Abstract
In a population-based cross-sectional study, we examined effects of sex and age on bone microstructure at the wrist using high-resolution 3-D pQCT. Compared with women, men had thicker trabeculae in young adulthood and had less microstructural damage with aging. These findings may contribute to the virtual immunity of men to age-related increases in wrist fractures.
Introduction
Although changes in bone microstructure contribute to fracture risk independently of BMD, it has not heretofore been possible to assess this noninvasively in population-based studies.
Materials and Methods
We used high-resolution 3-D pQCT imaging (voxel size, 89 μm) to define, in a random sample of women (n = 324) and men (n = 278) 21–97 years of age, sex and age effects on bone microstructure at the wrist.
Results
Relative to young women (age, 20–29 years), young men had greater trabecular bone volume/tissue volume (BV/TV; by 26%, p = 0.001) and trabecular thickness (TbTh; by 28%, p < 0.001) but similar values for trabecular number (TbN) and trabecular separation (TbSp). Between ages 20 and 90 years, cross-sectional decreases in BV/TV were similar in women (−27%) and in men (−26%), but whereas women had significant decreases in TbN (−13%) and increases in TbSp (+24%), these parameters had little net change over life in men (+7% and −2% for TbN and TbSp, respectively; p < 0.001 versus women). However, TbTh decreased to a greater extent in men (−24%) than in women (−18%; p = 0.010 versus men).
Conclusions
Whereas decreases with age in trabecular BV/TV are similar in men and women, the structural basis for the decrease in trabecular volume is quite different between the sexes. Thus, over life, women undergo loss of trabeculae with an increase in TbSp, whereas men begin young adult life with thicker trabeculae and primarily sustain trabecular thinning with no net change in TbN or TbSp. Because decreases in TbN have been shown to have a much greater impact on bone strength compared with decreases in TbTh, these findings may help explain the lower life-long risk of fractures in men, and specifically, their virtual immunity to age-related increases in distal forearm fractures.
Keywords: osteoporosis, aging, bone structure, pQCT
INTRODUCTION
Whereas areal BMD (aBMD) using DXA has been extremely useful for defining sex and age effects on bone mass and as a clinical tool for the prediction of fracture risk,(1) an inherent limitation of DXA is its inability to separate trabecular from cortical bone or to assess bone microstructure. Both central and peripheral QCT have been used in population studies to assess sex and age effects on trabecular and cortical bone,(2) but the limited resolution of standard peripheral (pixel size ~ 350 μm) or central (pixel size ~ 750 μm) CT instruments does not permit an evaluation of trabecular microstructure. Because changes in trabecular structure may impact bone strength independently of bone mass,(3) there is increasing clinical interest in assessing bone microstructure, with the ultimate goal of improving the prediction of fracture risk. However, until recently, this had only been possible using the invasive technique of bone biopsy followed by relatively tedious bone histomorphometric(4–6) or ex vivo μCT(7) analyses, which are not amenable for application either in population studies or for routine clinical use. The recent development(8) and validation(9–11) of high resolution 3-D pQCT instrumentation with a pixel size of <200 μm, and more recently <100 μm, has paved the way for evaluating trabecular microstructure noninvasively, at least at the wrist. Thus, in this study, we used 3-D pQCT imaging to define, in a relatively large (n = 602) population-based sample of women and men spanning a broad age range (21–97 years), sex and age effects on bone microstructure at the wrist. Our findings both provide novel insights into sex differences in alterations in trabecular structure with aging and establish the use of this technology in population studies.
MATERIALS AND METHODS
Study subjects
We recruited subjects from an age-stratified, random sample of Rochester, MN, residents who were selected using the medical records linkage of the Rochester Epidemiology Project.(12) This population is highly characteristic of the United States white population, but blacks and Asians are under-represented. The sample spanned ages from 21 to 97 years and included 324 women and 278 men. Reflecting the ethnic composition of the community, 98% of the subjects were white. There were 110 premenopausal women and 214 postmenopausal women; of the postmenopausal women, 76 were on estrogen therapy, 4 were on bisphosphonates, and 2 were on a selective estrogen receptor modulator. Of the 278 men, 2 were on bisphosphonates, 1 was on a selective estrogen receptor modulator, and 1 was on testosterone therapy.
3-D pQCT analysis
The nondominant wrist (or in the case of a prior wrist fracture, the nonfractured wrist) was scanned using a high-resolution 3-D pQCT device (a prototype of the Xtreme CT; Scanco Medical AG, Bassersdorf, Switzerland). The in vivo measurement protocol included the acquisition of a 3-D stack of 116 high-resolution CT slices at the distal end of the radius, as indicated in Fig. 1A, using an effective energy of 40 keV, slice thickness of 89 μm, field of view of 90 mm, image matrix of 1024 × 1024 pixels, and pixel size of 89 μm. Figure 1B shows representative cross-sectional images, with a 3-D representation shown in Fig. 1C.
FIG. 1.
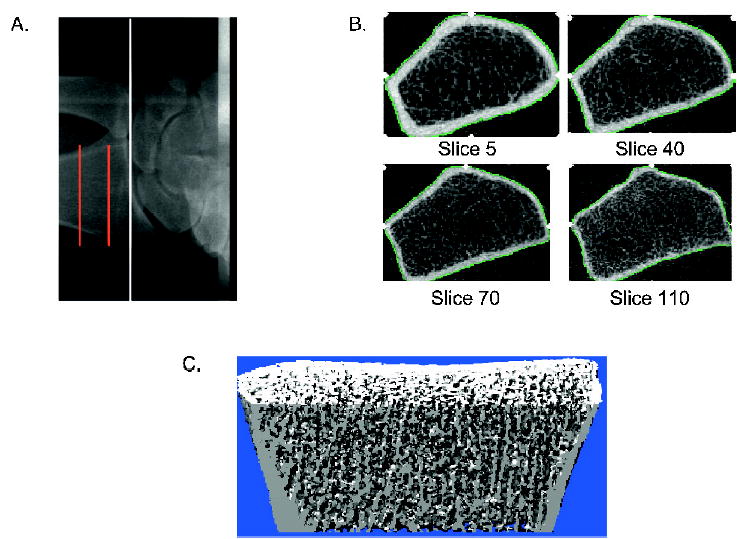
(A) Radiograph showing the site of imaging at the distal radius. The white line indicates the beginning of the joint space and the red lines indicate the section of bone over which images are acquired. (B) Representative cross-sectional images from the stack of CT slices from proximal (top left) to distal (bottom right). (C) Representative 3-D image.
The processing and analysis of the images has been extensively described and validated(9–11,13) and is summarized briefly here. The first step involves the determination of trabecular volumetric BMD (vBMD, mg/cm3) as the average mineral density within the trabecular region. From this, the trabecular bone volume/tissue volume (BV/TV) is derived, assuming a mineral density of fully mineralized bone of 1.2 g hydroxyapatite/cm3. Recognizing that individual trabeculae will not be resolved at their correct thickness because of partial volume effects, a thickness-independent structure extraction is used to assess trabecular microarchitecture. To this end, the 3-D ridges (the center points of the trabeculae) are detected in the gray-level images as described in detail in Laib et al.(9) Trabecular number (TbN, 1/mm) is taken as the inverse of the mean spacing of the ridges.(10) Combining TbN and BV/TV, trabecular thickness (TbTh, mm) is then derived as (BV/TV)/TbN, and trabecular separation (TbSp, mm) is derived as (1 - BV/TV)/TbN, as is done in standard histomorphometry.(14) The validity of this approach has been rigorously tested by comparing the 3-D pQCT methodology with 28-μm resolution μCT.(11) In this comparison, the correlation coefficients between the 3-D pQCT values for BV/TV, TbN, TbTh, and TbSp and the respective measures using μCT (n = 15 specimens) were 0.99, 0.96, 0.97, and 0.98, respectively (all p < 0.0001). Note that the older 3-D pQCT device used in that analysis had a voxel size of 165 μm (compared with the newer scanner used in this study, which has a voxel size of 89 μm), so the results represent a conservative bias. The key point here, and the major reason this methodology can be used to obtain the types of correlations with μCT noted above, is that the resolution here has to be sufficient to adequately resolve the distance between the trabecular ridges (1/TbN, or ~300–500 μm), and not necessarily to resolve individual trabeculae (~100 μm or less). In addition to the trabecular parameters, cortical measures, including cortical vBMD (mg/cm3), bone area (BA, mm2), endocortical area (EnA, mm2), and cortical thickness (CTh, mm) are also obtained.(10) To assess the short-term precision of the measurements, 20 volunteers (age, 19–40 years) were scanned twice on the same day after repositioning, and the following CVs were observed for the various parameters: cortical vBMD, 1.3%; CTh, 5.1%; BV/TV, 1.2%; TbN, 2.2%; TbTh, 1.8%; TbSp, 3.4%.
Statistical analyses
All results were analyzed with and without the inclusion of the 78 postmenopausal women who were receiving estrogen or a selective estrogen receptor modulator and the 6 subjects who were receiving bisphosphonate therapy. Because analysis with and without the inclusion of these subjects gave similar results, all were included. The relationships between the 3-D pQCT output parameters and age were studied using Pearson correlation and least squares regression, where age was modeled using natural splines. Each model was compared with a linear relationship, and the simplest model was used for analysis. Changes in variables between ages 20 and 90 years were based on predicted values from these models. Differences in changes over life between men and women were tested using an age–sex interaction term in a regression model. Variables in Table 2 were examined using a split point model where the slope was allowed to differ before and after age 50. An adjustment for the effect of differences in bone size on bone area was made by dividing the original values by height. The need for this adjustment was determined by a significant correlation of a given variable with height among subjects 20–39 years of age. The Student’s t-test was used to compare the mean values for young women (age, 20–29 years) versus young men. The S-plus function lowess,(15) essentially a type of moving average, was used to explore the data in Fig. 2.
Table 2.
Percent Changes in Selected 3-D-pQCT Variables Between Ages 20–49 and 50–90 Years in Rochester, MN, Women (W) and Men (M)
|
Percent change between ages 20 and 49 years |
Percent change between ages 50 and 90 years |
|||||
|---|---|---|---|---|---|---|
| Women | Men | p (W vs. M) | Women | Men | p (W vs. M) | |
| BV/TV | −12.3* | −17.0‡ | 0.249 | −14.6† | −9.8† | 0.783 |
| TbN | −1.5 | 13.6‡ | <0.001 | −10.5‡ | −8.2‡ | 0.411 |
| TbTh | −11.5* | −25.9‡ | 0.002 | −6.9 | −4.1 | 0.794 |
| TbSp | 4.1 | −8.2† | 0.036 | 17.1‡ | 9.3‡ | 0.079 |
| CTh | −6.8 | 11.2 | 0.052 | −50.7‡ | −44.8‡ | 0.195 |
| Cort vBMD | −2.7 | 4.4 | 0.024 | −22.2‡ | −17.6‡ | 0.009 |
p < 0.05;
p < 0.01;
p < 0.001.
BV/TV, bone volume/tissue volume; TbN, trabecular number; TbTh, trabecular thickness; TbSp, trabecular separation; CTh, cortical thickness; Cort vBMD, cortical volumetric BMD.
FIG. 2.
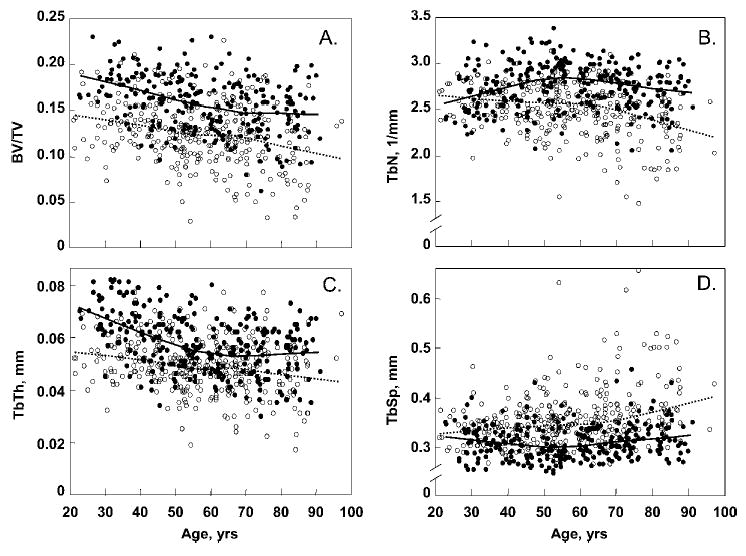
Age-related changes in trabecular bone microstructural variables at the wrist in Rochester, MN, women and men. (A) Bone volume/tissue volume (BV/TV). (B) Trabecular number (TbN). (C) Trabecular thickness (TbTh). (D) Trabecular separation (TbSp). Curve fitting was done with a smoother function. Individual values and smoother lines are given for women using open circles and dashed lines and for men using closed circles and solid lines.
RESULTS
Table 1 describes the age- and sex-specific changes over life for the 3-D pQCT measurements in the trabecular and cortical compartments of the distal forearm. Where appropriate (see the Materials and Methods section), both the unadjusted values and those divided by height are provided. Table 1 provides means and SD for the absolute values for variables in women (n =17) and men (n =19) 20–29 years of age. It also provides the absolute (in units of the variable) and relative (percentage) changes with age between 20 and 90 years as well as the significance of the age regression and of the differences between women and men.
Table 1.
3-D-pQCT Parameters in an Age-Stratified, Random Sample of Rochester, MN, Women (W) and Men (M)
|
Women |
Men |
p-values |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Change between 20 and 90 years |
Change between 20 and 90 years |
|||||||||
| Mean ± SD (20–29 years) (N = 17) | Absolute | Percentage | Age-Corr.* | Mean ± SD (20–29 years) (N = 19) | Absolute | Percentage | Age-Corr.* | M/W difference in young adults | M/W age difference† | |
| Trabecular parameters | ||||||||||
| BV/TV | 0.141 ± 0.028 | −0.039 | −27¶ | −0.27 | 0.178 ± 0.031 | −0.047 | −26¶ | −0.39 | 0.001 | 0.524 |
| TbN (1/mm) | 2.64 ± 0.17 | −0.35 | −13¶ | −0.29 | 2.60 ± 0.25 | 0.17 | 7‡ | 0.02 | 0.577 | <0.001 |
| TbTh (mm) | 0.053 ± 0.009 | −0.010 | −18¶ | −0.21 | 0.068 ± 0.008 | −0.018 | −24‡¶ | −0.43 | <0.001 | 0.010 |
| TbSp (mm) | 0.327 ± 0.031 | 0.076 | 24¶ | 0.31 | 0.320 ± 0.041 | −0.007 | −2‡ | 0.11 | 0.531 | <0.001 |
| Cortical parameters | ||||||||||
| BA (mm2) | 235.3 ± 29.5 | 27.4 | 11§ | 0.16 | 355.9 ± 62.7 | 40.2 | 11§ | 0.16 | <0.001 | 0.458 |
| BA (mm2/ht, m) | 143.9 ± 15.1 | 25.6 | 18¶ | 0.25 | 196.9 ± 29.9 | 37.8 | 19¶ | 0.28 | <0.001 | 0.201 |
| EnA (mm2) | 176.5 ± 27.3 | 46.4 | 27¶ | 0.26 | 277.8 ± 62.0 | 52.8 | 19¶ | 0.21 | <0.001 | 0.713 |
| EnA (mm2/ht, m) | 108.0 ± 15.1 | 35.5 | 34¶ | 0.33 | 153.6 ± 30.7 | 42.1 | 28¶ | 0.30 | <0.001 | 0.502 |
| CTh (mm) | 0.825 ± 0.136 | −0.437 | −52‡¶ | −0.58 | 0.852 ± 0.176 | −0.320 | −38‡¶ | −0.40 | 0.603 | 0.001 |
| Cort vBMD (mg/cm2) | 893.0 ± 45.2 | −192.7 | −22‡¶ | −0.64 | 850.3 ± 38.0 | −133.7 | −16‡¶ | −0.48 | 0.005 | <0.001 |
Where there was a significant relationship between height (Ht) and a given variable in young adults, both the unadjusted and adjusted values for all variables are given.
Pearson correlation coefficient.
Comparisons of slopes.
Indicates that regression model is nonlinear.
p < 0.01;
p < 0.001.
BV/TV, bone volume/tissue volume; TbN, trabecular number; TbTh, trabecular thickness; TbSp, trabecular separation; BA, bone area; EnA, endocortical area; CTh, cortical thickness; Cort vBMD, cortical volumetric BMD.
As is evident, relative to young women, young men had higher values for BV/TV (by 26%; p = 0.001), TbTh (by 28%; p < 0.001), BA (by 51%; p < 0.001), and EnA (by 57%; p < 0.001), but similar values for TbN, TbS, and CTh. Dividing by height still resulted in higher values for BA/ht (by 37%; p < 0.001) and EnA/ht (by 42%; p < 0.001) in the young men. Cortical vBMD was slightly, but significantly, higher in the young women (by 5%; p = 0.005).
Table 1 also shows that, over life, changes in BV/TV were similar in women and in men. However, whereas TbN declined (by 13%) and TbSp increased (by 24%) in the women, both parameters remained unchanged in the men when considering values between ages 20 and 90 years. In contrast, TbTh decreased to a greater extent in men compared with women (−24% versus −18%, respectively; p = 0.010). BA increased similarly in men and women (by 11%), but EnA increased to a somewhat greater extent in women (by 27% versus 19%; p = 0.713), whereas neither of these changes were different in women versus men; CTh (which represents the net difference between increases in BA and EnA) decreased to a greater extent in women compared with men (by 52% versus 38%; p = 0.001), as did cortical vBMD (by 22% versus 16%; p < 0.001).
We also examined in more detail the age- and sex-specific changes in the trabecular bone microstructural variables. As shown in Fig. 2A and Table 2, trabecular BV/TV, while higher in men compared with women at every age, declined similarly in both sexes. However, the pattern of change in TbN was quite different in women and men. Thus, in men, TbN increased by 13.6% between ages 20 and 49 years, whereas it remained stable in women at these ages (−1.5%; Fig. 2B; Table 2). In contrast, TbTh decreased more than twice as much in men between ages 20 and 49 years compared with age-matched women (−25.9% versus −11.5%, respectively; p = 0.002), as shown in Fig. 2C and Table 2. This increase in TbN and decrease in TbTh was associated with a significant decrease in TbSp in men between ages 20 and 49 years (−8.2%), whereas TbSp remained relatively stable in comparable aged women (4.1%; Fig. 2D; Table 2). In contrast to these differential changes in trabecular microstructure in women versus men between ages 20 and 49 years, these parameters changed very similarly in women and men between ages 50 and 90 years (Fig. 2; Table 2).
The above data suggested that, between ages 20 and 49 years, men may be converting thick trabeculae into more numerous, thinner trabeculae (resulting in the observed increase in TbN and decrease in TbTh over that age range). To visually assess this, we examined a number of 3-D reconstructions of scans from young versus middle-aged men and women, and Fig. 3 provides representative examples of these reconstructions. As indicated by the arrow in the scan from the 24-year-old man in Fig. 3, young men tended to have significant areas of apparent plate-like structures that were largely absent in young women or in middle-aged men or women. Figure 3 also shows representative reconstructions of scans from elderly men and women, showing the clear trabecular microarchitectural deterioration in the elderly woman, with less dramatic changes in the elderly man.
FIG. 3.
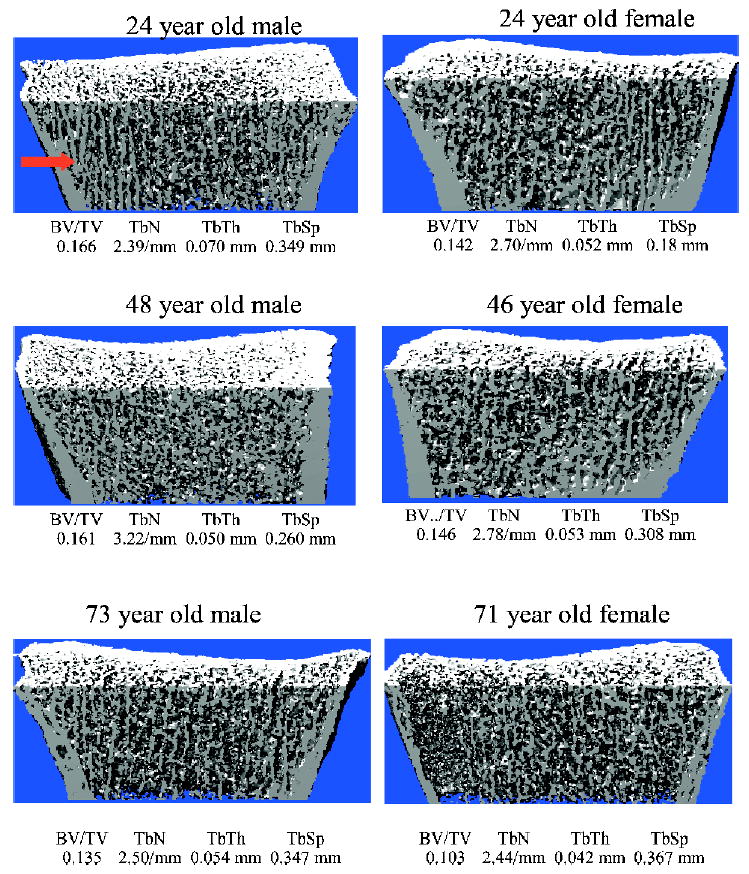
Representative 3-D reconstructions of images from young, middle-aged, and elderly men (left) and women (right). Also indicated are the values for trabecular microstructural variables in each of these subjects. The arrow at the top left indicates prominence of apparent plate-like trabeculae in the young man, which are absent or considerably less prominent in the other images.
Table 2 also shows that, in contrast to BV/TV, CTh and cortical vBMD did not change significantly in women or in men between ages 20 and 49 years, and both decreased markedly in both sexes after age 50 years, with greater decreases in women compared with men.
Finally, we also examined whether we could detect an acceleration of changes in trabecular microstructure in women associated specifically with the menopause. However, in this cross-sectional analysis, replotting the data in Fig. 2 based on years since menopause did not provide any clear evidence of a menopause effect beyond the overall age-related changes already shown in the figure (data not shown).
DISCUSSION
To our knowledge, this study represents the first application of noninvasive, high-resolution in vivo imaging to define, in a population-based sample, sex and age effects on bone microstructure. We found that, compared with young women, young men began adult life with indices of trabecular structure that predict stronger bones and a greater resistance to fracture.(3) Moreover, whereas BV/TV declined similarly in men and women over life, the microstructural basis for the decrease in trabecular volume differed between the sexes. Thus, in women, there appeared to be loss of trabeculae (resulting in the observed decrease in TbN and increase TbSp), whereas in men, the primary mechanism for the decrease in BV/TV was trabecular thinning. This, in turn, is likely to have a significant impact on age-related changes in bone strength, because based on finite element modeling, reductions in TbN have a 2- to 5-fold greater impact on bone strength compared with reductions in TbTh that result in similar decreases in bone volume.(16)
Even though we assessed trabecular microstructure noninvasively at the ultradistal radius, our results in women are consistent with the seminal findings of Parfitt and colleagues(4,6) on changes in bone histomorphometric indices in transiliac biopsies from pre- versus postmenopausal women. Thus, similar to our data, these investigators showed that, compared with premenopausal women, postmenopausal women had significant reductions in BV/TV, and this was associated primarily with reductions in TbN and increases in TbSp. This has formed the basis of the plausible hypothesis that menopause-associated increases in osteoclastic activity lead to losses of entire trabeculae, converting the continuous trabecular network characteristic of young women into the discontinuous network found in older women.(4,17) Whereas we were able to show similar changes in women over life at the radius using high-resolution 3-D pQCT, our cross-sectional study examined a broad age range, and we could not specifically resolve an effect of menopause on this process. It is likely that longitudinal analyses, which we are currently performing, will be necessary to directly show an effect of menopause on changes in trabecular structure in women. It should be noted that a relatively small study (n = 10 subjects) using μCT analysis of paired iliac crest biopsies from women going through menopausal transition(18) found relatively greater decreases in TbTh (−3.5%) than in TbN (−1.6%). In contrast, a second μCT study involving μCT analysis of iliac crest biopsies from early postmenopausal women in the placebo arm of a bisphosphonate trial (n = 12)(19) found fairly comparable decreases in TbTh (−12%) and in TbN (−14%). Thus, some of the μCT findings are discordant with the bone histomorphometry data,(4,6) highlighting the need for further longitudinal studies of changes in trabecular structure in women across the menopausal transition.
Our findings in men are also consistent with a previous study by Aaron et al.(5) using cadaveric transiliac bone biopsies. These investigators found, as we did, parallel decreases in BV/TV with age in men and women, and similar to our data, that study also found a significant decrease in TbN over life in women but not in men. Moreover, consistent with our findings at the wrist, TbTh at the iliac crest was higher in young men compared with age-matched women, but decreased more over life in men compared with women.(5) The concordance of our findings at the radius using 3-D pQCT with the previous work using bone histomorphometry at the iliac crest does suggest that the pattern of changes we observed at the radius in trabecular and in cortical bone may be generalizable to other skeletal sites, although further studies are clearly needed to address this issue definitively.
Whereas our findings using high-resolution pQCT at the radius are generally consistent with previous work using bone histomorphometry at the iliac crest, perhaps the most novel aspect of our data is the previously unrecognized increase in TbN and a decrease in TbSp in men between the ages of 20 and 49 years, which appeared to offset subsequent age-related decreases in TbN and increases in TbSp. In addition, between ages 20 and 49 years, TbTh also decreased markedly in men (more than twice as much as in women), and this was the principal reason for the greater overall decrease in TbTh over life in men compared with women. The most plausible explanation for these findings is that young adult men have thicker trabeculae than those present in young women, and that between ages 20 and 49 years, these thick trabeculae are converted into more numerous, thinner trabeculae, resulting in the observed marked decrease in TbTh, increase in TbN, and decrease in TbSp over that age range. Why this occurs in the men and not in the women, as well as the possible biomechanical or hormonal factors driving these changes, is unclear at this point.
In addition to better trabecular microstructure, men also have the obvious biomechanical advantage of larger bones (higher BA) and smaller age-related decreases in CTh. The latter is caused principally by somewhat smaller increases in EnA in men compared with women. Thus, because of the combined advantages in bone structure and size at the wrist in young adulthood, as well as less microstructural damage (in particular, preservation of TbN and TbSp) with aging, elderly men have better overall indices of trabecular and cortical bone than elderly women. This is reflected clinically in the virtual absence of an age-related rise in distal forearm (Colles’) fractures in men compared with the marked increase in these fractures in older women.(20)
The relatively continuous decrease in BV/TV over life in men and in women, which seems to begin in young adulthood, is similar to our recent findings on cross-sectional age-related changes in trabecular vBMD using lower-resolution central and pQCT.(2) Thus, we reported there that decreases in trabecular vBMD at multiple sites also began in young adulthood in both men and women,(2) and we have now confirmed these cross-sectional findings with longitudinal data on changes in trabecular vBMD at the wrist using pQCT.(21) The findings from this study and our previous work(2,21) are consistent with the study of Meier et al.,(22) who also found substantial decreases in vertebral trabecular vBMD not only in older men, but also in young men <50 years of age. In addition, Yu et al.(23) also showed significant decreases in vertebral trabecular vBMD in young and elderly women and men. Using peripheral and central QCT, Ito and colleagues(24,25) found that trabecular bone loss clearly increased during the menopausal years, with values in late (>5 years) postmenopausal women returning to levels close to those present in premenopausal women at most sites. In contrast to these findings in trabecular bone, CTh and vBMD in this study and cortical vBMD at multiple sites in our previous cross-sectional(2) and more recent longitudinal(21) studies remained relatively constant in men and in women until age 50 years, and all decreased subsequently with age.
It is likely that decreases in CTh and vBMD after age 50 years are driven largely by the menopause in women and, at least in part, by the age-related decrease in bioavailable sex steroids that begins in men around 50 years of age.(26) However, the cause(s) of the continuous decrease in trabecular BV/TV and vBMD even in young adult life remain unclear. These individuals, by definition, have “normal” sex steroid levels. We have previously suggested that cortical bone may be much more sensitive to estrogen than trabecular bone (i.e., that the “threshold” for estrogen deficiency in cortical bone is considerably lower than the threshold in trabecular bone),(27,28) and the present findings would be consistent with that notion. Thus, in the context of that hypothesis, estrogen would be able to prevent bone loss in cortical bone until midlife in women and in men, but the decline in bioavailable estrogen levels thereafter would result in the observed bone loss. In contrast, because of the postulated lower sensitivity of trabecular bone to estrogen, even “normal” estrogen levels in young adult life may be insufficient to completely prevent decreases in trabecular BV/TV and vBMD. Whereas the available evidence is consistent with this hypothesis, we recognize the limitations of our cross-sectional data, and these concepts clearly need to be tested in longitudinal studies. In addition, large population-based studies using other imaging techniques to assess bone microstructure, such as MRI,(29) should done to independently test the findings from the 3-D pQCT technology.
In summary, our study shows the use of high-resolution 3-D pQCT imaging for analyses of bone microstructure in population studies and potentially for the clinical assessment of fracture risk. We find that, relative to women, men begin adult life with better trabecular microstructure and have less microstructural damage with aging. Collectively, these findings may help explain the lower life-long risk of fractures in men, and specifically, their virtual immunity to age-related increases in wrist (Colles’) fractures.
Acknowledgments
The authors thank Sara Achenbach for performing the statistical analyses and Drs Bruno Koller, Stefan Hammerle, and Andres Laib (Scanco) for ongoing help and support with the 3-D pQCT imaging. This work was supported in part by NIH Grants AR-027065 and RR00585.
References
- 1.Miller PD, Zapalowski C, Kulak CAM, Bilezikian JP. Bone densitometry: The best way to detect osteoporosis and to monitor therapy. J Clin Endocrinol Metab. 1999;84:1867–1871. doi: 10.1210/jcem.84.6.5710. [DOI] [PubMed] [Google Scholar]
- 2.Riggs BL, Melton LJI, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 3.Bouxsein ML 2001 Biomechanics of age-related fractures. In: Marens R, Kelsey J, Feldman D (eds). Osteoporosis, 2nd ed. Academic Press, San Diego, CA, USA, pp. 509–534.
- 4.Parfitt AM, Mathews CHE, Villaneuva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. J Clin Invest. 1983;72:1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaron JE, Makins NB, Sagreiya K. The microanatomy of trabecular bone loss in normal aging men and women. Clin Orthop. 1987;215:260–271. [PubMed] [Google Scholar]
- 6.Han ZH, Palnitkar D, Rao S, Nelson D, Parfitt AM. Effect of ethnicity and age or menopause on the structure and geometry of iliac bone. J Bone Miner Res. 1996;11:1967–1975. doi: 10.1002/jbmr.5650111219. [DOI] [PubMed] [Google Scholar]
- 7.Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: Microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 8.Muller R, Hildebrand T, Ruegsegger P. Non-invasive bone biopsy: A new method to analyze and display the three-dimensional structure of trabecular bone. Phys Med Biol. 1994;39:145–164. doi: 10.1088/0031-9155/39/1/009. [DOI] [PubMed] [Google Scholar]
- 9.Laib A, Hilderbrand T, Hauselmann HJ, Ruegsegger P. Ridge number density: A parameter for in vivo bone structure analysis. Bone. 1997;21:541–546. doi: 10.1016/s8756-3282(97)00205-6. [DOI] [PubMed] [Google Scholar]
- 10.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6:329–337. [PubMed] [Google Scholar]
- 11.Laib A, Ruegsegger P. Calibration of trabecular bone structure measurements of in vivo three-dimensional peripheral quantitative computed tomography with 28-microm-resolution microcomputed tomography. Bone. 1999;24:35–39. doi: 10.1016/s8756-3282(98)00159-8. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 13.Muller R, Hildebrand T, Hauselmann HJ, Ruegsegger P. In vivo reproducibility of three-dimensional structural properties of noninvasive bone biopsies using 3D-pQCT. J Bone Miner Res. 1996;11:1745–1750. doi: 10.1002/jbmr.5650111118. [DOI] [PubMed] [Google Scholar]
- 14.Parfitt AM, Mathews CHE, Villanueva AR, Kleerekoper M, Frame B, Rao DS. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Calcif Tissue Int. 1983;72:1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venables WN, Ripley BD 1994 Modern Applied Statistics With S-Plus. Springer-Verlag, New York, NY, USA.
- 16.Silva MJ, Gibson LJ. Modeling the mechanical behavior of vertebral trabecular bone: Effects of age-related changes in microstructure. Bone. 1997;21:191–199. doi: 10.1016/s8756-3282(97)00100-2. [DOI] [PubMed] [Google Scholar]
- 17.Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: The critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res. 2005;20:177–184. doi: 10.1359/JBMR.041114. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Zhao J, Recker R, Draper MW, Genant HK. Longitudinal changes between premenopausal and postmenopausal in three-dimensional trabecular microstructural characteristics of human iliac crest bone biopsies. J Bone Miner Res. 2000;15:S184. [Google Scholar]
- 19.Dufresne TE, Chmielewski PA, Manhart MD, Johnson TD, Borah JB. Risedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomography. Calcif Tissue Int. 2003;73:423–432. doi: 10.1007/s00223-002-2104-4. [DOI] [PubMed] [Google Scholar]
- 20.Cooper C, Melton LJ. Epidemiology of osteoporosis. Trends Endocrinol Metab. 1992;3:224–229. doi: 10.1016/1043-2760(92)90032-v. [DOI] [PubMed] [Google Scholar]
- 21.Riggs BL, Melton LJ, III, Oberg AL, Atkinson EJ, Khosla S. Substantial trabecular bone loss occurs in young adult women and men: A population-based longitudinal study. J Bone Miner Res. 2005;20:S1–S4. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier DE, Orwoll ES, Keenan EJ, Fagerstrom RM. Marked decline in trabecular bone mineral content in healthy men with age: Lack of association with sex steroid levels. J Am Geriatr Soc. 1987;35:189–197. doi: 10.1111/j.1532-5415.1987.tb02308.x. [DOI] [PubMed] [Google Scholar]
- 23.Yu W, Qin M, Xu L, van Kuijk C, Meng X, Xing X, Cao J, Genant HK. Normal changes in spinal bone mineral density in a Chinese population: Assessment by quantitative computed tomography and dual-energy x-ray absorptiometry. Osteoporos Int. 1999;9:179–187. doi: 10.1007/s001980050133. [DOI] [PubMed] [Google Scholar]
- 24.Ito M, Nakamura T, Tsurusaki K, Uetani M, Hayashi K. Effects of menopause on age-dependent bone loss in the axial and appendicular skeletons in healthy Japanese women. Osteoporos Int. 1999;10:377–383. doi: 10.1007/s001980050243. [DOI] [PubMed] [Google Scholar]
- 25.Tsurusaki K, Ito M, Hayashi K. Differential effects of menopause and metabolic disease on trabecular and cortical bone assessed by peripheral quantitative computed tomography (pQCT) Br J Radiol. 2000;73:14–22. doi: 10.1259/bjr.73.865.10721315. [DOI] [PubMed] [Google Scholar]
- 26.Khosla S, Melton LJ, Atkinson EJ, O’Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 27.Khosla S, Melton LJ, III, Robb RA, Camp JJ, Atkinson EJ, Oberg AL, Rouleau PA, Riggs BL. Relationship of volumetric BMD and structural parameters at different skeletal sites to sex steroid levels in men. J Bone Miner Res. 2005;20:730–740. doi: 10.1359/JBMR.041228. [DOI] [PubMed] [Google Scholar]
- 28.Khosla S, Riggs BL, Robb RA, Camp JJ, Achenback SJ, Oberg AL, Rouleau PA, Melton LJ., III Relationship of volumetric bone density and structural parameters at different skeletal sites to sex steroid levels in women. J Clin Endocrinol Metab. 2005;90:5096–5103. doi: 10.1210/jc.2005-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majumdar S, Genant HK, Grampp S, Newitt DC, Truong V-H, Lin JC, Mathur A. Correlation of trabecular bone structure with age, bone mineral density, and osteoporosis status: In vivo studies in the distal radius using high resolution magnetic resonance imaging. J Bone Miner Res. 1997;12:111–118. doi: 10.1359/jbmr.1997.12.1.111. [DOI] [PubMed] [Google Scholar]


