The main clinical aim of neoadjuvant (also called primary or preoperative) treatment for operable breast cancer before surgery is to downstage large cancers to reduce the need for mastectomy. A research aim is to use the primary tumour as an in vivo measure of responsiveness to treatment. Trials of adjuvant treatments are expensive and take years to complete, and the aim therefore is to use neoadjuvant treatment to find short term surrogate markers (clinical, pathological, or biological) in small trials that can predict long term outcome accurately. For example, in the IMPACT (immediate preoperative Arimidex compared with tamoxifen) trial involving 300 patients, and comparing Arimidex and tamoxifen with the combination, biological changes in tumour proliferation were shown to predict correctly the superiority of adjuvant anastrozole over the other two treatments in the ATAC (Arimidex or tamoxifen alone or in combination) trial, which was a similarly designed adjuvant trial involving 9000 patients. If this can be confirmed in other trials, it may help identify new therapies quickly, and identify optimal treatment for patients.
Endocrine treatments
A randomised trial in postmenopausal women with large oestrogen receptor positive cancers that would otherwise require mastectomy showed that letrozole for four months is better than tamoxifen in terms of clinical response (55% v 36%) and breast conserving surgery (45% v 35%). A similar trial (IMPACT) compared three months of neoadjuvant anastrozole alone, tamoxifen alone, or the two in combination (neoadjuvant ATAC) and showed no significant difference in response rate (37% v 36% v 39%). In the subpopulation with large cancers that required mastectomy, however, anastrozole, like letrozole, was significantly more effective than tamoxifen or the combination in achieving breast conserving surgery (46% v 22% v 26%). A second study, the preoperative Arimidex compared with tamoxifen trial (PROACT), compared three months of anastrozole and tamoxifen. It showed similar response rates with the two drugs, but a higher rate of breast conserving surgery with anastrozole. Combined results of the PROACT and IMPACT trials showed a significantly greater response rate in tumours that were locally advanced or required a mastectomy at presentation.
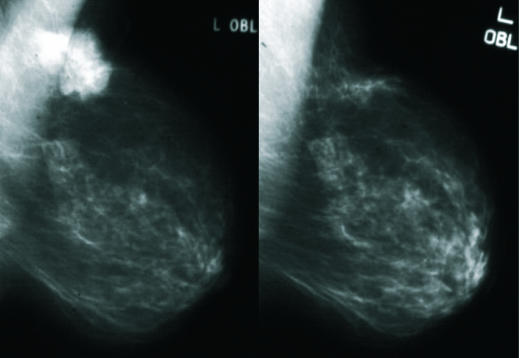
Figure 1.
Carcinoma of the left breast before (left) and after (right) three months of neoadjuvant letrozole
A study of 73 patients with exemestane showed a greater rate of conversion to breast conserving surgery with exemestane than with tamoxifen. A striking finding in the letrozole trial was that letrozole achieved a higher response rate than tamoxifen in the small subgroup of patients whose tumours overexpressed human epidermal growth factor receptor 1 (HER1) or human epidermal growth factor receptor 2 (HER2) (88% v 21%). The IMPACT trial showed a similar trend for anastrozole over tamoxifen or the combination against HER2 positive tumours (58% v 22% v 31%). Taken together, data from these trials provide compelling evidence that aromatase inhibitors are better than tamoxifen in downstaging large oestrogen receptor positive cancers in postmenopausal women to avoid mastectomy. In particular, aromatase inhibitors may be more effective in patients with oestrogen receptor positive tumours that also strongly overexpress HER2.
Figure 2.
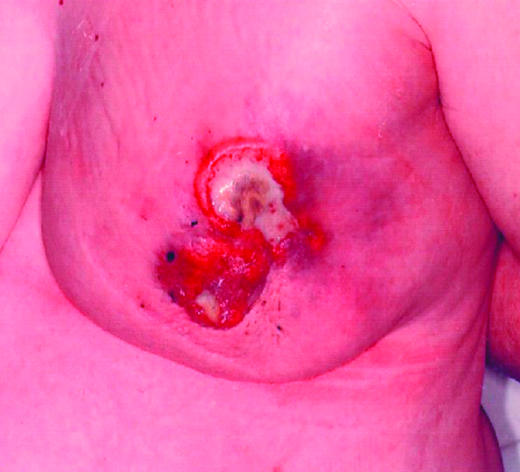
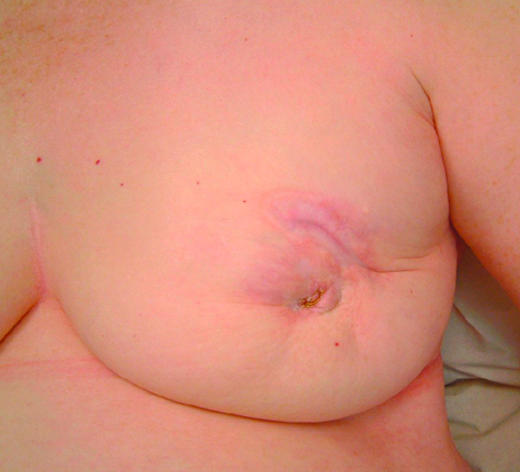
Locally advanced breast cancer showing re-epithelialisation and regression after treatment with letrozole
 This article is adapted from the 3rd edition of the ABC of Breast Diseases (Blackwell Publishing), available from all good medical bookshops, including www.hammicksbma.com
This article is adapted from the 3rd edition of the ABC of Breast Diseases (Blackwell Publishing), available from all good medical bookshops, including www.hammicksbma.com
In contrast with neoadjuvant chemotherapy, pathological complete remissions are rare with endocrine therapy, but easy administration and lack of side effects make it an attractive first line option for older women with large cancers
Chemotherapy
Neoadjuvant chemotherapy achieves clinical regression of tumours in about 70-80% of patients. This suggests that early cancers may be more chemosensitive than metastatic disease. Around 15-20% of patients achieve a complete pathological response of their tumour; this occurs more often in oestrogen receptor negative than oestrogen receptor positive tumours, and complete pathological response is a predictor for good long term outcome. Randomised trials show that survival is similar if chemotherapy is given before or after surgery. The neoadjuvant approach reduces the need for mastectomy and provides data on responsiveness to treatment. Regimens used for neoadjuvant chemotherapy are often similar to those used for adjuvant treatment. Continuous infusional chemotherapy regimens are no more effective than conventional schedules, but the NSABP B27 (national surgical adjuvant breast and bowel project B27) trial showed that four courses of sequential taxotere after four courses of anthracyclines (eight cycles) achieve higher clinical and pathological complete remissions than four cycles of anthracycline chemotherapy alone. Surprisingly, this gain in rates of pathological complete remissions did not translate into an improvement in survival for sequential taxotere usage. This challenges the hypothesis that differences in the rates of pathological complete remissions with neoadjuvant chemotherapy may be a short term surrogate predictor for long term outcome.
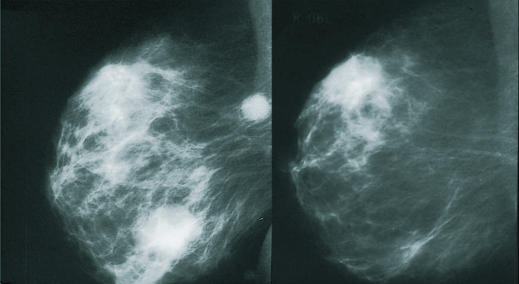
Figure 3.
Cancer of the breast before chemotherapy (left) and after (right), showing disappearance of mass lesion and axillary lymph node
Rates of breast conserving surgery are improved by neoadjuvant chemotherapy, but it can be difficult to map the extent of the disease after treatment. Magnetic resonance imaging may give a better assessment of response than a clinical approach or ultrasonography, but further investigation is needed. Some surgeons think that tumours seem to shrink more concentrically after neoadjuvant hormone therapy than chemotherapy, with low rates of incomplete excision.
Table 1.
Results from randomised trial of 2411 women with operable breast cancer enrolled in NSABP B27 protocol at median follow up of 68.8 months*†
| Group I | Group II | Group III | |
|---|---|---|---|
| Treatment | Adriamycin and cyclophosphamide × 4 cycles then surgery | Adriamycin and cyclophosphamide × 4 cycles then docetaxel × 4 cycles then surgery | Adriamycin and cyclophosphamide × 4 cycles then surgery then docetaxel × 4 cycles |
| Complete pathology response | 12.8% | 26.1% | 14.3% |
| Deaths any case | 150 | 143 | 163 |
| Contralateral breast cancer | 9 | 18 | 18 |
| Relapse free events | 247 | 210 (P=0.03) | 230 |
| Local recurrence | 67 | 43 | 44 (P=0.0014) |
All patients had four cycles of neoadjuvant chemotherapy and were randomised to have surgery alone, docetaxel then surgery, or surgery followed by docetaxel
Data from the NSABP study, presented at the San Antonio Breast Cancer Symposium 2004
Trastuzumab (Herceptin)
Trastuzumab achieved high response rates in combination with neoadjuvant chemotherapy in breast cancers that overexpressed HER2. It improved pathological complete remission rate significantly in one trial. It will probably become standard care with chemotherapy in the neoadjuvant treatment of large and locally advanced HER2 positive breast cancer, except in elderly women, where an aromatase inhibitor may be as effective.
Outcome
Progressive disease during neoadjuvant chemotherapy is rare (< 5% of patients); should it occur, the patient should be switched to second line chemotherapy or surgery. About half of patients with large cancers will have enough tumour regression to avoid mastectomy, but all patients need some form of surgery after neoadjuvant treatment, and usually radiotherapy (according to standard guidelines).
Selection of patients
Neoadjuvant systemic treatment was developed for women with locally advanced (inoperable) breast cancers. It is also used in women with large operable breast cancers to avoid mastectomy. In these women, neoadjuvant treatment is as effective as standard adjuvant treatment. In other patients, neoadjuvant treatment should be used only for clinical trials investigating surrogate clinical and biological markers for predicting treatment outcome.
The roles of adjuvant and neoadjuvant medical treatments in improving survival in early breast cancer emphasise the importance of patients being assessed and managed by multidisciplinary teams.
The ABC of Breast Diseases is edited by J Michael Dixon, consultant surgeon and senior lecturer in surgery, Edinburgh Breast Unit, Western General Hospital, Edinburgh.
Competing interests: Michael Dixon has received reimbursement for attending symposiums, fees for speaking, and educational grants from AstraZeneca, Novartis, and Pfizer. Ian Smith has received honoraria for lecturing, research grants, and fees for attending advisory boards from several companies involved in drugs for early breast cancer, including Novartis, AstraZeneca, Aventis, Pfizer, Eli Lilly, GlaxoSmithKline, and Roche.