Abstract
We have demonstrated that the bldB gene of Streptomyces coelicolor is required for the formation of aerial hyphae and the synthesis of antibiotics. We also found that BldB forms a higher-order complex (most likely a dimer) and that amino acid residues 20 to 78 are important for this interaction. This region is conserved in the BldB family, suggesting that dimer formation may be a common feature of these proteins.
The life cycle of the filamentous bacterium Streptomyces coelicolor begins with spore germination and the growth of filamentous, branching cells called substrate hyphae. After 24 to 36 h, a second cell type, the aerial hyphae, appears on the colony surface and grows up into the air. The two cell types have completely different fates: substrate hyphae produce secondary metabolites, including two pigmented antibiotics, and the aerial hyphae produce spores (1, 6, 14, 18).
Antibiotic synthesis and the formation of aerial hyphae commence at approximately the same time during colony development, and there is evidence that these events are coordinated at the molecular level. For example, several genes that are dispensable for cell viability are required for both of these developmental events to occur (5, 17, 23). Among these, mutations in the gene bldB have the most severe consequences (5, 17). While the developmental phenotypes of most bld mutants can be at least partially restored by growth on minimal medium containing the carbon source mannitol, bldB mutations block both the formation of aerial hyphae and the synthesis of antibiotics under all growth conditions (5, 17). bldB mutants are also defective in catabolite control (22) and do not fit into the hierarchy of extracellular complementation exhibited by many other bld mutants (19, 20, 21, 29, 30).
The bldB gene has been cloned and shown to encode a 98-amino-acid protein with a molecular mass of 10,899 Da (23) (Fig. 1). There are numerous homologues of bldB in the S. coelicolor genome (11), including abaA and whiJ, which are required for antibiotic synthesis and spore formation, respectively (9, 11, 24). At this time, no convincing bldB homologue has been detected in a nonactinomycete.
FIG. 1.
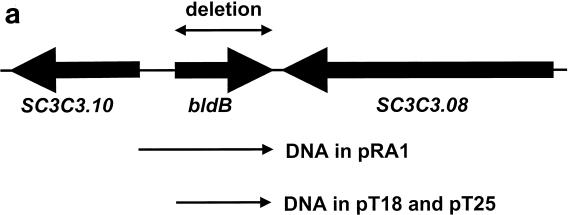
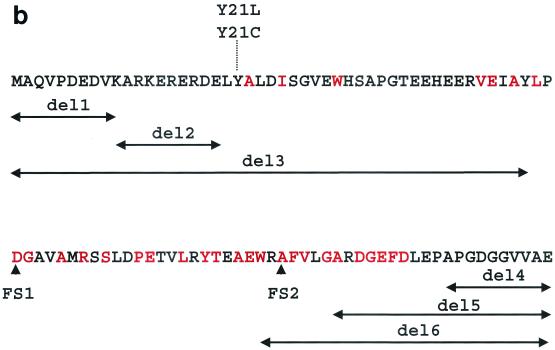
bldB gene and gene product. (a) bldB locus and relevant DNA fragments. The arrows below the chromosomal map indicate the DNA fragments used in bldB complementation (cloned into pRA1) and the two-hybrid analysis (cloned into pT18 and pT25). (b) BldB amino acid sequence, including the mutants tested for dimerization in the two-hybrid analysis. The red letters indicate highly conserved residues (Fig. 5). Deletion mutations (deletions of residues 1 to 10 [del1], 11 to 20 [del2], 1 to 47 [del3], 88 to 98 [del4], 78 to 98 [del5], and 71 to 98 [del6]) are represented by double-headed arrows, and frameshift mutations generated in random mutagenesis are indicated by FS1 and FS2.
The biochemical roles of BldB and its homologues are unknown. The remarkable pleiotropy of bldB mutants could suggest a role in controlling gene expression, and indeed, analysis of the BldB polypeptide sequence suggested that it might include a helix-turn-helix DNA binding motif (23). bldB expression, which is normally low during vegetative growth and increases at the time that aerial hyphae appear, is constitutive in bldB mutants, suggesting that BldB might regulate its own synthesis, like the developmental transcription factor BldD (8). No interaction between BldB and the bldB promoter has been detected in our laboratories, however, suggesting that BldB may affect bldB expression indirectly. The sequence of BldB provides no other clues to its function.
We have constructed a chromosomal deletion of the bldB open reading frame in S. coelicolor strain M145. The phenotype of this null mutant was identical to that of the previously identified point mutants. Using three experimental approaches, we have shown that BldB interacts with itself to form what is probably a dimer. We have further shown that residues in the conserved central core of the polypeptide are essential for this interaction. The similarity of BldB and its homologues suggests that dimerization may be a shared characteristic of these proteins.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains used in this work are listed in Table 1. Escherichia coli was grown on Luria-Bertani medium at 37°C for routine purposes. S. coelicolor was grown at 30°C in yeast extract with malt extract medium or on R2YE solid medium (15). For two-hybrid analysis, E. coli strain DHP-1 (13) was grown on MacConkey agar supplemented with 1% maltose at 30°C. S. coelicolor protoplasts were transformed (15) with unmethylated plasmid DNA isolated from ER2-1 cells. Ampicillin, apramycin, chloramphenicol, and neomycin were used at 100, 50, 25, and 10 μg/ml, respectively.
TABLE 1.
Strains employed in this study
| Strain | Genotype | Reference and/or source |
|---|---|---|
| S. coelicolor | ||
| N985 | bldB::aphI SCP1− SCP2− | This work |
| M145 | Prototroph SCP1− SCP2− | 15 |
| E. coli | ||
| BL21(DE3) | F−dcm ompT hsdS(rB− mB−) gal met λ(DE3) | Novagene |
| ER2-1 | F′ lacIqleuB6 thi-1 fhuA31 lacY1 tsx-78 galK2 galT22 supE44 hisG4 rpsLI36 (Strr) xyl-5 mtl-1 dam13::Tn9(Camr) dcm-6 mcrB1 mcrA hsdR2(rK− mK+) | J. McCormick |
| DHP-1 | F−cya glnv44(AS) recA endA1 gyrA96 Nalrthi-1 hsdR17 spoT1 rfbD1 | 13 |
| XL-1 Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac[F′ proAB lacIq ZΔM15 Tn10(Tetr)] | Stratagene |
| XL-10 Gold | Tetr Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac The [F′ proAB lacIq ZΔM15 Tn10(Tetr) Amy Camr] | Stratagene |
| XL-1 Red | endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac mutD5 mutS mutT Tn10(Tetr) | Stratagene; 7, 12, 26 |
Plasmids, primers, and sequencing.
The plasmids used in this study are listed in Table 2. The Mobix Laboratory at McMaster University performed primer syntheses and DNA sequencing. PCR was performed using Vent DNA polymerase from New England Biolabs and Pfu, Pfu turbo, and Herculase polymerases (Stratagene).
TABLE 2.
Plasmids employed in this study
| Plasmid | Description | Phenotypea | Reference or source |
|---|---|---|---|
| S. coelicolor | |||
| pRA1 | 0.62-kb EcoRI/XbaI amplicon from S. coelicolor containing bldB and its promoter inserted into pSET152 | Aprr | This work |
| pSET152 | lacZα MCSbreppUCoriT φC31 int attP aac(IV)3 | Aprr | 2 |
| pOJ260 | reppUC AmproriT lacZα MCS | Aprr | 2 |
| pBKO | 2-kb HindIII/NdeI amplicon from S. coelicolor chromosome of DNA upstream of bldB; aphI inserted at NdeI site; 1.2-kb NdeI/EcoRI amplicon of DNA downstream of bldB inserted into pOJ260 | Aprr | This work |
| E. coli | |||
| pPCR-Script Amp | Stratagene plasmid based on pBluescript II SK(+); unique SrfI site for insertion of blunt-end amplicons | Ampr | Stratagene |
| pBluescriptNeo | Derived from pBluescript II SK(+); 0.9-kb AseI amplicon containing aphI inserted into pBluescript | Ampr | This work |
| pT18 | bla ori colE1 f1 origin T18 MCS | Ampr | 13 |
| pT25 | cat ori p15A T25 MCS | Chlr | 13 |
| pBB801 | lacI bla ori pBR322; MRGSH6-BldB under T7 promoter/terminator expression | Ampr | R. Seyler |
| pT18BBH | bla ori colE1 f1 origin T18 MCS; 0.32-kb KpnI amplicon from pBB801 containing his6-bldB inserted into pT18 | Ampr | This work |
| pT25BBH | cat ori p15A T25 MCS his6-bldB; 0.32-kb KpnI amplicon from pBB801 containing his6-bldB inserted into pT25 | Chlr | This work |
| pT18NHB | Bla ori colE1 f1 origin T18 MCS; 0.32-kb KpnI amplicon from pBB801 containing bldB inserted into pT18 | Ampr | This work |
| pT25NHB | cat ori p15A T25 MCS his6-bldB; 0.32-kb KpnI amplicon from pBB801 containing bldB inserted into pT25 | Chlr | This work |
Antibiotic resistance markers are apramycin (Aprr), ampicillin (Ampr), and chloramphenicol (Chlr).
MCS, multiple cloning site.
Construction of a bldB null mutant.
Two- (Bup) and 1.2-kb (Bdown) DNA fragments upstream and downstream of bldB were amplified by PCR and introduced into pOJ260 so that they were separated by an NdeI site introduced during amplification. The resulting plasmid was cut with NdeI and ligated to an aphI gene with AseI ends to produce pBKO. Unmethylated pBKO was introduced into protoplasts of S. coelicolor strain M145, and transformants were selected with neomycin. These were screened for sensitivity to apramycin to identify strains in which bldB was replaced with aphI; 2% of the screened transformants exhibited the Neor Aprs phenotype. Chromosomal DNA from these candidates was digested with PstI and subjected to Southern analysis with the Bdown DNA fragment as a probe (25).
Complementation of the bldB null mutant.
A 0.62-kb DNA fragment containing bldB and its promoter region was amplified by PCR with primers Bcomp A and B. This fragment was inserted into pPCR-Script Amp (Stratagene), cut out with EcoRI, and ligated into plasmid pSET152 digested with the same enzyme to generate pRA1. The bldB null mutant N985 and its wild-type parent M145 were transformed with pRA1 and pSET152 and selected for resistance to apramycin. Phenotypic analysis was carried out by growth on solid R2YE medium for 2 days at 30°C.
Purification of BldB.
Plasmid pBB801 is based on pET15b. pET15b was digested with NcoI and BamHI to remove the His tag-encoding sequence. The open reading frame encoding BldB was inserted at these sites, and a His6 tag (MRGSHHHHHH-) was cloned into the NcoI site preceding the N terminus of the BldB coding region. This plasmid with His6-BldB under the control of a T7 promoter was introduced into E. coli BL21(DE3). Two liters of these cells was grown in liquid Luria-Bertani medium at 37°C until the optical density at 600 nm reached 0.6, at which point the cells were induced with 1 mM isopropyl-β-d-thiogalactopyranoside for 3 h. The induced cells were harvested by centrifugation and lysed by passage through a French pressure cell. The lysate was treated with DNase I and RNase A for 30 min, and insoluble material was pelleted by centrifugation. This lysate was applied to a 1-ml nickel sulfate column (Amersham Pharmacia). The column was washed with 50 mM imidazole and 500 mM NaCl in 50 mM Tris-HCl buffer (pH 7.5), and bound proteins were eluted with 0.5 M imidazole and 0.5 M NaCl in 50 mM Tris-HCl buffer (pH 7.5). Protein samples were concentrated, and the buffer was exchanged with 0.2 M NaCl and 1 mM dithiothreitol in 50 mM HEPES buffer (pH 7.5). The protein concentration was determined as described by Bradford (3).
BldB was further purified using a 1- by 30-cm Superdex H-75 column (Amersham Pharmacia). The mobile phase was 0.2 M NaCl and 1 mM dithiothreitol in 50 mM HEPES buffer (pH 7.5), and the flow rate was 0.1 ml/min. Blue dextran was used to determine the void volume. A single peak of BldB was detected, and its identity was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with both Coomassie staining and Western analysis with anti-His6 antibody.
Analytical ultracentrifugation of native BldB.
Velocity sedimentation analysis was performed at the Ontario Cancer Institute. Sedimentation was performed at 20°C in a Beckman XLI analytical ultracentrifuge using an AN50-Ti rotor and sapphire windows. The sedimentation equilibrium experiments using six-channel charcoal-Epon cells were performed at 23,000, 28,000, and 33,000 rpm. Samples were left for 24 h at each centrifugation speed before absorbance measurements were taken. Global analysis of the data was performed using XL-A and XL-I data analysis software (version 4.0) from Beckman Instruments.
Two-hybrid analysis.
The bldB gene, with and without a His6 tag, was amplified from pBB801 with primers HisB A and B and NHB, respectively, to introduce KpnI sites at the ends. These primers replaced the stop codon at the end of bldB with a GGG to permit expression of the carboxy-terminal T18 adenylate cyclase fusion. The amplified products were digested with KpnI and ligated into plasmids pT18 and pT25 digested with KpnI to yield plasmids pT18BBH and -NHB and pT25BBH and -NHB. All four plasmids were introduced into DHP-1, and the resulting cells were tested for a red-colony phenotype.
Mutational analysis of BldB-BldB interaction.
E. coli XL-1 Red (Stratagene) was transformed with pT25BBH. The mutagenized plasmid was isolated and digested with PstI and NcoI to release the mutated bldB gene. This was purified and ligated to fresh pT25BBH. The resulting clones were pooled, and the plasmid was recovered. DHP-1 containing the T18BBH plasmid was transformed with the pooled, mutagenized pT25BBH, and the transformants were screened for white-colony producers. pT25BBH plasmids from these were then retested, and DNA from those mutants that bred true was isolated and sequenced through the bldB region.
Amino-terminal deletions (Fig. 1b) were generated in bldB by PCR amplification with 5′-phosphorylated oligonucleotides. After the amplified products were digested with DpnI, the DNA ends were ligated together using T4 DNA ligase from New England Biolabs. Carboxy-terminal deletions in bldB were generated by the introduction of stop codons at relevant sites in bldB in pT25 using QuikChange mutagenesis (Stratagene). Point mutations altering Y21 were also generated by QuikChange PCR. All mutations were confirmed by sequencing with pT18seq and pT25seq. The mutated plasmids were introduced into DHP-1 cells and tested for BldB-BldB interaction as described above. The primers used for this work are listed in Table 3.
TABLE 3.
Oligonucleotides employed in this study
| Primer | Functiona | Sequences of oligonucleotide primer partnersb |
|---|---|---|
| Bup A and B | Amplification of BldB upstream region from the S. coelicolor chromosome | 5′-CAGGCGGCATATGAACGATCCCCGACAACCTTAC |
| 5′-GTCGAGAAGCTTTCGGGTGAGGCGCTG | ||
| Bdown A and B | Amplification of BldB downstream region from the S. coelicolor chromosome | 5′-CTCGACGAGCTCTGGCAGGGCACGCGC |
| 5′-GCGACGGGGCATATGTCGCCGAGTGAC | ||
| Bcomp A and B | Amplification of BldB and its promoter from the S. coelicolor chromosome | 5′-GCGACGCGAATTCGCTACGTCGCACCG |
| 5′-GTGCTCATCTCGGGTACGGGAGTGGTCCTGC | ||
| HisB A and B | Amplification of His-tagged bldB from pBB801 | 5′-GGAGGGTACCAATGAGAGGATCGCAT |
| 5′-TCGAGGTACCCCCTCGGCGACGACGCC | ||
| NHB | Amplification of non-His-tagged bldB from pBB801 | 5′-ATCAGGTACCAATGGCCCAGGTGCCG |
| pT18seq | Sequencing plasmid pT18 | 5′-GACCATGATTACGCCAAGCG |
| pT25seq | Sequencing plasmid pT25 | 5′-GATTCGGTGACCGATTACCTG |
| NT10 A and B | BldB aa 1-10 deletion in pT25BBH | 5′-GTGATGGTGATGGTGATGCGATC |
| 5′-GCCCGCAAGGAGCGCGAGC | ||
| NT20 A and B | BldB aa 11-20 deletion in pT25BBH | 5′-TTTGACGTCCTCGTCCGGCAC |
| 5′-TACGCGCTCGACATCTCGGGTG | ||
| NTVar | BldB aa 1-47 deletion in pT25BBH | 5′-CTGCCCGACGGAGCCGTGGCCATG |
| CT10 A and B | BldB aa 89-98 deletion in pT25BBH | 5′-ACCTGGAGCCGTAGCCGGGCGAC |
| 5′-GTCGCCCGGCTACGGCTCCAGGT | ||
| CT20 A and B | BldB aa 79-98 deletion in pT25BBH | 5′-TCGTCCTGGGTTAGCGGGACG |
| 5′-CGTCCCGCTAACCCAGGACGA | ||
| Framedel A and B | BldB aa 72-98 deletion in pT25BBH | 5′-GAGGCGGAGTGACGGGCTTTC |
| 5′-GAAAGCCCGTCACTCCGCCTC | ||
| T21L 1 and 2 | T21L mutagenesis in pT18BBH | 5′-CGAGCTGCTCGCGCTCGAC |
| 5′-GTCGAGCGCGAGCAGCTCG | ||
| T21C 1 and 2 | T21C mutagenesis in pT18BBH | 5′-CGAGCTGTGCGCGCTCGAC |
| 5′-GTCGAGCGCGCACAGCTCG |
aa, amino acids.
Nonhomologous regions and introduced restriction sites are in boldface type.
RESULTS AND DISCUSSION
Previously isolated mutations in bldB included two promoter mutations (bldB17 and bldB28), one Shine-Dalgarno mutation (bldB249), and four bldB sequence alterations (17). Of the last four, bldB112 and bldB186 caused gross alterations in the BldB polypeptide and two (bldB15 and bldB43) were point mutants, both of which altered the same amino acid residue: a tyrosine at position 21 (17). These mutants have similar phenotypes: defective morphogenesis and antibiotic synthesis. We constructed a deletion in the S. coelicolor chromosome, replacing the bldB open reading frame with the aphI gene, conferring resistance to the antibiotic neomycin (Fig. 1a; see Materials and Methods). The plates shown in Fig. 2 demonstrate the effects of this mutation. Aerial hyphae were plainly visible as a white fuzzy layer on top of wild-type colonies (M145 containing the control plasmid pSET152). In contrast, none of these spore-forming cells was produced by the bldB null mutant (N985 containing pSET152). Furthermore, the mutant produced a substantially reduced quantity of the pigmented antibiotics. These phenotypic alterations are essentially identical to those reported for the previously isolated point mutations, suggesting that those alterations do indeed prevent normal BldB function altogether.
FIG. 2.
Genetic analysis of bldB in S. coelicolor. The plates show wild-type S. coelicolor strain M145 (left) and the bldB null mutant N985 (middle), both containing the control plasmid pSET152, and the bldB null mutant containing pRA1 (right).
It was unlikely that this phenotype resulted from polar effects on any other genes, as bldB is believed to be monocistronic, and indeed, its downstream neighbor (SC3C3.08) is transcribed in the opposite orientation (23). To confirm the absence of polar effects, we constructed the plasmid pRA1 containing the bldB open reading frame and the 304 bp upstream of it (up to but not including the next predicted open reading frame, Sc3c3.10) that most likely contains its promoter (Fig. 1). We introduced pRA1 and the parent vector, pSET152, as a negative control into wild-type S. coelicolor cells (with no phenotypic effect) and into the strain N985 containing the bldB null mutation. As shown in Fig. 2, while pSET152 had no effect on the phenotype of the null mutant, pRA1 restored morphogenesis and antibiotic synthesis to near-wild-type levels.
To initiate structural analysis of the BldB polypeptide, we expressed it as a 12,154-Da amino-terminal His6 fusion protein. We carried out an initial round of purification using nickel affinity chromatography under nondenaturing conditions and then applied the purified protein to a Superdex H-75 gel filtration column. The BldB protein eluted from this column was judged to be >90% pure (data not shown).
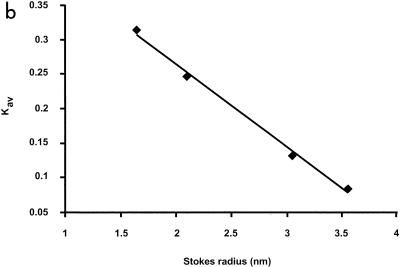
The BldB protein isolated by gel filtration chromatography behaved as though it was much larger than was predicted by its molecular weight. The elution profile of BldB, expressed in terms of milliabsorbance units, is shown in Fig. 3a, compared with those of the four standard proteins albumin (67 kDa), ovalbumin (43 kDa), chymotrypsinogen A (25 kDa), and RNase A (13.7 kDa), which have Stokes radii (rs) of 3.55, 3.05, 2.09, and 1.64 nm, respectively. To our surprise, BldB eluted at 52.5 min, between ovalbumin (50.5 min) and chymotrypsinogen A (59 min) (Fig. 3a), or with a Kav of 0.146 (16). A standard curve of Kav versus rs for the standard proteins (Fig. 3b) suggested an rs for BldB of ∼3.00, an exceptionally high number for a 98-amino-acid-residue protein. This suggested either that BldB was unfolded, and hence migrated more rapidly in gel filtration than it would if folded into a compact, globular shape, or that BldB formed a higher-order complex in solution.
FIG. 3.
In vitro analysis of BldB. (a) Gel filtration profiles of BldB, albumin (67 kDa), ovalbumin (43 kDa), chymotrypsinogen (25 kDa), and RNase A (13.7 kDa). mAU, milliabsorbance units. (b) Standard curve of rs relative to elution profile. Elution volume was expressed in terms of Kav (16), which was determined for each standard protein using the expression (Ve − Vo)/(Ve − Vt), where Ve is the elution volume, Vo is the void volume, and Vt is the total column volume.
To distinguish between these possibilities, we carried out analytical ultracentrifugation analysis with purified BldB (see Materials and Methods). For proteins that behave ideally in solution, this technique allows the calculation of the molecular mass of a protein simply on the basis of its hydrodynamic behavior. BldB did not behave in an ideal manner; however, its predicted molecular mass was 20,581 Da, 1.69-fold greater than what was expected given its sequence (see Materials and Methods). These data were more consistent with BldB oligomerization—an unfolded monomer would have been expected to yield a molecular mass equal to that predicted from the sequence.
Indeed, the results of these hydrodynamic experiments suggested that BldB might form an asymmetric dimer. Proteins having an elongated or highly asymmetric shape migrate more quickly during gel filtration, resulting in exaggerated estimates of their size (10). In contrast, such proteins move more slowly during high-speed centrifugation due to increased friction with buffer molecules, and this can result in an underestimation in the calculated molecular mass (10). The deviation of a protein from a spherical or globular shape can be estimated using the frictional ratio (f/f0), which compares the frictional behavior of a protein with that of an anhydrous sphere of the same molecular mass, and the Perrin factor, which corrects for hydration. Using an rs of 3.00, we calculated the f/f0 for BldB using the equation f/f0 = rs/(3vM/4πNA)1/3, where v is the partial specific volume (0.725 for BldB), M is the molecular mass predicted by the sequence (12,154, 24,308, 36,462, and 48,616 Da for the monomer, dimer, trimer, and tetramer), and NA is Avogadro's number (28). The resulting values were 1.97, 1.57, 1.40, and 1.24, respectively. For most proteins, the partial specific volume is between 0.7 and 0.75 ml/g (26); we assumed a value of 0.725 ml/g for BldB. Using the equation F = f/f0(1 + δ/ρv)−1/3, where f/f0 is the frictional ratio (see above), δ is protein hydration (0.35 ml/g), and ρ is 1 (solvent density estimated to be unity), the Perrin factor for each species was calculated to be 1.73, 1.37, 1.2, and 1.09, respectively, for the monomer, dimer, trimer, and tetramer (4). Folded proteins approximating a sphere typically have f/f0 values between 1.2 and 1.4 and Perrin factors close to 1.0. The values that we have calculated for BldB suggest, therefore, that it is either a monomer of extremely irregular shape, a dimer or trimer of moderately irregular shape, or a tetramer that approximates a perfect sphere. Given the results of the ultracentrifugation experiment and of work described below, we suggest that BldB forms an asymmetric dimer.
To investigate whether the BldB-BldB interaction observed in vitro could occur in vivo and to identify amino acids that are important for this interaction, we used a two-hybrid assay devised by Karimova and coworkers (13). This assay employs the restoration of adenylate cyclase activity to a cya null mutant strain of E. coli called DHP-1. The plasmids pT25 and pT18 encode the 238 amino-terminal residues and the 175 carboxy-terminal residues of the Bordetella pertussis adenylate cyclase catalytic domain, respectively. Genes of interest are fused, in frame, to the gene encoding either fragment of the B. pertussis adenylate cyclase, and the resulting plasmids are introduced into DHP-1. If the expressed fusion proteins interact, the amino- and carboxy-terminal fragments of the adenylate cyclase catalytic domain come together, restoring the synthesis of cyclic AMP. This in turn stimulates the expression of genes in E. coli involved in sugar metabolism, including the maltose regulon. Maltose fermentation can be easily visualized by growth on MacConkey medium containing maltose, where maltose fermentation confers a red-colony phenotype. Colonies unable to ferment maltose are white.
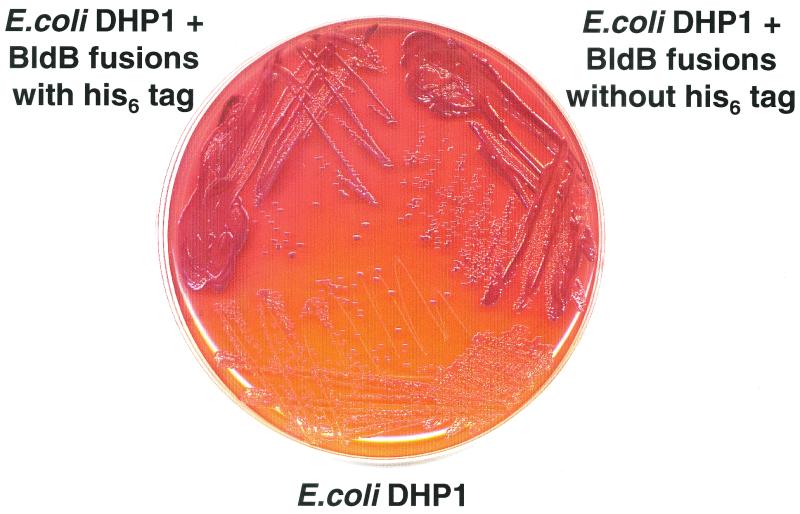
DNAs encoding BldB and BldB with an N-terminal His6 tag were inserted into both pT18 (to produce pT18BBH) and pT25 (to produce pT25BBH). These plasmids were introduced into the E. coli strain DHP-1 and tested for a BldB-BldB interaction (Fig. 4). While strains containing pT18BBH and pT25 or pT25BBH and pT18 had no capacity to ferment maltose, strains containing the two bldB fusions exhibited a strong red-colony phenotype (Fig. 4), suggesting that BldB had brought the two segments of adenylate cyclase together, as predicted by the in vitro analysis. The presence of the His6 tag had no effect on this interaction, and all subsequent work was carried out using the His-tagged fusions.
FIG. 4.
Two-hybrid analysis of BldB-BldB interaction. The cya mutant E. coli strain DHP-1 lacking the two-hybrid plasmids (bottom) or containing BldB cloned into both pT18 and pT25 with (top left) or without (top right) the His6 tag is shown.
To identify the amino acid residues required for the BldB-BldB interaction, we first carried out a random-mutagenesis experiment with the bldB gene in pT25BBH. The fusion plasmid was grown in the E. coli strain XL1-Red, which contains defective mutD, mutS, and mutT genes and therefore has an increased tendency to accumulate point mutations during DNA replication (7, 12, 27). We isolated pT25BBH from the mutagenized plasmid and screened for mutations in bldB that blocked the BldB-BldB interaction in DHP-1 containing pT18BBH. We screened ∼8,000 candidates from the pool of mutagenized genes and identified two bldB mutations. Both contained single-base-pair deletions causing frame shifts: one at position 49 (FS1) and the other at position 73 (FS2) (Fig. 1b). These replaced the last 49 and 25 amino acids of BldB with biologically irrelevant sequences of 54 and 30 amino acid residues, respectively.
To identify smaller fragments of BldB that retained dimerization capacity, we constructed a series of deletion mutations in pT25BBH (Fig. 1b) and tested them for the ability to confer the red-colony phenotype on E. coli strain DHP-1 containing the plasmid pT18BBH. Deletions of residues 1 to 10, 11 to 20, 78 to 98, or 88 to 98 of BldB had no discernible effect on the BldB-BldB interaction. In contrast, larger deletions that removed amino acids 1 to 47 and 71 to 98 both resulted in a loss of the red-colony phenotype, suggesting that these deletions had removed residues required either for BldB folding and stability or for the BldB-BldB interaction. We concluded from this analysis that the 58 amino acid residues at the core of BldB, between residues 20 and 78, were sufficient to bring about dimer formation.
One intriguing feature of the original bldB point mutants is that both alter the tyrosine at position 21, suggesting that this residue is important for BldB activity. To test whether this amino acid is important for dimerization, we introduced mutations into the bldB open reading frames in pT18BBH and pT25BBH to change this amino acid to either leucine or cysteine (the changes in bldB15 and bldB43, respectively) (23). When tested in the E. coli assay, neither of these mutations altered the red-colony phenotype either singly or when the bldB genes in both of the plasmids of the two-hybrid system were altered. This result suggests that Y21 is important for something other than BldB dimerization, the most likely possibility being either interaction with another protein or perhaps phosphorylation.
Finally, we aligned the sequence of BldB with four of its homologues, including AbaA, WhiJ, and two other homologues of unknown function (SCE9.31c and SC7A12.13 in the annotation of the S. coelicolor genome sequence). This alignment (Fig. 5) revealed a high degree of similarity among these polypeptides, including in particular the residues between positions 20 and 78, suggesting that these BldB-like proteins may also form dimers.
FIG. 5.
BldB homologues. The alignment illustrates conserved amino acid residues in BldB, AbaA, WhiJ, and two homologues of unknown function, SCE9.31c (E9.31c) and SCE7A12.13 (7A12.13). Conserved residues are indicated by asterisks.
Acknowledgments
We thank Eric Brown for assistance in the hydrodynamic analysis of the BldB protein.
This work was supported by the National Science and Engineering Research Council of Canada (grant no. 217540-99).
REFERENCES
- 1.Bibb, M. 1996. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 142:1335-1344. [DOI] [PubMed] [Google Scholar]
- 2.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Cantor, C. R., and P. R. Schimmel. 1980. Biophysical chemistry, part II. Techniques for the study of biological structure and function. W. H. Freeman & Co., New York, N.Y.
- 5.Champness, W. C. 1988. New loci required for Streptomyces coelicolor morphological and physiological differentiation. J. Bacteriol. 170:1168-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chater, K. F. 1993. Genetics of differentiation in Streptomyces. Annu. Rev. Microbiol. 47:685-713. [DOI] [PubMed] [Google Scholar]
- 7.Cox, E. C. 1976. Bacterial mutator genes and the control of spontaneous mutation. Annu. Rev. Genet. 10:135-156. [DOI] [PubMed] [Google Scholar]
- 8.Elliot, M. A., and B. K. Leskiw. 1999. The BldD protein from Streptomyces coelicolor is a DNA-binding protein. J. Bacteriol. 181:6832-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Moreno, M. A., A. J. Martin-Triana, E. Martinez, J. Niemi, H. M. Kieser, D. A. Hopwood, and F. Malpartida. 1992. abaA, a new pleiotropic regulatory locus for antibiotic production in Streptomyces coelicolor. J. Bacteriol. 174:2958-2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freifelder, D. 1984. Physical biochemistry. Applications to biochemistry and molecular biology, 2nd ed. W. H. Freeman & Co., New York, N.Y.
- 11.Gehring, A. M., J. R. Nodwell, S. M. Beverley, and R. Losick. 2000. Genomewide insertional mutagenesis in Streptomyces coelicolor reveals additional genes involved in morphological differentiation. Proc. Natl. Acad. Sci. USA 97:9642-9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glickman, B. W., and M. Radman. 1979. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc. Natl. Acad. Sci. USA 77:1063-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karimova, G., J. Pidoux, A. Ullmann, and D. Ladant. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA 95:5752-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelemen, G. H., and M. J. Buttner. 1998. Initiation of aerial mycelium formation in Streptomyces. Curr. Opin. Microbiol. 1:656-662. [DOI] [PubMed] [Google Scholar]
- 15.Kieser, T., M. J. Bibb, M. J. Buttner, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. John Innes Centre, Norwich, England.
- 16.Laurent, T. C., and J. Killander. 1964. A theory of gel filtration and its experimental verification. J. Chromatogr. 14:317-330. [Google Scholar]
- 17.Merrick, M. J. 1976. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J. Gen. Microbiol. 96:299-315. [DOI] [PubMed] [Google Scholar]
- 18.Miguelez, E. M., C. Hardisson, and M. B. Manzanal. 2000. Streptomycetes: a new model to study cell death. Int. Microbiol. 3:153-158. [PubMed] [Google Scholar]
- 19.Nodwell, J. R., K. McGovern, and R. Losick. 1996. An oligopeptide permease responsible for the import of an extracellular signal governing aerial mycelium formation in Streptomyces coelicolor. Mol. Microbiol. 22:881-893. [DOI] [PubMed] [Google Scholar]
- 20.Nodwell, J. R., and R. Losick. 1998. Purification of an extracellular signaling molecule involved in production of aerial mycelium by Streptomyces coelicolor. J. Bacteriol. 180:1334-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nodwell, J. R., M. Yang, D. Kuo, and R. Losick. 1999. Extracellular complementation and the identification of additional genes involved in aerial mycelium formation in Streptomyces coelicolor. Genetics 151:569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pope, M. K., B. D. Green, and J. Westpheling. 1996. The bld mutants of Streptomyces coelicolor are defective in the regulation of carbon utilization, morphogenesis and cell-cell signalling. Mol. Microbiol. 19:747-756. [DOI] [PubMed] [Google Scholar]
- 23.Pope, M. K., B. Green, and J. Westpheling. 1998. The bldB gene encodes a small protein required for morphogenesis, antibiotic production, and catabolite control in Streptomyces coelicolor. J. Bacteriol. 180:1556-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryding, N. J., M. J. Bibb, V. Molle, K. C. Findlay, K. F. Chater, and M. J. Buttner. 1999. New sporulation loci in Streptomyces coelicolor A3(2). J. Bacteriol. 181:5419-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Schachman, H. K. 1957. Ultracentrifugation, diffusion, and viscometry. Methods Enzymol. 4:32-71. [Google Scholar]
- 27.Scheuermann, R., S. Tam, P. M. Burgers, C. Lu, and H. Echols. 1983. Identification of the epsilon-subunit of Escherichia coli DNA polymerase III holoenzyme as the dnaQ gene product: a fidelity subunit for DNA replication. Proc. Natl. Acad. Sci. USA 80:7085-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel, L. M., and K. J. Monty. 1966. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim. Biophys. Acta 112:346-362. [DOI] [PubMed] [Google Scholar]
- 29.Willey, J., R. Santamaria, J. Guijarro, M. Geistlich, and R. Losick. 1991. Extracellular complementation of a developmental mutation implicates a small sporulation protein in aerial mycelium formation by Streptomyces coelicolor. Cell 65:641-650. [DOI] [PubMed] [Google Scholar]
- 30.Willey, J., J. Schwedock, and R. Losick. 1993. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 7:895-903. [DOI] [PubMed] [Google Scholar]