Abstract
Overproduction of the response regulator BaeR confers resistance to novobiocin and bile salts in a ΔacrAB mutant by stimulating drug exporter gene expression. The mdtABC (multidrug transporter ABC, formerly known as yegMNO) genes, which encode a resistance-nodulation-cell division (RND) drug efflux system, are responsible for resistance. The MdtABC system comprises the transmembrane MdtB/MdtC heteromultimer and MdtA membrane fusion protein. MdtAC also confers bile salt, but not novobiocin, resistance. This indicates that the evolution from an MdtC homomultimer to an MdtBC heteromultimer contributed to extend the drug resistance spectrum. A BLAST search suggested that such a heteromultimer-type RND exporter constitutes a unique family among gram-negative organisms.
The emergence of bacterial multidrug resistance has become an increasing problem in the treatment of infectious diseases. Multidrug resistance often results from the overexpression of multidrug efflux transporters (19). Recent genome sequence analysis revealed that bacteria have a lot of intrinsic drug exporter genes (20). We previously cloned all of the open reading frame (ORF) clusters encoding putative drug exporter genes in Escherichia coli and revealed that 20 genes encode exporters of known drugs and/or toxic compounds (16). During the course of these comprehensive studies, we found that the overexpression of a response regulator of a bacterial two-component signal transduction system confers multidrug resistance by stimulating the expression of multidrug exporter genes (15, 17).
Two-component signal transduction systems are major environmental sensing mechanisms of bacteria and are a component of sensor kinases and response regulators (2, 11). The vanS and vanR genes encode an Enterococcus two-component system which confers vancomycin resistance via upregulation of vanA and vanH, which make an altered peptidoglycan precursor to which vancomycin does not bind (3, 6). Similar two-component-system-mediated vancomycin tolerance was reported to occur in Streptococcus pneumoniae (18). Recently, it was found that some two-component systems confer drug resistance by upregulating drug exporter genes (4, 9, 15, 17). Since two-component systems are a mechanism for bacterial environmental adaptation and intrinsic drug exporters are a bacterial self-defense mechanism (23, 24), it is reasonable that some two-component systems could control drug exporter genes.
In previous papers, Nishino and Yamaguchi reported a mechanism for E. coli to express multidrug resistance by overexpression of the response regulator EvgA, which stimulates the expression of novel multidrug exporters (15, 17). EvgA regulates the expression of emrKY (7, 15), which encodes a major facilitator superfamily (MFS)-type bile salt-specific exporter, and yhiUV (17), which encodes a resistance-nodulation-cell division (RND)-type multidrug exporter. In this study, we characterize a novel multiple-membrane component RND-type drug transporter system, MdtABC, which comprises a transmembrane MdtB/MdtC heteromultimer and the membrane fusion protein MdtA. This system is also stimulated by overproduction of the response regulator BaeR.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. The chromosomal DNA of E. coli W3104 (25) was used as a template for PCR cloning of the mdt-bae ORF clusters. E. coli DH5α (Takara Shuzo Co., Kyoto, Japan) was used as a cloning host. E. coli KAM3 (12), a derivative of E. coli TG1 that lacks acrAB, and E. coli TG1ΔtolC, which lacks tolC, were used for drug susceptibility testing. E. coli KO6494 was constructed for this study from E. coli KAM3 by means of random knockout of the genes by Mu d1 phage integration. Plasmids pUC119, pHSG398, pQE30, pTrc99A, and pACYC177 were purchased from commercial sources. Other plasmids were constructed in this study.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant phenotype or genotype | Reference or origin |
|---|---|---|
| E. coli strains | ||
| W3104 | Wild-type strain | 25 |
| DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 gyrA96 thi-1 recA1 | Takara Shuzo Co. |
| TG1 | supE hsdΔ5 thi Δ(lac-proAB) F′ [traD36 proAB+lacIqlacZΔM15] | Takara Shuzo Co. |
| KAM3 | Derivative of TG1 that lacks a restriction system and acrAB | 12 |
| KO6494 | Derivative of KAM3 that lacks mdtB (formerly yegN) | This study |
| TG1ΔtolC | Derivative of TG1 that lacks tolC | This study |
| Plasmids | ||
| pUC119 | Vector; Apr; multiple cloning site in lacZ | Takara Shuzo Co. |
| pQE30 | His expression vector; Apr; multiple cloning site downstream of T5 promoter | Qiagen |
| pHSG398 | Derivative of pUC18 containing Cmr in place of Apr. | Takara Shuzo Co. |
| pUCyeg-mdt-bae | 14.2-kb SphI-EcoRI fragment containing yegKL (putative regulatory system), mdtABC (former name, yegMNO; putative MFP, putative RND transporter, putative RND transporter), mdtD (putative MF transporter), and baeSR (putative two-component system) genes cloned into pUC119, Apr | 16 |
| pUCmdtABCD | 9.5-kb PstI-SmaI fragment containing mdtABCD genes into pUC119, Apr | This study |
| pUCmdtABC | BglII-SmaI fragment was removed from pUCmdtABCD | This study |
| pUCyegLK | yegLK genes with the promoter region were amplified from pUCyeg-mdt-bae by PCR and cloned into pUC119 | This study |
| pUCmdtA | mdtA gene with the native promoter was cloned into pUC119 as above | This study |
| pUCmdtAB | mdtAB genes with the native promoter were cloned into pUC119 as above | This study |
| pUCbaeSR | baeSR genes with the native promoter were cloned into pUC119 as above | This study |
| pHSGbaeSR | SphI-EcoRI fragment of pUCbaeSR was subcloned into the multicloning site of pHSG398 | This study |
| pQEbaeS | baeS gene was amplified by PCR from pUCbaeSR and cloned into pQE30 under the control of T5 promoter | This study |
| pQEbaeR | baeR gene was cloned into pQE30 as above | This study |
| pTrc99A | Expression vector; multiple cloning site downstream of trc promoter, Apr | Amersham Pharmacia Biotech |
| pTrcmdtC | 3.1-kb EcoRI-PstI fragment containing mdtC gene cloned into pTrc99A, Apr | This study |
| pTrcmdtBC | 6.2-kb EcoRI-PstI fragment containing mdtB and mdtC genes cloned into pTrc99A, Apr | This study |
| pACYC177 | Vector; Apr, Kmr | MBI Fermentas |
| pACYCmdtA | 2.9-kb PstI-BamHI fragment containing mdtA gene cloned into pACYC177, Kmr | This study |
Subcloning and expression of individual ORFs in the mdt-bae ORF cluster.
The mdt-bae ORF cluster was previously cloned from the chromosomal DNA of E. coli W3104 in our laboratory (16). Each ORF or ORF pair was amplified from pUCyeg-mdt-bae (16) by PCR with a pair of primers containing a restriction enzyme site that exists in the multicloning sites of the pUC119 and pQE30 vectors. The DNA fragments were digested with restriction enzymes and then ligated into the multicloning sites of pUC119 and pQE30. As for mdtABCD, the chromosomal DNA of E. coli W3104 was digested by restriction enzymes PstI and SmaI followed by separation by agarose gel electrophoresis. The DNA fragments of around 9.5 kb were extracted from the gel and ligated into the multicloning site of pUC119 followed by transformation of E. coli KAM3. The colonies that carried the mdtABCD genes were detected by colony hybridization, with the PCR fragment of mdtC used as a probe. The PCR fragment was obtained by amplification of the mdtC gene from the E. coli W3104 chromosome with primers mdtC-F (CCGAATTCAAGTTTTTTGCCCTCTTCATTT) and mdtC-R (GGCTGCAGCTCGGTTACCGTTTGTTTAGGT). After purification, the PCR product was labeled with a digoxigenin labeling kit (Takara Shuzo Co.). The resulting cells carried plasmids encoding mdtABCD and the putative promoter region. pUCmdtABC was constructed from pUCmdtABCD by deletion of the BglII-SmaI fragment. The resulting plasmid lacked the mdtD gene.
Random knockout and selection of strains lacking the BaeR-responsive gene.
Random knockout of the E. coli KAM3 chromosomal genes was performed by the method of Mu d1 (Apr) phage integration as described previously (22). In brief, KAM3 cells were infected by Mu d1 phage followed by selection with 50 μg of ampicillin/ml. The phage-integrated KAM3 cells were then transformed with pHSGbaeSR (Cmr). The ampicillin- and chloramphenicol-resistant clones were isolated on agar plates containing 150 μg of ampicillin/ml and 10 μg of chloramphenicol/ml. Of these clones, the strains that were sensitive to 4 μg of novobiocin/ml were screened. The Mu d1 phage integration site was then determined as follows. In order to construct the plasmid library of this strain, the genomic DNA of E. coli KO6494 was subjected to partial Sau3AI digestion and then ligated into the pUC118 vector that had been digested with BamHI and treated with alkaline phosphatase. From this plasmid library, the plasmids containing Mu d1 DNA fragments were selected by colony hybridization with a PCR fragment of Mu d1 DNA as a probe. This PCR fragment was obtained from Mu d1 DNA by using primers MuF (GGTTGTGGTTAATTTGTTTATCA) and MuR (GGTAAATTCCTTTGATTACTGAT). The PCR product was labeled with a digoxigenin labeling kit (Takara Shuzo Co.). The plasmid obtained, which contained the Mu d1 fragment, was sequenced by using a BigDye DNA sequencing kit (PE Applied Biosystems) with M13, M4, and M13RV sequencing primers (Takara Shuzo Co.).
Construction of tolC in-frame deletion mutants.
To construct the tolC deletion mutant from E. coli TG1 cells, the precise in-frame deletions were generated by crossover PCR. The following oligonucleotide primers were used: tolC-No (CGCGGATCCTCATCCCGGCAACCATCTC), tolC-Ni (CACGCAATAACCTTCACACTCCAAATTTATAACCATTCCTTGTGGTGAAGCAGTAT),tolC-Co (CGCGGATCCGCTGGATTGCTGGGCC), and tolC-Ci, (GTTATAAATTTGGAGTGTGAAGGTTATTGCGTGTGATGACGACGACGGGG).The fragment containing the deletion was then cloned into the BamHI site (underlined in the primer sequences) of the pKO3 vector (10), which is a gene replacement vector that contains a temperature-sensitive origin of replication and markers for positive and negative selection for chromosomal integration and excision. The deletion was introduced into the chromosome by use of the pKO3 gene replacement protocol as described previously (10). The plasmid obtained was then electroporated into TG1. Cells were then recovered in 1 ml of SOC (2% Bacto Tryptone, 0.5% yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 20 mM glucose) for 1 h at 30°C. The cells were then plated on prewarmed chloramphenicol (20 μg/ml)-containing Luria broth (LB) plates and incubated at 43°C. From these plates, five colonies were picked up and inoculated into 1 ml of LB. After that, the cells were plated at 30°C on 5% (wt/vol) sucrose plates. The outgrowing bacteria were plated on LB plates with or without chloramphenicol at 30°C. Chloramphenicol-sensitive mutants were selected. Chromosomal insertions and deletions were confirmed by PCR.
Drug resistance determination.
The MICs of drugs and toxic compounds were determined as concentrations that largely prevented bacterial growth on L-agar (1% tryptone, 0.5% yeast extract, 0.5% NaCl) plates by sequential twofold dilutions as described previously (16).
Determination of the amount of mRNA by quantitative real-time PCR.
Total RNA was purified from KAM3 cells harboring pUC119 and pUCbaeSR by using RNA Protect bacterial reagent (Qiagen) and an SV total RNA isolation system (Promega). cDNA samples were synthesized from total RNA by using TaqMan reverse transcription reagents (PE Applied Biosystems) and random nucleotide hexamers. Then, specific primer pairs were designed with ABI PRISM Primer Express software (PE Applied Biosystems), and a real-time PCR was performed with each specific primer pair by using SYBR Green PCR Master Mix and run on an ABI PRISM 7000 sequence detection system.
RESULTS
ORF clusters responsible for the drug resistance phenotype of the genomic mdt and bae region.
The genomic yeg, mdt, and bae region, which is located at 46.5 to 46.6 min in the E. coli chromosome and contains eight ORFs (Fig. 1) (20a), when cloned into a multicopy vector, gave E. coli KAM3 (ΔacrAB) cells a drug resistance phenotype against bile salt derivatives, sodium dodecyl sulfate (SDS), and novobiocin. The MICs of deoxycholate, cholate, taurocholate, SDS, and novobiocin were increased by factors of 64, 8, >4, 4, and 8, respectively (Table 2). The MICs of 17 other drugs and toxic compounds (tetracycline, chloramphenicol, minocycline, erythromycin, enoxacin, kanamycin, vancomycin, doxorubicin, rifampin, trimethoprim, acriflavine, crystal violet, ethidium bromide, rhodamine 6G, methylviologen, tetraphenylphosphonium bromide, and carbonyl cyanide m-chlorophenylhydrazone) were unaffected. This region contains some putative drug exporter genes (16). According to the sequence similarities, mdtB and mdtC were suggested to encode RND-type drug exporters and mdtA was postulated to be a membrane fusion protein gene. In addition, mdtD is a putative MFS-type drug exporter gene. On the other hand, yegK and yegL are putative regulator genes, and baeS and baeR encode a putative sensor kinase and a response regulator, respectively, in a two-component signal transduction system (13). In order to determine which ORFs are responsible for the drug resistance phenotype, we subcloned the ORF clusters into the multicopy plasmid pUC119. As shown in Table 2, a plasmid carrying mdtABCD conferred the same drug resistance phenotype as a plasmid carrying the entire region. The genes mdtABC also showed the same resistance phenotype, while mdtAB and mdtA did not. The putative regulator genes, yegLK, conferred no resistance. Surprisingly, the baeSR two-component regulator genes gave the same resistance phenotype as mdtABC. The baeR gene alone also conferred the same resistance, while baeS alone conferred no resistance, indicating that overexpression of the response regulator is enough for the resistance phenotype. This result is similar to that obtained with evgA reported previously (15).
FIG. 1.
Physical map of the yeg-mdt-bae region located at 46.5 to 46.6 min in the E. coli chromosome (20a) and the regions cloned into plasmids. The drug resistance of cells carrying these plasmids is shown at the right. The Mu d1 phage insertion position of E. coli KO6494 is depicted.
TABLE 2.
Drug resistance of E. coli KAM3 (ΔacrAB) and KO6494 [ΔacrAB mdtB::Apr (Mu d1)] cells harboring a pUC119 or pHSG398 plasmid carrying an ORF cluster(s) in the genomic yeg, mdt, and bae regiona
| Drug | MIC (μg/ml) against KAM3 harboring multicopy plasmid pUC119 carrying:
|
MIC (μg/ml) against KO6494b harboring pHSG398 carrying:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| None | yeg/mdt/baec | yegLK | mdtA | mdtAB | mdtABC | mdtABCD | baeSR | baeS | baeR | None | baeSR | |
| Deoxycholate | 1,000 | 64,000 | 1,000 | 1,000 | 1,000 | 64,000 | 64,000 | 64,000 | 1,000 | >64,000 | 1,000 | 1,000 |
| Cholate | 8,000 | 64,000 | 8,000 | 8,000 | 8,000 | 64,000 | 64,000 | 64,000 | 8,000 | 64,000 | 8,000 | 8,000 |
| Taurocholate | 16,000 | >64,000 | 16,000 | 16,000 | 16,000 | >64,000 | >64,000 | >64,000 | 16,000 | 64,000 | 16,000 | 16,000 |
| SDS | 64 | 256 | 64 | 64 | 64 | 128 | 256 | 256 | 64 | 256 | 64 | 64 |
| Novobiocin | 1 | 8 | 1 | 1 | 1 | 8 | 8 | 8 | 1 | 8 | 1 | 1 |
MICs more than fourfold higher than those against host KAM3 cells are indicated by boldface. MICs of 17 different drugs and toxic compounds other than those listed here (tetracycline, chloramphenicol, minocycline, erythromycin, enoxacin, kanamycin, vancomycin, doxorubicin, rifampin, trimethoprim, acriflavine, crystal violet, ethidium bromide, rhodamine 6G, methylviologen, tetraphenylphosphonium bromide, and carbonyl cyanide m-chlorophenylhydrazone) were measured. However, no change in MIC was observed when the cells were transformed with any plasmid carrying ORFs listed above.
KO6494 is a Mu d1 phage-integrated mdtB-disrupted derivative of KAM3.
yeg/mdt/bae contains yegKL, mdtABCD (yegMNOB), and baeSR genes as shown in Fig. 1.
Genes responsible for baeR-modulated drug resistance.
In order to determine the genes modulated by baeR and responsible for drug resistance, the chromosomal genes of E. coli KAM3 were randomly knocked out by transposition of Mu d1 phage, which carries an ampicillin resistance gene as a marker. A mixture of cells with random insertions was transformed with pHSGbaeSR, which is a pHSG398 plasmid carrying baeSR genes and a chloramphenicol resistance gene as a marker. We obtained 7,200 clones of phage-integrated-plasmid-carrying strains by selection for ampicillin and chloramphenicol resistance. These clones were transplanted on the agar plate containing 4 μg of novobiocin/ml, and we obtained one novobiocin-sensitive strain, KO6494. The phage integration site, which was determined as described in Materials and Methods, was in the mdtB gene (data not shown). The MICs of bile salt derivatives, SDS, and novobiocin against strain KO6494 [ΔacrAB mdtB::Apr(Mu d1)] were the same as those against the host KAM3 cells (Table 2). Thus, phage Mu d1 insertion in the mdtB gene of strain KO6494 resulted in the loss of the drug resistance phenotype, even when the strain carried the multicopy baeSR genes (pHSGbaeSR).
Expression control of mdtABCD genes by BaeR.
In order to determine whether the expression of the mdtABC genes is controlled by BaeR, we performed Northern blot analysis with a DNA fragment containing the mdtC region as a probe. Total cellular RNA isolated from KAM3 cells carrying pUCbaeSR showed a radioactive band corresponding to the mdtC mRNA, whereas KAM3 cells showed no such band (data not shown). The size of the radioactive band was 13 kb, which covers the entire mdt-bae locus, suggesting that these six genes (mdtABCD and baeSR) are transcribed as one operon (Fig. 1).
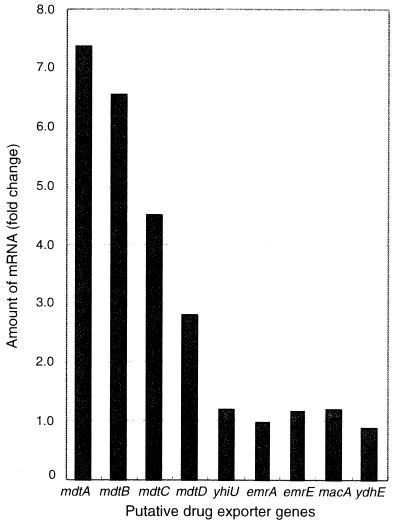
The amounts of mRNAs of individual genes of the mdtABCD region and of other multidrug exporter genes were determined by quantitative reverse transcription-PCR assay. As shown in Fig. 2, BaeR overexpression increased the amounts of mdtA, mdtB, mdtC, and mdtD mRNA by factors of about 7.4, 6.6, 4.5, and 2.8, respectively. The order of these values is consistent with the transcription of mdtABCD from the same promoter of the mdt-bae operon under the control of BaeR. On the other hand, the expression of the yhiUV, emrA, emrE, macA, and ydhE genes was not affected by BaeR overexpression.
FIG. 2.
Fold induction of transcripts attributed to baeR amplification as determined by amplification of cDNA samples from E. coli KAM3/pUCbaeSR (for details, see Materials and Methods).
MdtABC is a novel RND-type drug exporter complex with unique subunit composition.
To determine whether any single transporter gene can confer drug resistance, the mdtA, mdtB, and mdtC genes were separately cloned into pACYC177 and pTrc99A. None of these plasmids gave any drug resistance to KAM3 cells, except for the very-low-level deoxycholate and SDS resistance of pTrcmdtC (Table 3). The effect of combinations of the transporter genes, mdtB and mdtC, and/or the membrane fusion protein gene, mdtA, on drug resistance was checked (Table 3). The pair of pACYCmdtA and pTrcmdtBC conferred the same resistance pattern as pUCmdtABC, except that the deoxycholate resistance was slightly low. On the other hand, the drug resistance pattern of the combination of pACYCmdtA and pTrcmdtC was unique; that is, the combination conferred resistance only to deoxycholate and its derivatives and not to novobiocin or SDS at all (Table 3). Since pUCmdtAB conferred no resistance (Table 2), there is no possibility that MdtABC is a mixture of independent drug exporters, i.e., bile salt-specific MdtAC and novobiocin-specific MdtAB. In light of the high (45%) amino acid sequence similarity of MdtB and MdtC, a complex of MdtB and MdtC is likely to be replaced with an MdtC homomultimer in the absence of MdtB. Thus, it seems that the complex of MdtC homomultimer and MdtA may have narrower drug specificity than the complex of MdtB/MdtC heteromultimer and MdtA. The very low resistance shown by pTrcmdtC and pTrcmdtBC alone may be caused by a trace amount of MdtA from leaky expression of the chromosomal gene in E. coli KAM3.
TABLE 3.
Drug resistance of E. coli KAM3 cells harboring combinations of plasmids carrying mdtA and mdtBCa
| Drug | MIC (μg/ml) against KAM3 cells harboring combinations of plasmids:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| pTrcmdtC
|
pACYCmdtA and pTrcmdtC
|
pTrcmdtBC
|
pACYCmdtA and pTrcmdtBC
|
|||||
| −IPTG | +IPTG | −IPTG | +IPTG | −IPTG | +IPTG | −IPTG | +IPTG | |
| Deoxycholate | 2,000 | 2,000 | 8,000 | 8,000 | 1,000 | 2,000 | 4,000 | 32,000 |
| Cholate | 16,000 | 16,000 | 32,000 | 64,000 | 8,000 | 16,000 | 16,000 | 64,000 |
| Taurocholate | 32,000 | 32,000 | 32,000 | 64,000 | 32,000 | 32,000 | 64,000 | >64,000 |
| SDS | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 128 |
| Novobiocin | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 8 |
MICs more than fourfold higher than those against host KAM3 cells are indicated by boldface. The gene expression from pTrc plasmids was induced with 10 μM IPTG (isopropyl-β-d-thiogalactopyranoside).
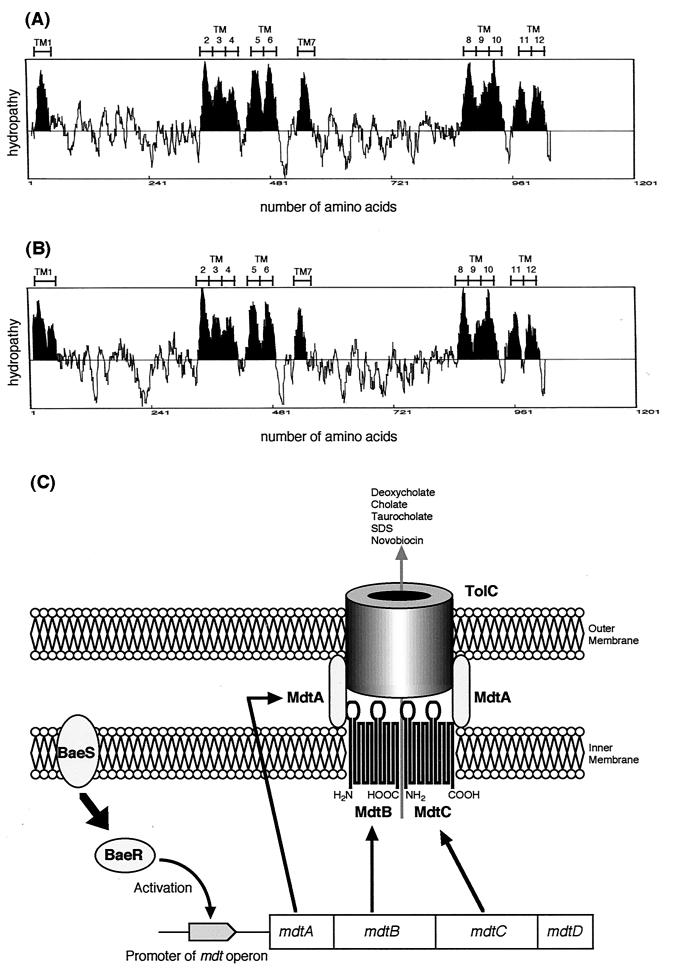
Finally, we found that pUCmdtABC did not confer any resistance to E. coli TG1 cells from which the tolC gene was deleted (data not shown), indicating that TolC is required for the resistance phenotype of MdtABC (Fig. 3).
FIG. 3.
Hydropathy profiles of MdtB (A) and MdtC (B). Putative transmembrane segments are indicated. (C) Schematic model of the molecular construction of MdtABC-TolC transporter complex and the expression regulation.
DISCUSSION
In this study, we showed that overexpression of the response regulator BaeR stimulates the drug resistance phenotype by upregulating the expression of a novel RND-type drug exporter, MdtABC. This is the second case in E. coli of response regulator overexpression-stimulated drug resistance. The first one involves EvgA, which regulates drug exporter genes (7, 15). The evgAS operon is upstream of the evgA-controlled emrKY operon, which encodes an MFS-type bile salt-specific drug exporter. These operons are divergently transcribed (15). EvgAS also controls the remote genes yhiUV, which encode an RND-type multidrug exporter conferring the multidrug phenotype of evgA-overexpressing strains (17). On the other hand, the baeSR genes are downstream of the mdtABC genes and are transcribed in the same direction. baeSR was first reported by Nagasawa et al. (13) as a novel two-component signal transduction system, but neither BaeR-controlled genes nor signals sensed by BaeS had been known. We found in this study that overexpression of BaeR upregulates the expression of MdtABC. The baeSR-mediated resistance can be assigned to mdtABC. Recently, Li et al. (9) reported that the two-component signal transduction system SmeSR in Stenotrophomonas maltophilia controls the smeABC genes, which encode a putative RND-type transporter system, and confers multidrug resistance. However, the gene responsible for smeSR-mediated resistance is smeC and not smeAB. Since SmeC is an outer membrane channel protein like E. coli TolC, some other genes encoding an inner membrane transporter may cooperate with SmeC.
Neither MdtA, MdtB, nor MdtC provided drug resistance when overexpressed individually. MdtAB also did not confer drug resistance, but MdtAC conferred limited resistance against bile salt derivatives. MdtABC conferred resistance against novobiocin and bile salt derivatives. These results strongly suggest that the transmembrane functional unit is an MdtB/MdtC heteromultimer and that MdtB contributes to extend the substrate specificity of the transporter to novobiocin. From an evolutionary point of view, MdtB might be derived from MdtC and the transporter might gain the extension of the substrate range by the replacement of the ancestral MdtC homomultimer with the MdtB/MdtC heteromultimer. Such transporters with heteromultimer-type transmembrane subunits are unique as RND-type drug exporters, but they are common in other bacterial multicomponent substrate transporters, such as maltose transporter (14) and phosphotransferase systems (21). We found that MdtABC homologues are present in Pseudomonas aeruginosa and Xylella fastidiosa. PA2526, PA2527, and PA2528 of P. aeruginosa showed sequence identities with MdtC, MdtB, and MdtA of about 56, 62, and 45%, respectively. XF2384, XF2385, and XF2386 of X. fastidiosa showed identities with MdtA, MdtC, and MdtB of about 37, 46, and 40%, respectively. Although none of these putative RND-type transporters has been analyzed yet, they may constitute a new type subfamily of RND-type drug efflux transporters.
MdtABC requires a multifunctional outer membrane channel TolC for its function, much like YhiUV (17), EmrKY (17), MacAB (8), and AcrAB (5). Of these, MdtABC, YhiUV, and AcrAB have the RND-type inner membrane transporters MdtB/MdtC, YhiV, and AcrB, respectively. On the other hand, EmrKY and MacAB have different types of inner membrane subunit, EmrY and MacB, which are MFS- and ABC (ATP binding cassette family)-type transporters, respectively. The common features of these transport systems are the presence of their own membrane fusion proteins. Thus, it seems that the membrane fusion protein-dependent drug exporter systems in E. coli generally require TolC as an outer membrane subunit, irrespective of the types of their inner membrane proteins. These results suggest that the TolC dependence may be determined by a membrane fusion protein.
It is likely that the two-component signal transduction system-mediated upregulation of the expression of intrinsic drug exporter genes will become a source of multidrug resistance in pathogens.
ADDENDUM
After submitting this report, we became aware that the report of Baranova and Nikaido (1), which deals with the same subject, was submitted to this journal at almost the same time. The results of their study are consistent with ours, except for the limited resistance of MdtAC against deoxycholate and SDS. The lack of resistance of MdtAC-carrying cells against deoxycholate reported in their study may be due to the absence of the translation products. Their results showing BaeR binding to the mdtABCD promoter region also support our conclusion that BaeR is a transcriptional regulator of the mdtABCD operon.
Acknowledgments
Satoshi Nagakubo and Kunihiko Nishino contributed equally to this work.
We thank Tomofusa Tsuchiya for strain KAM3 and George M. Church for plasmid pKO3. We thank Hiroshi Nikaido for helpful discussions. We thank Mary Berlyn and Kenneth Rudd for suggestions on genetic nomenclature.
K. Nishino is supported by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists. This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Baranova, N., and H. Nikaido. 2002. The BaeSR two-component regulatory system activates transcription of the yegMNOB (mdtABCD) transporter gene cluster in Escherichia coli and increases its resistance to novobiocin and deoxycholate. J. Bacteriol. 184:4168-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coote, J. G. 2001. Environmental sensing mechanisms in Bordetella. Adv. Microb. Physiol. 44:141-181. [DOI] [PubMed] [Google Scholar]
- 3.Evers, S., and P. Courvalin. 1996. Regulation of VanB-type vancomycin resistance gene expression by the VanSB-VanRB two-component regulatory system in Enterococcus faecalis V583. J. Bacteriol. 178:1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fournier, B., R. Aras, and D. C. Hooper. 2000. Expression of the multidrug resistance transporter NorA from Staphylococcus aureus is modified by a two-component regulatory system. J. Bacteriol. 182:664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fralick, J. A. 1996. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J. Bacteriol. 178:5803-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haldimann, A., S. L. Fisher, L. L. Daniels, C. T. Walsh, and B. L. Wanner. 1997. Transcriptional regulation of the Enterococcus faecium BM4147 vancomycin resistance gene cluster by the VanS-VanR two-component regulatory system in Escherichia coli K-12. J. Bacteriol. 179:5903-5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato, A., H. Ohnishi, K. Yamamoto, E. Furuta, H. Tanabe, and R. Utsumi. 2000. Transcription of emrKY is regulated by the EvgA-EvgS two-component system in Escherichia coli K-12. Biosci. Biotechnol. Biochem. 64:1203-1209. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi, N., K. Nishino, and A. Yamaguchi. 2001. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 183:5639-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, X.-Z., L. Zhang, and K. Poole. 2002. SmeC, an outer membrane multidrug efflux protein of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 46:333-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuno, T. 1998. His-Asp phosphotransfer signal transduction. J. Biochem. (Tokyo) 123:555-563. [DOI] [PubMed] [Google Scholar]
- 12.Morita, Y., K. Kodama, S. Shiota, T. Mine, A. Kataoka, T. Mizushima, and T. Tsuchiya. 1998. NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob. Agents Chemother. 42:1778-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagasawa, S., K. Ishige, and T. Mizuno. 1993. Novel members of the two-component signal transduction genes in Escherichia coli. J. Biochem. 114:350-357. [DOI] [PubMed] [Google Scholar]
- 14.Nikaido, H. 1994. Maltose transport system of Escherichia coli: an ABC-type transporter. FEBS Lett. 346:55-58. [DOI] [PubMed] [Google Scholar]
- 15.Nishino, K., and A. Yamaguchi. 2001. Overexpression of the response regulator evgA of the two-component signal transduction system modulates multidrug resistance conferred by multidrug resistance transporters. J. Bacteriol. 183:1455-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino, K., and A. Yamaguchi. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishino, K., and A. Yamaguchi. 2002. EvgA of the two-component signal transduction system modulates production of the YhiUV multidrug transporter in Escherichia coli. J. Bacteriol. 184:2319-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak, R., B. Henriques, E. Charpentier, S. Normark, and E. Tuomansen. 1999. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature 399:590-593. [DOI] [PubMed] [Google Scholar]
- 19.Okusu, H., D. Ma, and H. Nikaido. 1996. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 178:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulsen, I. T., L. Nguyen, M. K. Sliwinski, R. Rabus, and M. H. Saier. 2000. Microbial genome analyses: comparative transport capabilities in eighteen prokaryotes. J. Mol. Biol. 301:75-100. [DOI] [PubMed] [Google Scholar]
- 20a.Rudd, K. E. 1998. Linkage map of Escherichia coli K-12, edition 10: the physical map. Microbiol. Mol. Biol. Rev. 62:985-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siebold, C., K. Flukiger, R. Beutler, and B. Erni. 2001. Carbohydrate transporters of the bacterial phosphoenolpyruvate: sugar phosphotransferase system (PTS). FEBS Lett. 504:104-111. [DOI] [PubMed] [Google Scholar]
- 22.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Thanassi, D. G., L. W. Cheng, and H. Nikaido. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White, D. G., J. D. Goldman, B. Demple, and S. B. Levy. 1997. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J. Bacteriol. 179:6122-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto, T., M. Tanaka, C. Nohara, Y. Fukunaga, and S. Yamagishi. 1981. Transposition of the oxacillin-hydrolyzing penicillinase gene. J. Bacteriol. 145:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]