet 1987;2: 779-82. [DOI] [PubMed] [Google Scholar]
- 20.Thompson S, Barber J. How should cost data in pragmatic randomised trials be analysed? BMJ 2000;320: 1197-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone MA, Patel H, Panja KK, Barnett DB, Mayberry JF. Results of Helicobacter pylori screening and eradication in a multi-ethnic community in central England. Eur J Gastroenterol Hepatol 1998;10: 957-62. [DOI] [PubMed] [Google Scholar]
- 22.Lassen A, Hallas J, Schaffalitzky De Muckadell OB. Eradication of Helicobacter pylori and use of anti-secretory drugs: population based cohort study. BMJ 2005;327: 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mason J, Axon AT, Forman D, Duffet S, Drummond M, Crocombe W, et al. The cost-effectiveness of population Helicobacter pylori screening and treatment: a Markov model using economic data from a randomized controlled trial. Aliment Pharmacol Ther 2002;16: 559-68. [DOI] [PubMed] [Google Scholar]
- 24.Chiba N, Veldhuyzen van Zanten SJ, Escobedo S, Grace E, Lee J, Sinclair P, et al. Economic evaluation of Helicobacter pylori eradication in the CADET-HP randomised controlled trial of H. pylori positive primary care patients with uninvestigated dyspepsia. Aliment Pharmacol Ther 2005;19: 349-58. [DOI] [PubMed] [Google Scholar]
- 25.El-Serag HB, Talley NJ. Systematic review: health-related quality of life in functional dyspepsia. Aliment Pharmacol Ther 2003;18: 387-93. [DOI] [PubMed] [Google Scholar]
- 26.Forman D, Goodman KJ. The epidemiology of stomach cancer: correlating the past with the present. BMJ 2000;320: 1682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tytgat GN. Helicobacter pylori: where are we and where are we going? Aliment Pharmacol Ther 2000;14(S3): 55-8. [DOI] [PubMed] [Google Scholar]
- 28.Netten A, Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit, 2002.