Abstract
Biosynthesis of the isoprenoid precursor isopentenyl diphosphate (IPP) proceeds via two distinct pathways. Sequence comparisons and microbiological data suggest that multidrug-resistant strains of gram-positive cocci employ exclusively the mevalonate pathway for IPP biosynthesis. Bacterial mevalonate pathway enzymes therefore offer potential targets for development of active site-directed inhibitors for use as antibiotics. We used the PCR and Enterococcus faecalis genomic DNA to isolate the mvaS gene that encodes 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) synthase, the second enzyme of the mevalonate pathway. mvaS was expressed in Escherichia coli from a pET28 vector with an attached N-terminal histidine tag. The expressed enzyme was purified by affinity chromatography on Ni2+-agarose to apparent homogeneity and a specific activity of 10 μmol/min/mg. Analytical ultracentrifugation showed that the enzyme is a dimer (mass, 83.9 kDa; s20,w, 5.3). Optimal activity occurred in 2.0 mM MgCl2 at 37oC. The ΔHa was 6,000 cal. The pH activity profile, optimum activity at pH 9.8, yielded a pKa of 8.8 for a dissociating group, presumably Glu78. The stoichiometry per monomer of acetyl-CoA binding was 1.2 ± 0.2 and that of covalent acetylation was 0.60 ± 0.02. The Km for the hydrolysis of acetyl-CoA was 10 μM. Coupled conversion of acetyl-CoA to mevalonate was demonstrated by using HMG-CoA synthase and acetoacetyl-CoA thiolase/HMG-CoA reductase from E. faecalis.
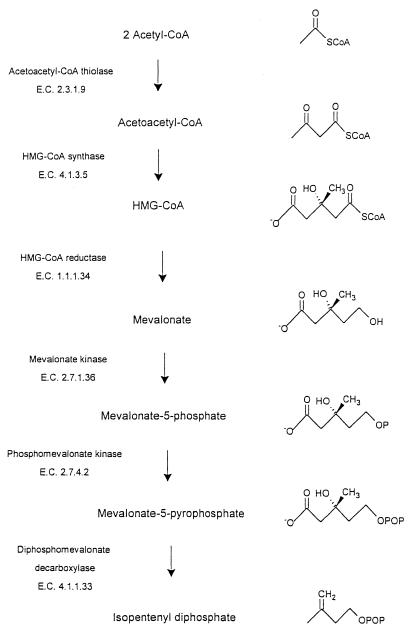
Two distinct pathways, the mevalonate pathway (Fig. 1) and the nonmevalonate pathway (20), give rise to isopentenyl diphosphate (IPP), the monomer unit for isoprenoid biosynthesis. Mammals and the archaea studied use exclusively the mevalonate pathway (3), whereas plants employ both pathways (1). Inspection of the sequences of microbial genomes has revealed that, whereas many gram-negative eubacteria contain genes that encode enzymes of the nonmevalonate pathway, the gram-positive cocci, Borrelia burgdorferi (27), and Streptomyces species (10, 24) possess genes that appear to encode the enzymes of the mevalonate pathway.
FIG. 1.
Intermediates and enzymes of the mevalonate pathway for IPP biosynthesis.
The mevalonate pathway is essential for the survival of representative gram-positive cocci. Wilding et al. (28) established that the survival of knockout mutants of the genes that encode 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (EC 1.1.1.34) of Streptococcus pneumoniae and Staphylococcus aureus depended on externally supplied mevalonate. While the mevalonate pathway is also essential for human subjects, there are two classes of HMG-CoA reductase that differ both with respect to their structure and their inhibition characteristics (4). In addition, there may be significant differences between the bacterial and human forms of other enzymes of the pathway. The enzymes of the mevalonate pathway may therefore represent potential targets for the development of new antibiotics.
An unusual feature of the mevalonate pathway of Enterococcus faecalis is that a dual-function polypeptide, acetoacetyl-CoA thiolase/HMG-CoA reductase (11, 27), catalyzes the first and third reactions of the pathway (Fig. 1). The second reaction of the mevalonate pathway, the functionally irreversible condensation of acetoacetyl-CoA and acetyl-CoA to form HMG-CoA, is catalyzed by HMG-CoA synthase (EC 4.1.3.5). Our interest in E. faecalis HMG-CoA synthase, which appears to link the activities of the dual-function E. faecalis enzyme, reflects both the potential of the mevalonate pathway enzymes of enterococci as targets for antibiotics and the fact that no bacterial HMG-CoA synthase has previously been characterized.
MATERIALS AND METHODS
Reagents.
Purchased reagents and kits included T4 DNA ligase, Vent DNA polymerase, and restriction enzymes (New England Biolabs); deoxynucleoside triphosphates (Invitrogen); 40% acrylamide (Amresco); Bradford reagent and MicroSpin BioP-30 columns (Bio-Rad); IPTG (isopropyl-β-d-thiogalactopyranoside; Gibco-BRL); glass fiber filters (Millipore); [1-14C]acetyl-CoA (Amersham-Pharmacia); and nickel-nitrilotriacetic acid (Ni-NTA) agarose, a QIAprep spin miniprep kit, and a gel extraction kit (Qiagen). E. faecalis chromosomal DNA was a gift of Michael Gwynn at GlaxoSmithKline. Synthetic oligonucleotides were prepared by Integrated DNA Technologies, Coralville, Iowa. E. faecalis acetoacetyl-CoA thiolase/HMG-CoA reductase was prepared as previously described (11). Unless otherwise specified, all other reagents were from Sigma.
Construction of the expression plasmid.
The mvaS gene was PCR amplified from E. faecalis chromosomal DNA with primers that introduced an NdeI site (5′-CGTAAAGGAGTTAAACATATGACAATTGGG-3′) and a BamHI site (5′-GAATCGGGGGATCCAAATACTTAGTTCG-3′) at the ends of the amplified fragment. The 1,176-bp PCR fragment was cloned into NdeI/BamHI-cut pET28 in frame with the N-terminal histidine tag and thrombin cleavage site to give the expression plasmid MvaS-pET28-6H. The insert was sequenced by the Iowa State University DNA Sequencing Facility, Ames, to verify that no mutations had been introduced during PCR amplification.
Expression and purification of the gene product.
Escherichia coli BL21(DE3) cells transformed with MvaS-pET28-6H were grown at 37°C, with shaking, on Luria-Bertani broth (22) containing 10 μg of kanamycin per ml. Cells harvested by centrifugation were washed with 0.9% saline, suspended in Buffer A (10 mM imidazole, 300 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol, 20 mM HEPES; pH 8.0), and lysed in a French press. The supernatant liquid (cytosol) obtained after centrifugation of the cell lysate in a Beckman L8-70 ultracentrifuge (30,000 rpm, 60 min, 4°C) was applied to an Ni-NTA column. The column was washed with Buffer A and then eluted successively with 50 and 100 mM imidazole in Buffer A. Fractions with high activity were combined and stored in liquid nitrogen. Protein concentration was determined by the Bradford method (5).
Analytical ultracentrifugation.
E. faecalis HMG-CoA synthase, i.e., 430 μl of a 1-mg/ml solution in 100 mM NaCl-10 mM Tris (pH 8.0), was loaded in a Beckman analytical ultracentrifuge cell with sapphire windows and an aluminum-filled epoxy centerpiece. The cell was placed in a Beckman Model XL-I centrifuge and allowed to come to thermal equilibrium at 20°C for 1 h. The sample was then spun at 50,000 rpm for 4 h. Rayleigh interference scans were taken at 1-min intervals.
HMG-CoA synthase activity.
Determination of HMG-CoA synthase activity employed a Hewlett-Packard model 8452 diode array spectrophotometer with the cell compartment maintained at 37°C to monitor the change in absorbance at 302 nm that accompanies the acetyl-CoA-dependent disappearance of the enolate form of acetoacetyl-CoA (21). The standard assay medium included 500 μM acetyl-CoA, 20 μM acetoacetyl-CoA, 5.0 mM MgCl2, and 50 mM Tris (pH 9.75). Assays employed a final volume of 220 μl. One enzyme unit (eu) represents the disappearance in 1 min of 1 μmol of acetoacetyl-CoA. The reported data represent mean values for at least triplicate determinations.
Acetyl-CoA hydrolase activity.
Acetyl-CoA hydrolase activity was determined by measuring the release of coenzyme A with DTNB [5,5′-dithiobis(2-nitrobenzoic acid)]. The assay employed a Hewlett-Packard model 8452 diode array spectrophotometer with the cell compartment maintained at 37°C to monitor the change in absorbance at 412 nm that accompanies the reaction of CoA with DTNB. The assay employed modifications of the procedures of Weitzman (26) and Miziorko et al. (19). Standard assay conditions consisted of 1 mM DTNB, 20 μM acetyl-CoA, and 50 mM Tris (pH 9.8) in a final volume of 220 μl. One eu represents the release in 1 min of 1 μmol of CoA. The reported data represent mean values for at least triplicate determinations.
RESULTS
Expression and purification of the gene product of E. faecalis mvaS.
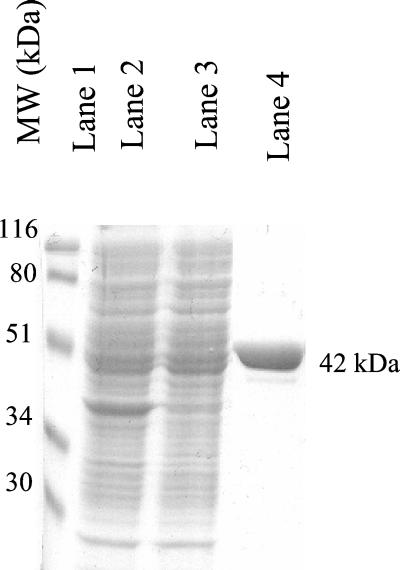
The mvaS gene of E. faecalis, which encodes HMG-CoA synthase, was cloned into pET28 in frame with an N-terminal histidine tag. A soluble protein was expressed in E. coli to high levels at 37°C and was readily purified 20-fold on a nickel-chelating column. The yield was 4 mg per liter of >98% homogeneous protein, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2).
FIG. 2.
SDS-PAGE of fractions obtained during purification of E. faecalis HMG-CoA synthase. Lane 1, prestained standards of the indicated molecular weight; lane 2, cell extract; lane 3, cytosol; lane 4, Ni-NTA fraction.
Sequence alignments.
Animal HMG-CoA synthases are cytosolic (cholesterogenic) or mitochondrial (ketogenic). CLUSTALW (www.expasy.ch) was used to align the amino acid sequences of HMG-CoA synthases from known animal and putative bacterial forms of the enzyme. The cysteine (17), histidine (16), and glutamate (8) implicated as catalytic residues in the avian enzyme are conserved across all forms of the enzyme (Table 1). The sequence identities were 75 and 80% for the mitochondrial and cytosolic isozymes, respectively, and ca. 50% across the two classes. The bacterial HMG-CoA synthases share >20% identity among themselves but only 10% identity with the animal enzymes. The relatively low identity between the bacterial and animal forms might indicate differences in their active sites that might ultimately be exploited for antibiotic design.
TABLE 1.
Selected sequences and conserved residues of animal and bacterial HMG-CoA synthasesa
| Sequence set and sourceb | Sequence (residue position) |
|---|---|
| Set 1 | (95) |
| Animal | LEVGTETIIDKSKxVK |
| Bacterial | VIVATESxIDxxKAxx |
| Set 2 | (129) |
| Animal | DTTNACYGGT |
| Bacterial | ExxxACYxAT |
| Set 3 | (264) |
| Animal | MIFHxPxxK |
| Bacterial | xxFHxPxCK |
Capital letters indicate conserved residues. Residues in boldface are conserved in both animal and bacterial HMG-CoA synthases. An “x” indicates a nonconserved residue. Glu95 (8), Cys129 (17), and His264 (16) have been identified as catalytic residues in the avian enzyme. For E. faecalis HMG-CoA synthase these residues are Glu79, Cys111, and His233.
Animal cytosolic HMG-CoA synthases aligned are from rats, hamsters, humans, and chickens. Animal mitochondrial HMG-CoA synthases aligned are from mouse, rat, human, and pig. Aligned bacterial HMG-CoA synthase sequences are from Streptococcus pneumoniae, Streptococcus pyogenes, Enterococcus faecalis, Enterococcus faecium, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus aureus, and a Streptomyces sp.
Multimeric state.
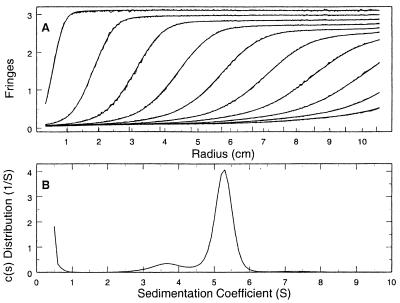
The multimeric state of E. faecalis HMG-CoA synthase was investigated by analytical ultracentrifugation and gel filtration chromatography. Approximately 90% of the total protein had a sedimentation constant of ca. 5.3 S (Fig. 3), which is consistent with a molecular mass of 83.9 kDa. The dimer mass calculated from the amino acid sequence is 84.3 kDa. Material migrating at 3.6 S probably represents the monomer, although the calculated mass of 45 kDa is somewhat larger than the predicted size of 42 kDa. Gel filtration on a Superdex 200 column confirmed these results (data not shown.)
FIG. 3.
Sedimentation velocity ultracentrifugation of E. faecalis HMG-CoA synthase. (A) Sedimentation boundaries measured by using Rayleigh interference optics plotted against radial position. The data shown are for 25-min intervals and represent one-fifth of the data used in the analysis. The jagged lines represent the observed fringes, and the smooth lines represent the calculated best-fit distribution calculated by using Sedfit 8.3 and the Lamm equation model (23). (B) Best-fit c(s) sedimentation coefficient distribution, allowing for systematic time-invariant noise. The uncorrected values for the sedimentation coefficients for the minor and major peaks are 3.6 and 5.3 S, respectively.
Effect of temperature, magnesium ion concentration, and hydrogen ion concentration.
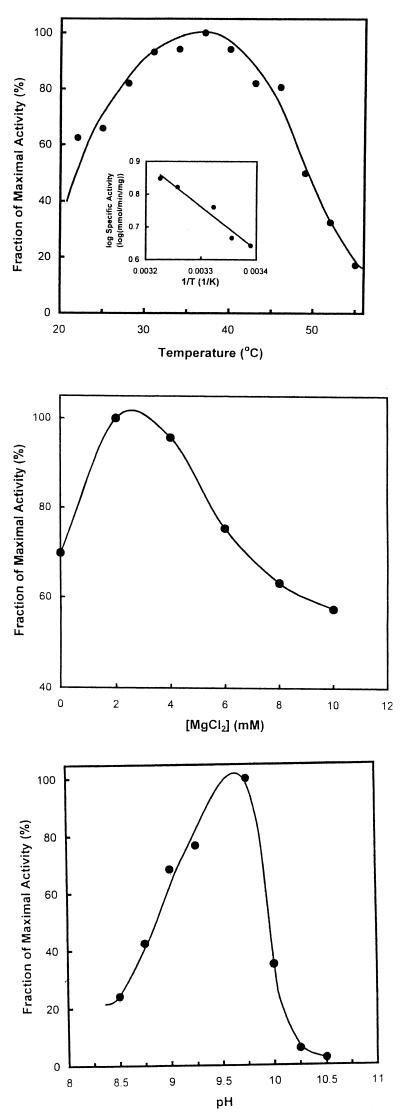
Enzymes of the mevalonate pathway are present in mesophiles, thermophiles, and halophiles. We therefore investigated the effect of temperature, magnesium ion concentration, and hydrogen ion concentration on E. faecalis HMG-CoA synthase activity. Optimum activity occurred at ca. 37°C (Fig. 4, top). A ΔHa of 6,000 cal was calculated from the Arrhenius plot (Fig. 4, top [inset]). Activity was optimal at 2 mM MgCl2 (Fig. 4, middle). Neither 5 to 10 mM EDTA nor 2 to 10 mM KCl affected activity. Optimal activity occurred at pH 9.8 (Fig. 4, bottom). Modeling of the data for pH values from pH 8.5 to 9.25 gave an estimated value for the pKa of a dissociating group of 8.8.
FIG. 4.
Effect of temperature, MgCl2 concentration, and hydrogen ion concentration. (Top) Temperature. Assays were conducted at the indicated temperatures under otherwise standard conditions. The inset shows selected data shown as an Arrhenius plot. (Middle) MgCl2.Assays were conducted at the indicated concentrations of MgCl2 under otherwise standard conditions. (Bottom) Hydrogen ion concentration. Assays were conducted in 50 mM sodium acetate, 50 mM glycine, 50 mM Tris, and 50 mM 2-(N-morpholino)ethanesulfonic acid at the indicated pH under otherwise standard conditions.
Kinetic parameters.
For synthesis of HMG-CoA, the Km for acetyl-CoA was 350 μM. Since acetoacetyl-CoA competes with acetyl-CoA for the catalytic cysteine, only a Kmapp can be determined (14). At a concentration of 500 μM acetyl-CoA, the Kmapp for acetoacetyl-CoA was 10 μM. Table 2 summarizes these kinetic parameters.
TABLE 2.
Kinetic constants for selected HMG-CoA synthases
Detection of an acetyl-S-enzyme intermediate.
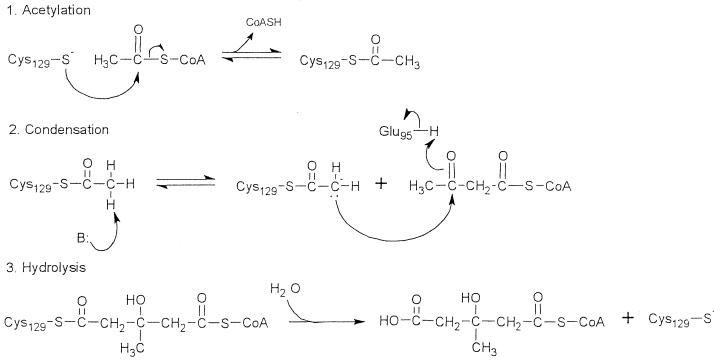
Formation of HMG-CoA proceeds in three stages (Fig. 5), the first and third of which can be studied experimentally. Detection of covalently bound acetyl-CoA documents the formation of an acetyl-S-enzyme intermediate in stage 1. Determination of the stoichiometry of acetyl-CoA bound indicates the number of active sites. Covalent acetylation of HMG-CoA synthase was determined by the procedure of Miziorko et al. (19), and acetyl-CoA binding was determined by the procedure of Vollmer et al. (25). E. faecalis HMG-CoA synthase bound 1.2 ± 0.2 mol of acetyl-CoA per mol of active sites, 60% of which were covalently associated with the enzyme (Table 3). All of the active sites appear to be associated with acetyl-CoA, and at least half of the sites contain an acetyl-S-intermediate.
FIG. 5.
Proposed mechanism for catalysis of the HMG-CoA synthase reaction. Active site residues of the avian mitochondrial enzyme include Glu95, Cys129, and His264. During the acetylation step, Cys129 attacks the carbonyl group of acetyl-CoA, forming an acetyl-S-intermediate (17). After the addition and condensation of acetoacetyl-CoA, HMG-CoA is released by hydrolysis. Glu95 acts as the general acid in the condensation step (8), and His264 anchors binding of acetoacetyl-CoA (16).
TABLE 3.
Parameters for the partial reactions that model the first and third stages of the overall reaction catalyzed by HMG-CoA synthase
| Source (reference) | Acetyl-CoA bound (mol/mol of monomer)a | Acetyl-CoA covalently bound (mol/mol of monomer)b | Hydrolysis of acetyl-CoA
|
|
|---|---|---|---|---|
| Vmax (eu/mg) | Km (μM) | |||
| E. faecalis | 1.2 ± 0.2c | 0.60 ± 0.02c | 0.02 | 8.5 |
| Avian liver cytosol (7, 8) | 1.1 | 0.63 | 0.016 | 11 |
To determine the stoichiometry of acetyl-CoA binding, 100 μg of HMG-CoA synthase and 0.5 mM or 1 mM [1-14C]acetyl-CoA (specific activity, 150 cpm/nmol) in 50 mM Tris (pH 9.75) were combined in a volume 70 μl and incubated at room temperature for 5 min. The entire reaction mixture was then applied to a MicroSpin BioP-30 gel filtration column and centrifuged for 4 min at 1,000 × g. The filtrate was then assayed for protein concentration (5) and radioactivity. The stoichiometry was determined by dividing the moles of acetyl-CoA by the moles HMG-CoA synthase monomer present.
The stoichiometry of covalently bound acetyl-CoA is presented. HMG-CoA synthase (100 μg), 200 μg of bovine serum albumin as a carrier protein, and 0.5, 1.0, 2.0, or 3.0 mM [1-14C]acetyl-CoA (specific activity, 150 cpm/nmol) in 50 mM Tris buffer (pH 9.75) at a final volume of 150 μl were mixed on ice. After 2 min, 1 ml of 10% trichloroacetic acid was added, and each incubation mixture was applied to a glass fiber filter. Filters were washed with 10% trichloroacetic acid and then with 1 ml of ice-cold absolute ethanol, allowed to dry, and counted. The stoichiometry of covalently bound acetyl-CoA was determined by dividing the moles of acetyl-CoA by the moles HMG-CoA synthase monomer present.
Mean ± the standard deviation.
Hydrolytic release of enzyme-bound acetyl-CoA.
The hydrolytic release of enzyme-bound HMG-CoA cannot readily be measured directly because the overall reaction is irreversible. Stage 3 can, however, be modeled by the hydrolysis of the acetylated enzyme, albeit at only 1% the rate of the overall reaction (7, 19). A Km of 10 μM for acetyl-CoA and a Vmax of 2 × 10−2 μmol/min/mg were determined for the hydrolysis of acetyl-CoA. These parameters approximate those previously reported for avian HMG-CoA synthase (Table 3).
Coupled conversion of acetyl-CoA to mevalonate.
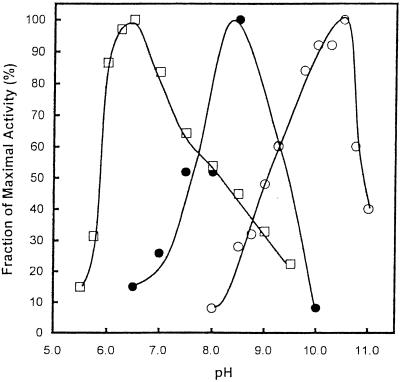
The first and third reactions of the mevalonate pathway in E. faecalis, the synthesis of acetoacetyl-CoA and the formation of mevalonate, are catalyzed by a single bifunctional enzyme (11). Since HMG-CoA synthase catalyzes the second reaction of the pathway, we investigated the coupled conversion of acetyl-CoA to mevalonate. The pH optima for HMG-CoA synthase, acetoacetyl-CoA thiolase, and HMG-CoA reductase range from pH 6.5 to 10.5. We therefore studied the coupled conversion of acetyl-CoA to mevalonate at several pH values. Coupled conversion was indeed observed. Optimal synthesis of mevalonate from acetyl-CoA occurred at pH 8.5 (Fig. 6).
FIG. 6.
Coupled conversion of acetyl-CoA to mevalonate catalyzed by E. faecalis acetoacetyl-CoA thiolase/HMG-CoA reductase and HMG-CoA synthase. Shown is the effect of hydrogen ion concentration on the conversion of acetyl-CoA to acetoacetyl-CoA (○), of HMG-CoA to mevalonate (□), and of acetyl-CoA to mevalonate (•). All assays employed 50 mM sodium acetate, 50 mM glycine, 50 mM Tris, and 50 mM 2-(N-morpholino)ethanesulfonic acid at the indicated pH. Assays of acetoacetyl-CoA thiolase and HMG-CoA reductase activity were conducted essentially as previously described (11), but under the above conditions. For the conversion of acetyl-CoA to mevalonate, the additions were: 1 mM acetyl-CoA, 0.4 mM NADPH, 9 nM E. faecalis acetoacetyl-CoA thiolase/HMG-CoA reductase subunit, and 16 nM E. faecalis HMG-CoA synthase.
DISCUSSION
Only recently has it been possible to deduce from inspection of the sequences of bacterial genomes that gram-positive bacteria employ the mevalonate pathway to synthesize IPP (27). Of the enzymes of the mevalonate pathway in enterococci, only S. aureus HMG-CoA reductase (28) and the acetoacetyl-CoA thiolase/HMG-CoA reductase of E. faecalis (11) have been expressed and characterized. We report here the cloning, expression, purification, and characterization of the first bacterial HMG-CoA synthase.
Analytical ultracentrifugation and gel filtration revealed that E. faecalis HMG-CoA synthase is a dimer and thus has the same multimeric state as other HMG-CoA synthases (14). Optimal activity occurred at 37°C in the presence of 2.0 mM MgCl2 (Fig. 4), which stabilizes acetoacetyl-CoA (9). The free energy of activation, ΔHa, was 6,000 cal. A pKa of 8.8 was estimated from the effect of hydrogen ion concentration on activity (Fig. 4). This value is similar to the pKa of 8.6 reported for the avian enzyme (15) that was subsequently attributed to Glu95 and to its presumably hydrophobic environment (8). Glu95, and by inference its conjugate residue Glu78 of the E. faecalis enzyme, acts as a general acid during the condensation stage of the HMG-CoA synthase reaction (Fig. 5).
As for the avian enzyme (18), the stoichiometry of acetyl-CoA binding was 1.2 ± 0.2 per monomer and of covalent acetylation was 0.60 ± 0.02 per monomer (Table 3). The difference in stoichiometry may indicate that the presence of an acetyl-S-enzyme intermediate at one active site may induce conformational changes that preclude simultaneous formation of an acetyl-S-enzyme intermediate at the other site. Km for the hydrolysis of acetyl-CoA, a reaction that models the third or hydrolytic stage of the overall reaction, was 10 μM. The Vmax of 10 eu/mg and the specific activity of 6 μmol/min/mg of protein for E. faecalis HMG-CoA synthase are only twofold greater than those for the avian enzyme (Table 2). However, the 10 μM Kmapp for acetoacetyl-CoA, which is almost an order of magnitude higher than that for the avian enzyme, may be indicative of subtle differences at their active sites.
The overall conversion of acetyl-CoA to mevalonate comprises the first three reactions of the mevalonate pathway (Fig. 1). The coupled conversion of acetyl-CoA to mevalonate was demonstrated by using the dual enzyme acetoacetyl-CoA thiolase/HMG-CoA reductase and HMG-CoA synthase of E. faecalis. Optimal activity for the coupled reactions occurred at pH 8.5.
The enzymes of the mevalonate pathway of IPP biosynthesis represent potential targets for metabolic intervention. The mevalonate pathway is essential for the survival of S. aureus and, by inference, for other gram-positive bacteria (28). Although the mevalonate pathway is also essential for human subjects, there are two classes of HMG-CoA reductase that differ both with respect to their structure and their inhibition characteristics (4). The Ki values for statin drug inhibition of the class I HMG-CoA reductases of eukaryotes are nanomolar (2) but are millimolar for the class II HMG-CoA reductases of S. aureus (28) and E. faecalis (11). Significant differences also characterize the crystal structures of the class I human enzyme (12) and the class II enzyme of Pseudomonas mevalonii (13). It thus may be possible to develop inhibitors, i.e., “class II statins,” specific to class II HMG-CoA reductases. Furthermore, the existence of two classes of one enzyme of the mevalonate pathway suggests that significant differences may also characterize the bacterial and human forms of additional enzymes of the pathway. Although the glutamate (8), cysteine (17), and histidine (15) implicated as participating in catalysis in the avian form of the enzyme are conserved in E. faecalis HMG-CoA synthase (Table 1), the bacterial HMG-CoA synthases and their animal counterparts exhibit only ca. 10% overall sequence identity. The sequences of bacterial HMG-CoA synthases also cluster away from those of the eukaryotic synthases (27). Although low sequence identity and remote clustering of the enzymes from different kingdoms are indicative of different classes of HMG-CoA synthase, this inference must await confirmation by detailed structural investigations.
Acknowledgments
This research was funded by American Heart Association grant 0150503N (V.W.R.) and by National Institutes of Health grants HL 47113 (V.W.R.) and 52115 (C.V.S.).
We thank Michael Gwynn and Imogen Wilding of Glaxo SmithKline for the gift of E. faecalis genomic DNA and Kevin Lehnbeuter for technical assistance.
Footnotes
Journal paper 16732 from the Purdue Agricultural Experiment Station.
REFERENCES
- 1.Bach, T. J. 1995. Some new aspects of isoprenoid biosynthesis in plants: a review. Lipids 30:191-202. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff, K. M., and V. W. Rodwell. 1996. 3-Hydroxy-3-methylglutaryl-CoA reductase from Haloferax volcanii: purification, characterization, and expression in Escherichia coli. J. Bacteriol. 178:19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochar, D. A., J. A. Friesen, C. V. Stauffacher, and V. W. Rodwell. 1999. Biosynthesis of mevalonic acid from acetyl-CoA, p. 15-44. In David Cane (ed.), Isoprenoids including carotenoids and steroids. Pergamon Press, Oxford, United Kingdom.
- 4.Bochar, D. A., C. V. Stauffacher, and V. W. Rodwell. 1999. Sequence comparisons reveal two classes of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol. Genet. Metab. 66:122-127. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Cabano, J., C. Buesa, F. G. Hegardt, and P. F. Marrero. 1997. Catalytic properties of recombinant 3-hydroxy-3-methylglutaryl coenzyme A synthase-1 from Blattella germanica. Insect Biochem. Mol. Biol. 27:499-505. [DOI] [PubMed] [Google Scholar]
- 7.Chun, K. Y., D. A. Vinarov, and H. M. Miziorko. 2000. 3-Hydroxy-3-methylglutaryl-CoA synthase: participation of invariant acidic residues in formation of the acetyl-S-enzyme reaction intermediate. Biochemistry 39:14670-14681. [DOI] [PubMed] [Google Scholar]
- 8.Chun, K. Y., D. A. Vinarov, J. Zajicek, and H. M. Miziorko. 2000. 3-Hydroxy-3-methylglutaryl-CoA synthase: a role for glutamate 95 in general acid/base catalysis of C-C bond formation. J. Biol. Chem. 275:17946-17953. [DOI] [PubMed] [Google Scholar]
- 9.Clinkenbeard, K. D., T. Sugiyama, and M. D. Lane. 1975. Cystolic 3-hydroxy-3-methylglutaryl-CoA synthase from chicken liver. Methods Enzymol. 35:160-167. [PubMed] [Google Scholar]
- 10.Hamano, T., T. Dairi, M. Yamamoto, T. Kawasaki, H. Kaneda, T. Kuzuyama, N. Itoh, and H. Seto. 2001. Cloning of a gene cluster encoding enzymes responsible for the mevalonate pathway from a terpenoid antibiotic-producing Streptomyces strain. Biosci. Biotechnol. Biochem. 65:1627-1635. [DOI] [PubMed] [Google Scholar]
- 11.Hedl, M., A. Sutherlin, E. I. Wilding, M. Mazzulla, D. McDevitt, P. Lane, J. W. Burgner II, K. R. Lehnbeuter, C. V. Stauffacher, M. N. Gwynn, and V. W. Rodwell. 2002. Enterococcus faecalis acetoacetyl-CoA thiolase/3-hydroxy-3-methylglutaryl coenzyme A reductase, a dual-function protein of isopentenyl diphosphate biosynthesis. J. Bacteriol. 184:2116-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Istvan, E. S., M. Palnitkar, S. K. Buchanan, and J. Deisenhofer. 2000. Crystal structure of the catalytic portion of human HMG-CoA reductase: insights into regulation of activity and catalysis. EMBO J. 19:819-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence, C. M., V. W. Rodwell, and C. V. Stauffacher. 1995. Crystal structure of Pseudomonas mevalonii HMG-CoA reductase at 3.0 angstrom resolution. Science 268:1758-1762. [DOI] [PubMed] [Google Scholar]
- 14.Lowe, D. M., and P. K. Tubbs. 1985. 3-Hydroxy-3-methylglutaryl-coenzyme A synthase from ox liver. Biochem. J. 227:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra, I., H. A. Charlier, Jr., and H. M. Miziorko. 1995. Avian cytosolic 3-hydroxy-3-methylglutaryl-CoA synthase: evaluation of the role of cysteines in reaction chemistry. Biochim. Biophys. Acta 1247:253-259. [DOI] [PubMed] [Google Scholar]
- 16.Misra, I., and H. M. Miziorko. 1996. Evidence for the interaction of avian 3-hydroxy-3-methylglutaryl-CoA synthase histidine 264 with acetoacetyl-CoA. Biochemistry 35:9610-9616. [DOI] [PubMed] [Google Scholar]
- 17.Misra, I., C. Narasimhan, and H. M. Miziorko. 1993. Avian 3-hydroxy-3-methylglutaryl-CoA synthase: characterization of a recombinant cholesterogenic isozyme and demonstration of the requirement for a sulfhydryl functionality in formation of the acetyl-enzyme reaction intermediate. J. Biol. Chem. 268:12129-12135. [PubMed] [Google Scholar]
- 18.Miziorko, H. M., and M. D. Lane. 1977. 3-Hydroxy-3-methylglutaryl-CoA synthase. Participation of acetyl-S-enzyme and enzyme-S-hydroxymethylglutaryl-S-CoA intermediates in the reaction. J. Biol. Chem. 252:1414-1420. [PubMed] [Google Scholar]
- 19.Miziorko, H. M., K. D. Clinkenbeard, W. D. Reed, and M. D. Lane. 1975. 3-Hydroxy-3-methylglutaryl-CoA synthase: evidence for an acetyl-S-enzyme intermediate and identification of a cysteinyl sulfhydryl as the site of acetylation. J. Biol. Chem. 250:5768-5773. [PubMed] [Google Scholar]
- 20.Rohmer, M. 1999. A mevalonate-independent root to isopentenyl diphosphate, p. 45-67. In David Cane (ed.), Isoprenoids including carotenoids and steroids. Pergamon Press, Oxford, United Kingdom.
- 21.Rokosz, L. L., D. A. Boulton, E. A. Butkiwicz, G. Sanyal, M. A. Cueto, P. A. Lachance, and J. D. Hermes. 1994. Human cytoplasmic 3-hydroxy-3-methylglutaryl coenzyme A synthase: expression, purification, and charaterization of recombinant wild-type and Cys129 mutant enzymes. Arch. Biochem. Biophys. 312:1-13. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 23.Schuck, P., M. A. Perugini, N. R. Gonzales, G. J. Howlett, and D. Schubert. 2002. Size-distribution analysis of proteins by analytical ultracentrifugation: strategies and application to model systems. Biophys. J. 82:1096-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takagi, M., T. Kuzuyama, S. Takahashi, and H. Seto. 2000. A gene cluster for the mevalonate pathway from Streptomyces sp. strain CL190. J. Bacteriol. 182:4153-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vollmer, S. H., L. M. Mende-Mueller, and H. M. Miziorko. 1988. Identification of the site of acetyl-S-enzyme formation on avian liver mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase. Biochemistry 27:4288-4292. [DOI] [PubMed] [Google Scholar]
- 26.Weitzman, P. D. J. 1969. Citrate synthase from Escherichia coli. Methods Enzymol. 13:22-26. [Google Scholar]
- 27.Wilding, E. I., J. R. Brown, A. P. Bryant, A. F. Chalker, D. J. Holmes, K. A. Ingraham, S. Iordanescu, C. Y. So, M. Rosenberg, and M. N. Gwynn. 2000. Identification, evolution, and essentiality of the mevalonate pathway for isopentenyl diphosphate biosynthesis in gram-positive cocci. J. Bacteriol. 182:4319-4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilding, E. I., D.-Y. Kim, A. P. Bryant, M. N. Gwynn, R. D. Lunsford, D. McDevitt, J. E. Myers, Jr., M. Rosenberg, D. Sylvester, C. V. Stauffacher, and V. W. Rodwell. 2000. Essentiality, expression and characterization of the class II 3-hydroxy-3-methylglutaryl coenzyme A reductase of Staphylococcus aureus. J. Bacteriol. 182:5147-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]