Abstract
The UhpABC regulatory system in enterobacteria controls the expression of the hexose phosphate transporter UhpT. Signaling is initiated through sensing of extracellular glucose 6-phosphate by membrane-bound UhpC, which in turn modulates the histidine-protein kinase UhpB. Together with the cytoplasmic response regulator UhpA, they constitute a typical two-component regulatory system based on His-to-Asp phosphoryl transfer. Activated (i.e., phosphorylated) UhpA binds to the promoter region of uhpT, resulting in initiation of transcription. We have investigated the contribution of transmembrane signaling (through UhpBC) and intracellular activation (through UhpA) to the overall Uhp response (UhpT expression) in vivo. UhpA activation could be made independent of transmembrane signaling when ΔuhpBC cells were grown on pyruvate. Inorganic phosphate interfered with glucose 6-phosphate-dependent, UhpBC-mediated, as well as pyruvate-mediated activation of UhpA. The relationship between the concentration of inducer (glucose 6-phosphate) and the Uhp induction rate was nonhyperbolic, indicating positive cooperativity. The degree of cooperativity was affected by the carbon or energy source available to the cells for growth. As pyruvate-mediated activation of UhpA in ΔuhpBC cells could result in considerably stronger UhpT expression than glucose 6-phosphate-dependent activation through UhpBC, the observed positive cooperativity for the overall pathway in wild-type cells may reflect the previously described cooperative binding of UhpA to the uhpT promoter (J. L. Dahl et al., J. Biol. Chem. 272:1910-1919, 1997).
Bacteria must be able to accurately respond to a large number of extracellular signals in their continuously changing surroundings. Therefore, they have evolved sophisticated sensory mechanisms coupled to intracellular signal transduction pathways. The majority of these prokaryotic intracellular signaling routes are based on the reversible phosphorylation of sensor kinases and response regulators in so-called two-component regulatory systems (23, 28, 30). According to this concept, sensory input affects autophosphorylation on a conserved histidine residue in the transmitter module of the kinase. The phosphoryl group is subsequently transferred to a conserved aspartate residue in the (amino-terminal) receiver domain of the cognate response regulator. This covalent modification elicits a molecular switch, and above a certain threshold amount of phosphorylated regulator, a specific response is turned on, which is in most cases gene transcription via (enhanced) affinity of the carboxyl-terminal output domain of the regulator for the target promoter sequence.
To provoke a cellular response, the environmental physical or chemical stimulus has to cross the membrane. Therefore, in most cases the first step in a signal transduction cascade is the activation of a membrane-bound protein. In order to be able to quantitatively study specific transmembrane (receptor) signaling leading to cellular activation, the first (ligand-induced) step and the subsequent response should be well defined and straightforward to quantify. However, despite the huge number of characterized signaling pathways, such examples are not abundant.
An exception is the Uhp regulatory system in Escherichia coli (for a review, see reference 13). This signaling pathway is triggered by external glucose 6-phosphate, which is recognized by the membrane-bound receptor protein UhpC. UhpC interacts with a second membrane-bound protein, UhpB, the kinase/phosphatase of the Uhp two-component system. Sensing of glucose 6-phosphate is supposed to cause a conformational change in the supposed UhpBC complex, leading to histidine autophosphorylation in the (cytoplasmic) transmitter domain of UhpB. Upon phosphoryl transfer, the response regulator UhpA (presumably phosphorylated on the conserved Asp-54) shows enhanced affinity for the uhpT promoter and, together with the catabolite gene activator protein, initiates uhpT transcription. Glucose 6-phosphate is the only ligand that can activate signaling through UhpABC, which results in UhpT expression and thus in the uptake of a broad range of phosphorylated sugars by this transport protein, in exchange for intracellular phosphate. This enables enterobacteria to use these compounds as a carbon and energy source.
In this study we used E. coli strains with a chromosomal uhpT-lacZ fusion. Such strains facilitated quantitative measurements of the output of signaling through the Uhp system (i.e., uhpT transcription rates measured through β-galactosidase expression), as a function of the added glucose 6-phosphate concentration [G6P], together with the effect of inorganic phosphate (Pi) and the carbon or energy source used for growth. We obtained stimulus-response relationships indicative of positive cooperativity in the Uhp signaling pathway. Furthermore, Uhp induction rates reached significantly higher levels in ΔuhpBC cells, via pyruvate-mediated activation of UhpA, than in wild-type cells (maximally) induced with glucose 6-phosphate.
It has been shown previously in vitro that binding of UhpA to the uhpT promoter exhibits positive cooperativity and that binding occurs with much higher affinity when UhpA is phosphorylated (5, 6, 22). Therefore, the overall positive cooperativity derived from our whole-cell assay may reflect the contribution at the level of transcription regulation affected by signaling through UhpBC and/or phosphoryl transfer from UhpB to UhpA and/or the energy status. We discuss our findings in light of the cooperative phenomena observed in chemotaxis regulation (29).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The E. coli strains (K-12 derivatives) used in this study were RK5115 [Φ(uhpT-lacZ) ΔuhpT], RK5163 [Φ(uhpT-lacZ)], and RK1307 [Δuhp(B60-C437) Φ(uhpT-lacZ[Km])], kindly provided by R. J. Kadner (27, 34). Cells were grown aerobically at 37°C in Luria-Bertani (LB) medium (20) or in MOPS (morpholinepropanesulfonic acid) minimal medium (21) containing either 30 mM succinate, 10 mM glucose, or 40 mM pyruvate as the carbon source, 14 mM NH4Cl as the nitrogen source, and (unless indicated otherwise) 2 mM K2HPO4 as the phosphate source and supplemented with thiamine (20 μg ml−1).
Uhp induction.
Overnight cultures (RK5115) in LB or MOPS minimal medium were diluted in the same fresh medium to an optical density at 600 nm (OD600) of ∼0.075 and grown until mid-exponential phase. Prior to inducing the Uhp system with glucose 6-phosphate (Boehringer Mannheim), the main culture was distributed into appropriate volumes in small culture flasks, which were incubated in a 37°C shaking water bath. At an OD600 of 0.8 to 0.9, glucose 6-phosphate from a 50-fold-concentrated stock solution was added. To study Uhp inhibition, potassium phosphate (pH 7.5; 25-fold concentrated) was added 12.5 min after addition of glucose 6-phosphate.
To measure Uhp induction in RK1307, cells were grown in MOPS minimal medium with 30 mM succinate and the indicated phosphate concentration. Overnight-grown cells were harvested by centrifugation (10 min at 3,000 × g) and resuspended to a final OD600 of 0.1 in MOPS minimal medium with 40 mM pyruvate and the same phosphate concentration. Culture aliquots were collected on ice at the indicated time points for determination of OD600 and for measurement of β-galactosidase activity. Samples were stored at −80°C.
Uhp reporter assay.
β-Galactosidase (uhpT-lacZ) activity was assayed as described before (20) with only minor modifications. Hydrolysis of o-nitrophenyl-β-d-galactopyranoside, the substrate for β-galactosidase, was assayed in cells permeabilized with sodium dodecyl sulfate-chloroform in Z buffer (20). Reactions were stopped by addition of 1 M Na2CO3. The resulting absorbance (O-nitrophenol formed) was measured spectrophotometrically (OD420).
Data analysis.
β-Galactosidase activities (Miller units [MU]), normalized for cell density (OD600), were calculated by using the equation (OD420 × 1,000)/(reaction time in minutes × culture volume in milliliters × OD600). The induction rate (VUhp, in MU per minute) per glucose 6-phosphate concentration was obtained by calculating the slope of the linear trendline through the MU-versus-time data series between 5 and ∼40 min after glucose 6-phosphate addition. Activity determinations were performed on culture samples obtained from at least two independent induction experiments. Where indicated, a statistical evaluation of three experiments (mean ± standard deviation [SD]) is shown. Plotted data (glucose 6-phosphate concentration versus VUhp) were fitted (using Origin 6.0, Microcal Software) to the Michaelis-Menten equation, VUhp = Vmax × [G6P]/(K + [G6P]) as well as to the Hill equation, VUhp = Vmax × ([G6P])n/[(K)n + ([G6P])n], where K is K0.5G6P and n is the Hill coefficient (nH). For calculations assuming competitive inhibition, the KG6P, Pi = KG6P × (1 + [Pi]/KiPi) relationship was used, derived from Michaelis-Menten (Lineweaver-Burk) kinetics, in which the Pi concentration is the inhibiting phosphate concentration and KiPi is the inhibition constant.
RESULTS
Energy- and carbon source-dependent Uhp induction.
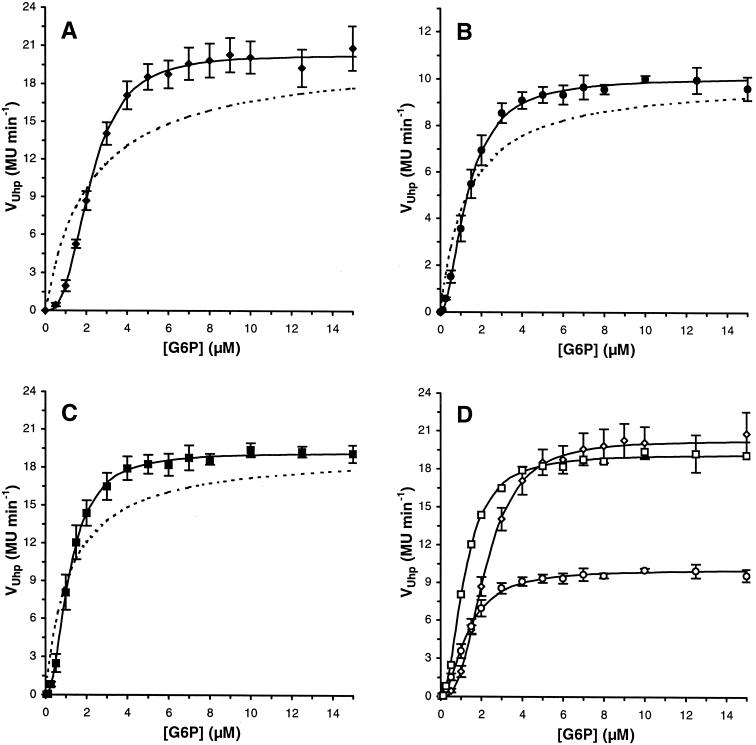
Addition of increasing amounts of glucose 6-phosphate to exponentially growing E. coli RK5115 (uhpT-lacZ ΔuhpT) cultures gave rise to a linear increase in β-galactosidase activity. As expected, it was observed that it took about 2 min after initiation of Uhp signaling before β-galactosidase activity could be detected (not shown). This reporter of UhpT expression reached higher levels in a strain in which functional UhpT was missing, due to the absence of concomitant uptake of inducer by the synthesized transporter, which occurs in RK5163 after 10 to 15 min (data not shown) (27). When the induction rate of uhpT-driven β-galactosidase expression (VUhp) was plotted as a function of the glucose 6-phosphate concentration, this resulted in the dose-response curves shown in Fig. 1A (succinate as the carbon source) and Fig. 1B (glucose as the carbon source), both in MOPS minimal medium, and in Fig. 1C in LB medium.
FIG. 1.
Uhp induction rates in E. coli RK5115. Rates (VUhp) per glucose 6-phosphate concentration were obtained by determining the slope of the linear trendline of the β-galactosidase activity-versus-time data between 5 and 30 min after glucose 6-phosphate addition. Error bars represent the SD of three independent experiments (mean ± SD). Uhp induction rates for E. coli RK5115 in MOPS minimal medium with 30 mM succinate (♦) (A), MOPS minimal medium with 10 mM glucose (•) (B), Luria-Bertani medium (▪) (C). The data in panels A to C are compiled in panel D (now with open symbols). Each correlation was fitted (solid lines) to the Hill equation, as described in Materials and Methods. The dotted curve in graphs A to C represents a Michaelis-Menten curve with the same K0.5G6P and Vmax as the Hill fit.
Half-maximal induction rates were reached at 1 to 2.5 μM glucose 6-phosphate (called K0.5G6P). For comparison, the three curves are depicted in one graph in Fig. 1D. In MOPS minimal medium, growth on succinate resulted in higher induction rates (Vmax = 20 MU min−1), albeit with a higher threshold sensitivity (i.e., lower initial rates; K0.5G6P = 2.2 μM), than obtained during growth on glucose (K0.5G6P = 1.3 μM; Vmax = 10 MU min−1). The 50% reduction in maximal induction during growth on glucose has been measured previously and can be ascribed to catabolite repression (18). Cells grown in rich medium (LB) showed the same sensitivity (K0.5G6P = 1.2 μM) as in MOPS minimal medium with glucose, but reached similar maximal Uhp induction rates (19 MU min−1) as in MOPS minimal medium with succinate.
The dose-response curves obtained appeared to be sigmoid rather than hyperbolic, i.e., deviated from ideal Michaelis-Menten kinetics (dotted lines in Fig. 1A to C, with K0.5G6P and Vmax as mentioned above). Because this implied an allosteric effect, the data were fitted to a sigmoidal function, i.e., the Hill equation (lines in Fig. 1), as well as linearized into a Hill plot (not shown). The magnitude of nH in the Hill equation, being the slope of the line in the Hill plot (log [V/(Vmax − V]) versus n × log[G6P] − n × log K), is related to the type of cooperative behavior and can be greater than (positive cooperativity), less than (negative cooperativity), or equal to (no cooperativity) 1. We obtained Hill coefficients (± SD; R2 ≥ 0.99) of 2.11 (± 0.03) in LB and of 2.78 (± 0.08) and 1.95 (± 0.17) for cells grown on succinate and glucose as the carbon source in MOPS minimal medium, respectively. These values indicate positive cooperativity in overall Uhp induction kinetics, with a cooperative strength depending on the carbon and energy source.
Inhibition of Uhp induction.
Induction studies were performed on cells grown in a culture medium with a relatively low phosphate concentration (2 mM) compared to other (minimal) media (21), since a high concentration of phosphate in the medium inhibits Uhp induction either partly or completely (depending on the concentration of glucose 6-phosphate and phosphate); maximal induction is reached at higher concentrations of glucose 6-phosphate (data not shown) (27). It has been proposed that this (competitive) inhibition may occur at the regulatory sensor (27), later established to be UhpC (35).
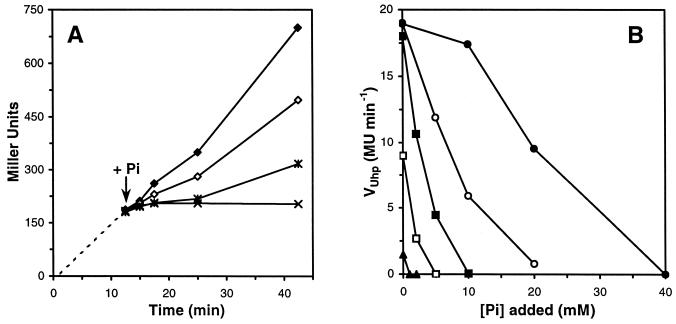
We addressed this (supposedly extracellular) regulatory inhibition in more detail to investigate whether it could be used to control the intracellular signal transfer through UhpABC. Therefore, we added phosphate to cultures in which uhpT was already induced and followed the subsequent response over time (Fig. 2). Inhibition occurred within 3 min following addition of phosphate, as shown in Fig. 2A for an inducing glucose 6-phosphate concentration of 5 μM and three concentrations of phosphate. We established a correlation between the added minimal phosphate concentration for total inhibition ([Pi]0 when VUhp = 0) and the glucose 6-phosphate concentration, i.e., [Pi]0 = 2,000 × [G6P]. Thus, we were able to determine the affected induction rates at different glucose 6-phosphate concentrations for added phosphate concentrations below each [Pi]0 (Fig. 2B). This resulted in an apparent Ki Pi for competitive inhibition of 2.9 ± 0.7 mM (via KG6P, Pi = K0.5G6P × [1 + [Pi]/KiPi]) for cells grown in MOPS minimal medium with succinate as the carbon source and an initial phosphate concentration in the medium of 2 mM.
FIG. 2.
Inhibition of Uhp induction by addition of inorganic phosphate. (A) RK5115 cells in MOPS minimal medium with 30 mM succinate were induced with 5 μM glucose 6-phosphate at time zero. Phosphate was added at 12.5 min (arrow), and samples were analyzed as described in Materials and Methods. Symbols: ♦, 0 mM Pi; ⋄, 2 mM Pi; ∗, 5 mM Pi; ×, 10 mM Pi. (B) Rates (VUhp) at various glucose 6-phosphate concentrations were obtained by determining the slope of the linear trendlines of β-galactosidase activity versus time, as in A, between 15 and 42.5 min for each phosphate concentration tested. Glucose 6-phosphate concentrations: ▴, 1 μM; □, 2.5 μM; ▪, 5 μM; ○, 10 μM; •, 20 μM. [Pi]0, intersection of the line representing VUhp with the horizontal axis (VUhp = 0).
Uhp induction stimulated by growth on pyruvate.
Extracellular glucose 6-phosphate is the only known inducing signal for the Uhp system in wild-type E. coli. However, recently we showed that UhpT was expressed in cells without the UhpBC sensor and in the absence of glucose 6-phosphate when cells were grown on pyruvate (33). This phenomenon strongly indicates that UhpA can use acetyl phosphate as phosphoryl donor in vivo, like many other response regulators, and thus can be activated in the absence of kinase and phosphatase activity by UhpB. Acetyl phosphate is present in large amounts during growth on pyruvate (17) due to an increased flux into the Pta-AckA (phosphotransacetylase-acetate kinase) pathway (10).
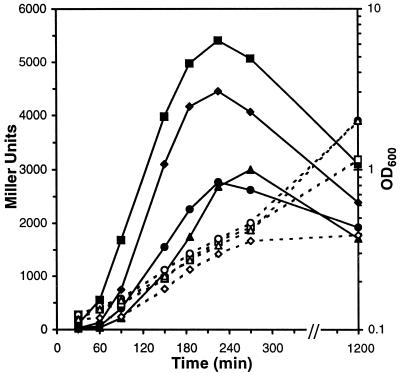
Here, we have further characterized this pyruvate-stimulated Uhp induction in cells with a ΔuhpBC background (uhpT-lacZ). As shown in Fig. 3, Uhp was induced after 30 min upon switching the carbon source to pyruvate and reached a maximum in the early exponential growth phase, in agreement with the observed growth phase-dependent synthesis of acetyl phosphate (24). The Uhp induction rate (up to 40 MU min−1) as well as the maximum expression level (MUmax) in pyruvate-grown ΔuhpBC cells was considerably higher than in wild-type cells. We also addressed the effect of the concentration of inorganic phosphate in the growth medium. Uhp induction appeared to be more sensitive to suboptimal levels of phosphate for growth, in a phase of growth (i.e., early exponential) at which these phosphate concentrations were not limiting growth yet (Fig. 3). High phosphate concentrations decreased maximal Uhp induction rates in ΔuhpBC cells but did not block Uhp induction as in wild-type cells.
FIG. 3.
Uhp induction profile in ΔuhpBC cells grown on pyruvate. RK1307 cells (ΔuhpBC uhpT-lacZ) were grown in MOPS minimal medium with the indicated Pi concentration and switched from succinate to pyruvate (40 mM) as the carbon source at time zero, as described in Materials and Methods. Pi concentrations (open symbols, OD600; solid symbols, MU): ♦, ⋄, 50 μM; ▪, □, 200 μM; •, ○, 2.5 mM; ▴, ▵, 20 mM.
Next, we addressed Uhp induction in early-exponential-phase (OD600 ∼0.3) wild-type cells (RK5115) grown on pyruvate (compared with growth on succinate) and a low optimal phosphate concentration (400 μM) upon addition of glucose 6-phosphate to find out whether Uhp induction rates were affected when UhpA was activated by UhpB (cells plus glucose 6-phosphate, i.e., kinase on) and (supposedly) acetyl phosphate simultaneously. In contrast to pyruvate-grown ΔuhpBC cells (Fig. 3), pyruvate-grown wild-type cells showed very low (basal) UhpT expression (20 to 40 MU in the absence of glucose 6-phosphate, though not zero like during growth on succinate). But their sensitivity to glucose 6-phosphate (Ksuc/Kpyr = 1.31 ± 0.17 for K0.5G6P) and Uhp induction rates were higher (Vpyr/Vsuc = 1.15 ± 0.08 at Vmax) than in succinate-grown wild-type cells. When they were grown in the presence of 2 mM phosphate and induced at an OD600 of 0.8 to 0.9, the pyruvate-mediated additive effect on Uhp induction was not apparent. This is in agreement with the phosphate effect in ΔuhpBC cells as well as with the growth phase dependence of the intracellular acetyl phosphate level, as shown in Fig. 3 and previously (24).
DISCUSSION
We have investigated induction of UhpT expression (uhpT-lacZ) through the Uhp regulatory system in cells with a wild-type regulatory background as well as in cells with a ΔuhpBC background. These two strains enabled us to separate the contribution of transmembrane signaling (through UhpBC and glucose 6-phosphate) and intracellular activation (through UhpA) to the Uhp response in vivo.
Uhp induction curves ([G6P] versus UhpT expression rates) were determined in rich medium (LB) and in minimal medium (MM) with glucose or succinate as the carbon source (Fig. 1). This resulted in K0.5G6Ps of 1 to 2.5 μM, in agreement with the value derived from single-time-point β-galactosidase (MU) measurements per unit of glucose 6-phosphate (27), but 10 times lower than in earlier measurements (37), due to the method previously used (based on glucose 6-phosphate uptake), as well as the phosphate concentration in the medium (8).
The difference in maximal induction rates between cells grown on succinate and cells grown on glucose reflects the known catabolite repression effect on uhpT transcription (8, 18, 19). This 50% reduction during growth on glucose, as shown previously (18), can be ascribed to a small amount of cyclic AMP receptor protein (the catabolite gene activator protein) available, which is required (together with UhpA) to activate uhpT transcription. However, glucose-grown cells were more sensitive to threshold concentrations of glucose 6-phosphate than succinate-grown cells (Fig. 1D). Apparently, at these glucose 6-phosphate concentrations, Uhp induction is not affected by catabolite repression. In rich medium (carbon source independent; LB), the catabolite repression effect was not observed (Fig. 1C), and cells were more sensitive to threshold concentrations of glucose 6-phosphate due to a lower phosphate concentration in LB medium (∼1 mM; J. Tommassen, personal communication).
It has been shown previously (27) that cells grown in medium with high inorganic phosphate concentrations required higher glucose 6-phosphate-concentrations to induce UhpT expression than cells grown in medium with low inorganic phosphate, i.e., the strict extracellular effect of glucose 6-phosphate was inhibited. We quantified the inhibitory effect of phosphate on signal transfer through UhpABC in induced cells (Fig. 2), resulting in an apparent Ki of ∼3 mM. Addition of a maximally inhibiting amount of phosphate to an induced culture resulted in an off-response within 3 min (presumably required to complete translation of uhpT mRNA). Therefore, UhpBC is quickly desensitized to the kinase off/phosphatase on state, causing phosphorylated UhpA (P-UhpA) dephosphorylation. It is likely that phosphate acts extracellularly, i.e., it is competitive with glucose 6-phosphate for the binding site in UhpC, as its inhibitory effect is rather instantaneous, and it can be mimicked to a certain extent by other anions such as sulfate (Verhamme et al., unpublished data). Furthermore, a high phosphate concentration decreases pyruvate-mediated Uhp induction in ΔuhpBC cells but does not block it (Fig. 3).
We observed that the Uhp system was more sensitive (than strictly Michaelis-Menten-type) for inducer concentrations above threshold levels, i.e., the dose-response curves were steeper than hyperbolic (Fig. 1). By further analysis of this phenomenon, we derived Hill coefficients (nH) greater than 1. Apparently, this value is influenced by the carbon or energy source available to the cells for growth, resulting in an nH of 2 in LB medium and in minimal medium with glucose and an nH of 2.8 in minimal medium with succinate. These values imply a carbon source-dependent overall positive cooperativity (nH >1) in signaling through UhpABC to uhpT.
As we are dealing with a typical signal transduction system, starting with transmembrane signaling, followed by intracellular phosphoryl transfer, and leading to modulation of specific gene transcription, the contribution of each of these separate levels of signal processing will be discussed. First, cooperativity could occur at the membrane level, i.e., at the sensor components UhpC and UhpB. Glucose 6-phosphate may bind to UhpC in a cooperative manner (either positively or negatively), and this may also occur in the interaction of UhpC with UhpB to transduce the signal through the membrane. The model for induction by external glucose 6-phosphate holds that it binds to and alters the conformation of UhpC and that this information is communicated to UhpB by direct protein-protein interaction (11). There is no evidence that this occurs in higher-order clusters such as the chemoreceptor supramolecular complex (15, 16), but at least minimal lateral interaction between UhpC and UhpB molecules has to occur to achieve histidine autophosphorylation in trans, catalyzed through the two transmitter domains of a sensory kinase dimer (7). Future in vivo protein distribution and localization studies in membranes may reveal spatial organization. However, UhpB and the turgor sensor KdpD are the only two (functionally characterized) sensory kinases in E. coli with more than two transmembrane domains (36), possibly restricting spatial movement of these sensors upon signal recognition. For KdpD, it has been shown that it functions as a homodimer and that there is no change in the oligomeric state upon activation (9). Moreover, in vitro phosphorylation assays with UhpBC-enriched membranes did not reveal any apparent cooperativity in either glucose 6-phosphate concentration-dependent transmembrane signaling output (i.e., UhpB autophosphorylation) or the subsequent phosphoryl flow towards UhpA (32).
It has been shown previously that UhpA binds to the uhpT promoter in oligomerized form and that this occurs with positive cooperativity (6). The Hill coefficient for this effect ranged from 2.5 to 5 for unphosphorylated UhpA in vitro (22). Phosphorylated UhpA exhibits a strongly increased affinity for the uhpT promoter (5, 6), implying increased positive cooperativity (6), possibly approaching the upper value of this range. Our numbers indicate positive cooperativity for the overall pathway in vivo, strictly depending on phosphorylated UhpA. They do not exceed the lowest value of the 2.5 to 5 range. Therefore, the regulatory steps preceding and impairing transcriptional activation, like the recently demonstrated phosphatase activity and sequestration ability of UhpB (38), may negatively affect (via control of the level of P-UhpA) the entire signal transduction route in vivo, resulting in a more moderate positive cooperativity. This may explain why UhpT expression can be higher in the absence of UhpBC, during growth on pyruvate, when UhpA is supposed to be phoshorylated by acetyl phosphate (Fig. 3). In addition, these data show that phosphate, besides its extracellular impact on Uhp induction in wild-type cells, also affects pyruvate-mediated Uhp induction in ΔuhpBC cells, possibly due to its metabolic control of the Pta-Ack pathway.
Besides further understanding of signaling through Uhp, the possibility of UhpT expression without UhpBC (and without UhpA overexpression) offers a new biotechnological opportunity to exploit glucose 6-phosphate production by E. coli (31). Furthermore, we measured a small additive pyruvate-mediated effect, presumably due to acetyl phosphate, on glucose 6-phosphate-triggered UhpA activation in early-exponential-phase wild-type cells, which adds to the relevance of acetyl phosphate as a physiologically significant signaling molecule (33). This should be further addressed in a Δpta mutant.
Allosteric effects have been studied in detail at different levels in chemotaxis. Binding of aspartate to its chemoreceptor dimer occurs with strong negative cooperativity in vitro (2). However, in the complete transmembrane signaling step, positively cooperative interactions dominate, as shown by in vitro chemoreceptor-coupled kinase (CheA) assays (4, 14). In addition, in vivo studies on the interaction between P-CheY and the flagellar motor reveal positive cooperativity (1, 25). Also, the phosphatase CheZ interacts with P-CheY in a positively cooperative manner (3). Moreover, the overall description of chemotactic signaling has recently been changed, from a relationship between ligand binding and the output response with limited positive cooperativity (26) to a sigmoidal stimulus-response relation with strong positive cooperativity (12).
A quantitative overall description of signaling intensity has hitherto been possible only for a small minority of transmembrane signaling pathways. To find out where in a regulatory system allosteric phenomena take place and what their effect is on the final output, single pathways have to be dissected. The detailed characterization of such pathways and their mutual interactions (33) is a prerequisite for a better understanding of the integration of the complex signal transduction machinery that bacteria exploit to survive and compete.
Acknowledgments
We thank R. J. Kadner for making strains available.
This work was supported by the Netherlands Organization for Scientific Research (NWO), through the division for Earth and Life Sciences (Gebied ALW).
REFERENCES
- 1.Alon, U., L. Camarena, M. G. Surette, B. Aguera y Arcas, Y. Liu, S. Leibler, and J. B. Stock. 1998. Response regulator output in bacterial chemotaxis. EMBO J. 17:4238-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biemann, H. P., and D. E. Koshland, Jr. 1994. Aspartate receptors of Escherichia coli and Salmonella typhimurium bind ligand with negative and half-of-the-sites cooperativity. Biochemistry 33:629-634. [DOI] [PubMed] [Google Scholar]
- 3.Blat, Y., B. Gillespie, A. Bren, F. W. Dahlquist, and M. Eisenbach. 1998. Regulation of phosphatase activity in bacterial chemotaxis. J. Mol. Biol. 284:1191-1199. [DOI] [PubMed] [Google Scholar]
- 4.Bornhorst, J. A., and J. J. Falke. 2000. Attractant regulation of the aspartate receptor-kinase complex: limited cooperative interactions between receptors and effects of the receptor modification state. Biochemistry 39:9486-9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Q., and R. J. Kadner. 2000. Effect of altered spacing between uhpT promoter elements on transcription activation. J. Bacteriol. 182:4430-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahl, J. L., B. Y. Wei, and R. J. Kadner. 1997. Protein phosphorylation affects binding of the Escherichia coli transcription activator UhpA to the uhpT promoter. J. Biol. Chem. 272:1910-1919. [DOI] [PubMed] [Google Scholar]
- 7.Dutta, R., L. Qin, and M. Inouye. 1999. Histidine kinases: diversity of domain organization. Mol. Microbiol. 34:633-640. [DOI] [PubMed] [Google Scholar]
- 8.Ezzell, J. W., and W. J. Dobrogosz. 1978. Cyclic AMP regulation of the hexose phosphate transport system in Escherichia coli. J. Bacteriol. 133:1047-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heermann, R., K. Altendorf, and K. Jung. 1998. The turgor sensor KdpD of Escherichia coli is a homodimer. Biochim. Biophys. Acta 1415:114-124. [DOI] [PubMed] [Google Scholar]
- 10.Holms, H. 1996. Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol. Rev. 19:85-116. [DOI] [PubMed] [Google Scholar]
- 11.Island, M. D., and R. J. Kadner. 1993. Interplay between the membrane-associated UhpB and UhpC regulatory proteins. J. Bacteriol. 175:5028-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasuja, R., Y. Lin, D. R. Trentham, and S. Khan. 1999. Response tuning in bacterial chemotaxis. Proc. Natl. Acad. Sci. USA 96:11346-11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadner, R. J. 1995. Expression of the Uhp sugar-phosphate transport system of Escherichia coli, p. 263-274. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. American Society for Microbiology, Washington, D.C.
- 14.Li, G., and R. M. Weis. 2000. Covalent modification regulates ligand binding to receptor complexes in the chemosensory system of Escherichia coli. Cell 100:357-365. [DOI] [PubMed] [Google Scholar]
- 15.Liu, Y., M. Levit, R. Lurz, M. G. Surette, and J. B. Stock. 1997. Receptor-mediated protein kinase activation and the mechanism of transmembrane signaling in bacterial chemotaxis. EMBO J. 16:7231-7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maddock, J. R., and L. Shapiro. 1993. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science 259:1717-1723. [DOI] [PubMed] [Google Scholar]
- 17.McCleary, W. R., and J. B. Stock. 1994. Acetyl phosphate and the activation of two-component response regulators. J. Biol. Chem. 269:31567-31572. [PubMed] [Google Scholar]
- 18.Merkel, T. J., J. L. Dahl, R. H. Ebright, and R. J. Kadner. 1995. Transcription activation at the Escherichia coli uhpT promoter by the catabolite gene activator protein. J. Bacteriol. 177:1712-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merkel, T. J., D. M. Nelson, C. L. Brauer, and R. J. Kadner. 1992. Promoter elements required for positive control of transcription of the Escherichia coli uhpT gene. J. Bacteriol. 174:2763-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 21.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olekhnovich, I. N., J. L. Dahl, and R. J. Kadner. 1999. Separate contributions of UhpA and CAP to activation of transcription of the uhpT promoter of Escherichia coli. J. Mol. Biol. 292:973-986. [DOI] [PubMed] [Google Scholar]
- 23.Parkinson, J. S., and E. C. Kofoid. 1992. Communication modules in bacterial signaling proteins. Annu. Rev. Genet. 26:71-112. [DOI] [PubMed] [Google Scholar]
- 24.Prüss, B. M., and A. J. Wolfe. 1994. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol. Microbiol. 12:973-984. [DOI] [PubMed] [Google Scholar]
- 25.Scharf, B. E., K. A. Fahrner, L. Turner, and H. C. Berg. 1998. Control of direction of flagellar rotation in bacterial chemotaxis. Proc. Natl. Acad. Sci. USA 95:201-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segall, J. E., S. M. Block, and H. C. Berg. 1986. Temporal comparisons in bacterial chemotaxis. Proc. Natl. Acad. Sci. USA 83:8987-8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shattuck-Eidens, D. M., and R. J. Kadner. 1981. Exogenous induction of the Escherichia coli hexose phosphate transport system defined by uhp-lac operon fusions. J. Bacteriol. 148:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 29.Stock, J. 1999. Sensitivity, cooperativity and gain in chemotaxis signal transduction. Trends Microbiol. 7:1-4. [DOI] [PubMed] [Google Scholar]
- 30.Stock, J. B., A. J. Ninfa, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53:450-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Zee, J. R., P. W. Postma, and K. J. Hellingwerf. 1996. Quantitative conversion of glucose into glucose 6-phosphate by intact Escherichia coli cells. Biotechnol. Appl. Biochem. 24:225-230. [PubMed] [Google Scholar]
- 32.Verhamme, D. T., J. C. Arents, P. W. Postma, W. Crielaard, and K. J. Hellingwerf. 2001. Glucose-6-phosphate-dependent phosphoryl flow through the Uhp two-component regulatory system. Microbiology 147:3345-3352. [DOI] [PubMed] [Google Scholar]
- 33.Verhamme, D. T., J. C. Arents, P. W. Postma, W. Crielaard, and K. J. Hellingwerf. 2002. Investigation of in vivo cross-talk between key two-component systems of Escherichia coli. Microbiology 148:69-78. [DOI] [PubMed] [Google Scholar]
- 34.Webber, C. A., and R. J. Kadner. 1997. Involvement of the amino-terminal phosphorylation module of UhpA in activation of uhpT transcription in Escherichia coli. Mol. Microbiol. 24:1039-1048. [DOI] [PubMed] [Google Scholar]
- 35.Weston, L. A., and R. J. Kadner. 1987. Identification of Uhp polypeptides and evidence for their role in exogenous induction of the sugar phosphate transport system of Escherichia coli K-12. J. Bacteriol. 169:3546-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams, S. B., and V. Stewart. 1999. Functional similarities among two-component sensors and methyl-accepting chemotaxis proteins suggest a role for linker region amphipathic helices in transmembrane signal transduction. Mol. Microbiol. 33:1093-1102. [DOI] [PubMed] [Google Scholar]
- 37.Winkler, H. H. 1971. Kinetics of exogenous induction of the hexose-6-phosphate transport system of Escherichia coli. J. Bacteriol. 107:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright, J. S., and R. J. Kadner. 2001. The phosphoryl transfer domain of UhpB interacts with the response regulator UhpA. J. Bacteriol. 183:3149-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]