Abstract
Collagens are abundant proteins in higher organisms, and are formed by a complex biosynthetic pathway involving intracellular and extracellular post-translational modifications. Starting from simple soluble precursors, this interesting pathway produces insoluble functional fibrillar and non-fibrillar elements of the extracellular matrix. The present review highlights recent progress and new insights into biological regulation of extracellular procollagen processing, and some novel functions of byproducts of these extracellular enzymatic transformations. These findings underscore the notion that released pro-peptides and other proteolytic products of extracellular matrix proteins have important biological functions, and that structural proteins are multifunctional. An emerging concept is that a dynamic interplay exists between extracellular products and byproducts with cells that helps to maintain normal cellular phenotypes and tissue integrity.
The biosynthesis of collagen proteins includes the formation of mRNA/ribosome complexes bound to the endoplasmic reticulum, fully consistent with the normal secretory pathway. Once in the lumen of the endoplasmic reticulum, procollagen chains undergo proline and lysine hydroxylation of some residues by prolyl-3-hydroxylase, prolyl-4-hydroxylase; and by lysyl hydroxylases encoded by three related genes producing four proteins due to alternative splicing [Walker et al., 2005]. The intracellular modifications of proline and lysine hydroxylation are unique to collagens and require ascorbate, iron, and 2-oxo-glutarate. Hydroxylation of proline and lysine residues occurs before triple helix formation and hydroxyproline is critical for ultimate stabilization of the helical structure of triple helical procollagens. Prolyl 4-hydroxlase is a multifunctional enzyme, and has protein disulfide isomerase (PDI) activity [Pihlajaniemi et al., 1987]. PDI activity may aid in collagen triple helix formation in the endoplasmic reticulum, as initial chain association depends upon correct disulfide formation in the C-propeptide region of procollagens I – III [Noiva and Lennarz, 1992]. Lysyl hydroxylase family members, respectively, favor either helical or non-helical regions of procollagen polypeptide chains as substrates [Mercer et al., 2003; Pornprasertsuk et al., 2004; van der Slot et al., 2003]. Hydroxylysine residues, in addition, undergo glycosylation of some of the hydroxylysine residues by galactosyl transferase and galactosylhydroxylysysl glucosyl transferase. This glycosylation is distinct from O- and N-glycosylation that occurs on most other proteins, and is another unique posttranslational modification of collagen precursors. Recent findings show that the lysyl hydroxylase 3 isoform has hydroxylysyl galacosylytransferase and hydroxylysylglycosyl transferase activity, indicating that this enzyme, like prolyl-4-hydroxylase, is multifunctional [Heikkinen et al., 2000; Wang et al., 2002a; Wang et al., 2002b].
Triple helical procollagen containing the proline and lysine modifications described above is next secreted into the extracellular environment, possibly as small aggregates of procollagen triple helical units [Fleischmajer et al., 1987; Fleischmajer et al., 1988]. There are now indications that the intracellular trafficking from the endoplasmic reticulum to ultimate secretion may have some unique features, as recently reviewed [Canty and Kadler, 2005]. Once in the extracellular environment, further modifications occur that ultimately lead to deposition and cross-linking required for normal extracellular matrix formation. It is these extracellular modifications that are the primary focus of the present review.
Extracellular events and biological control of collagen deposition
The notion that extracellular steps in the collagen biosynthetic pathway could be regulated in biologically significant ways is supported by observations of osteoblasts that differentiate in vitro. These cultures undergo a well characterized differentiation program in vitro in which the highest amount of collagen synthesis occurs early, whereas insoluble collagen deposition occurs primarily at a later stage [Franceschi and Iyer, 1992; Franceschi et al., 1994; Gerstenfeld et al., 1988; Quarles et al., 1992; Stein et al., 1996]. An example of this phenomenon is shown in Figure 1, where collagen mRNA levels are very high early in the life of these cultures and then diminishes, while collagen deposition is initially low and increases with time. Similarly, TGF-β1 increases collagen deposition in osteoblasts to a greater degree than collagen synthesis without significantly affecting proteolytic activity [Centrella et al., 1992]. This suggests that extracellular biosynthetic enzymes could be up-regulated by this growth factor. TNF-α decreases collagen deposition without significantly diminishing collagen synthesis [Panagakos et al., 1994; Pischon et al., 2004], and could down-regulate collagen processing activities. In addition, connective tissue growth factor (CCN2/CTGF) stimulates collagen deposition without effect on collagen mRNA levels in gingival fibroblasts [Hong et al., 1999]. These observations suggest that certain differentiating cell types and certain cytokines could regulate collagen deposition by a mechanism that includes control of extracellular biosynthetic activities.
Figure 1.
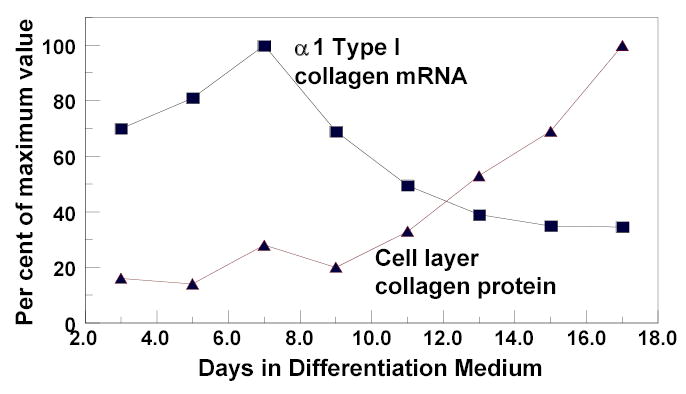
Relationship between collagen mRNA levels and cell layer collagen protein accumulation in differentiating osteoblasts. MC3T3-E1 pre-osteoblasts were cultured in differentiation medium [Hong et al., 2004], and RNA isolated at intervals. Cell layer collagen based on cell layer hydroxyproline measurements were determined from separate cultures grown at the same time. ▪, α 1-type I collagen mRNA levels determined by Northern blotting and densitometry normalized to 18S rRNA, ▴, cell layer collagen based on hydroxyproline measurements as described [Hong et al., 2004].
Procollagen pro-peptide processing
Secreted procollagens have C-terminal and N-terminal non-helical pro-peptides that are removed in the extracellular environment by specific enzymes [Prockop et al., 1998]. A major consequence of pro-peptide removal is diminished solubility of the resulting tropocollagen [Prockop and Kivirikko, 1995], thus facilitating higher order fibril assembly made up of triple helical tropocollagen units organized in the characteristic quarter stagger array that has been visualized by electron microscopy [Mould et al., 1990].
Removal of the C-terminal pro-peptides of fibrillar pro-collagens including types I, II, and III is accomplished by procollagen C-proteinases (PCP’s) [Kessler and Goldberg, 1978]. Removal of C-terminal pro-peptides is more efficient than removal of N-terminal propeptides, and is critical for the assembly of collagen fibrils [Kadler et al., 1987]. PCP’s are zinc metalloproteinases and are members of the astacin family of enzymes. They are products of the Bmp1 gene and the structurally related genes mTll1 and mTll2 [Kessler et al., 1996; Li et al., 1996; Scott et al., 1999]. It is of considerable interest that procollagen C-proteinases process several other extracellular matrix pro-proteins [Amano et al., 2000; Colombo et al., 2003; Imamura et al., 1998; Kessler et al., 2001; Scott et al., 2000; Steiglitz et al., 2004; Wolfman et al., 2003], including the secreted proenzyme form of lysyl oxidase [Cronshaw et al., 1995; Panchenko et al., 1996; Uzel et al., 2001], the final extracellular enzyme that contributes to the biosynthesis of mature collagens [Kagan, 1986; Kagan and Trackman, 1991]. The processing of fibrillar procollagens by procollagen C-proteinases is highly stimulated by another extracellular protein family, the procollagen C-proteinase enhancers (PCPE’s) [Adar et al., 1986; Steiglitz et al., 2002; Takahara et al., 1994]. PCPE’s bind to procollagens and induce a conformational change, and thereby facilitate proteolytic processing by PCP’s [Adar et al., 1986; Ricard-Blum et al., 2002; Steiglitz et al., 2002]. PCPE may be specific for fibrillar collagens, and CUB domains in PCPE bind triple helical regions of procollagens [Ricard-Blum et al., 2002]; [Steiglitz et al., 2002]. PCPE’s are encoded by two genes, Pcolce1 and 2 that have structural similarity and activity, though Pcolce2 is less widely expressed. It can be appreciated from this information that removal of C-terminal pro-peptides from secreted procollagens requires the collaboration of at least three extracellular proteins, procollagen polypeptides, PCPE, and procollagen C-proteinases. Given that procollagen C-proteinases themselves are produced as pro-enzymes activated by proteolytic processing [Leighton and Kadler, 2003], it is clear that still additional enzymatic processes contribute to this process. As indicated below, it is likely that cell surface proteins including integrins are required to help orchestrate and coordinate extracellular collagen processing and deposition.
Removal of N-terminal pro-peptides from secreted fibrillar procollagens is accomplished by members of the ADAMTS family of proteases (a disintegrin and metalloproteinase with thrombospondin type I repeats) [Colige et al., 2002]. There is tissue specificity of expression of these enzymes. ADAMTS-3 is the principal cartilage and type II procollagen N-proteinase [Fernandes et al., 2001]; whereas ADAMTS-2 and -14 are more important in skin and other tissues [Colige et al., 2002]. All three enzymes process procollagen types I, II and III in vitro [Colige et al., 2002; Fernandes et al., 2001; Wang et al., 2003]. Genetic deficiency of ADAMTS-2 leads to Ehlers-Danlos Syndrome Type VII C in humans, and was first seen in animals as dermatosparaxis [Colige et al., 1999]. Collagen fibril formation is abnormal and is known as hieroglyphic in appearance in transmission electron micrographs. Affected individuals have weak skin, joints, blood vessels, and ligaments [Malfait et al., 2004] but generally normal cartilage [Fernandes et al., 2001]. A certain degree of normal retention of procollagen N-pro-peptides occurs in a tissue specific manner, and reports indicate that retained procollagen N-propeptide inhibits lateral fibril growth [Fleischmajer et al., 1987; Fleischmajer et al., 1988; Silver et al., 2003].
Collagen cross-linking; last but not least
Collagens must be cross-linked to exhibit the normal physical properties of tensile strength. This understanding stems from observations made in early recorded history including those by Hippocrates himself as previously reviewed [Siegel, 1979]. Sheep that grazed on a certain type of sweet pea exhibited abnormalities including aortic aneurism, skin abnormalities, bone and joint weakness. These findings were reproduced in laboratory animals fed a diet rich in Lathyrus oderatus peas [Geiger et al., 1933], and the condition became known as osteolathyrism. The active agent in Lathyrus oderatus peas that causes osteolathyrism was identified as β-aminopropionitrile (BAPN) [McKay et al., 1954]. In 1961 it was understood that BAPN inhibited collagen and elastin cross-linking in some way [Martin et al., 1961]; and Pinnell and Martin later reported that BAPN inhibited an enzyme that catalyzed oxidative deamination of lysine residues in elastin, and named it lysyl oxidase [Pinnell and Martin, 1968]. This enzyme activity was shown to oxidize collagen substrates as well [Siegel and Martin, 1970]. As reviewed previously [Kagan, 1986; Kagan and Trackman, 1991], lysyl oxidase catalyzes the final enzymatic step required for collagen cross-linking, generating peptidyl-δ -hydroxy-α -aminoadipic-δ -semialdehyde and peptidyl-α -aminoadipic-δ -semialdehyde from hydroxylysine and lysine residues, respectively. These aldehydes then spontaneously react with other aldehydes or unmodified lysine and hydroxylysine residues to form a variety of intra- and intermolecular cross-links found in collagens and elastin. Lysyl oxidase is a copper-dependent amine oxidase [Kagan, 1986]. It is secreted from fibrogenic cells as a pro-enzyme that appears to have little or no enzyme activity, and is processed in the extracellular environment to produce the active 32 kDa enzyme and an 18 kDa propeptide [Trackman et al., 1992].
Four additional lysyl oxidase genes have since been identified [Ito et al., 2001; Jourdan-Le Saux et al., 1999; Kenyon et al., 1993; Kim et al., 1995; Maki and Kivirikko, 2001; Maki et al., 2001], and are now known as lysyl oxidase like 1 – 4 (LOXL1 – 4). These proteins have significant sequence identity with mature lysyl oxidase, but have no similarity to the pro-peptide region of lysyl oxidase [Csiszar, 2001]. Relatively little is known regarding the substrate specificity of LOXL-1 – 4, except that LOXL-1 oxidizes an elastin rich substrate [Borel et al., 2001], and LOXL-1 null animals survive and have abnormalities principally in elastin rich tissues [Liu et al., 2004]. By contrast, lysyl oxidase null animals die late in gestation or soon after birth and exhibit a wide range of connective tissue abnormalities [Hornstra et al., 2003; Maki et al., 2002]. LOXL-4 oxidizes a collagen-rich substrate and does not require proteolytic processing [Ito et al., 2001]. Like lysyl oxidase, LOXL-1 and LOXL-4 are inhibited by BAPN [Borel et al., 2001; Ito et al., 2001]. As noted, relatively little is known regarding the substrate specificity of LOXL1-4. This is due in part to the fact that reported recombinant bacterial expression systems for all lysyl oxidases have generally failed to reproducibly yield active enzymes [Di Donato et al., 1997; Jung et al., 2003; Kim et al., 2003; Ouzzine et al., 1996].
A remarkable finding is that secreted pro-lysyl oxidase is processed by procollagen C-proteinases [Panchenko et al., 1996; Uzel et al., 2001], the same enzymes that remove the C-terminal pro-peptides from procollagens I, II, and III [Kessler et al., 1996; Prockop et al., 1998]. This finding raised the interesting possibility that procollagen C-proteinases could serve as a regulatory switch to either promote or inhibit collagen processing and deposition. Procollagen C-proteinases are considered as therapeutic targets in the development of anti-fibrosis drugs as they potentially could interfere both with procollagen processing, and collagen cross-linking and interfere with extracellular matrix formation and development of fibrosis [Pischon et al., 2005; Prockop and Kivirikko, 1995].
Cellular control of collagen deposition
Control of expression and activation of extracellular collagen processing activities alone cannot fully explain fine tuning of the extracellular matrix deposition in different tissues, although removal of the C-propeptides of fibrillar procollagens is absolutely required for normal collagen deposition and fibril growth [Silver et al., 2003]. It is now appreciated that cells and cell surface proteins and a variety of extracellular proteins, glycosaminoglycans, and proteoglycans contribute in significant ways to the control of extracellular matrix deposition and organization, and to the control of collagen deposition in particular [Canty and Kadler, 2005]. For example, a pre-existing fibronectin network in the extracellular matrix has been shown to collaborate with α 11β1 and α 2β1 integrins to promote the efficient deposition of type III and type I collagens by mouse fibroblasts [Velling et al., 2002]. It is interesting that type V collagen is required for normal type I collagen fibril formation [Wenstrup et al., 2004], and that type V collagen itself is processed by BMP-1, a procollagen C-proteinase [Imamura et al., 1998; Kessler et al., 2001]. Additional extracellular matrix elements bind to collagens and contribute to regulation of fibril formation, diameter and uniformity. For example thrombospondins bind to collagens and affect organization and turnover of collagen fibers [Narouz-Ott et al., 2000]. Small leucine rich proteoglycans (SLRP’s) bind to collagens and control fibril diameter and length, and disruption of these interactions results in dramatic connective tissue abnormalities in vivo [Corsi et al., 2002]. Additional non-collagenous proteins influence collagen fibril assembly, and regulation of expression or turnover of these proteins potentially could have important effects on collagen deposition [Cronshaw et al., 1993].
Procollagen C-proteinase and lysyl oxidase as biological control points
As noted, cultured differentiating osteoblasts, and osteoblasts treated with TNF-α , or TGF-β1, deposit insoluble collagen in amounts that diverge from direct regulation of pro-collagen gene expression and procollagen protein synthesis. Recent studies have investigated the mechanism of some of these findings. First, five osteosarcoma cell lines that differed in their ability to deposit a mineralized collagenous bone-like extracellular matrix were studied in order to gain information related to the regulation of collagen deposition by osteoblastic cells [Uzel et al., 2000]. Two clones that produced collagen and BMP-1 and lysyl oxidase activity were the best producers of insoluble collagen, as expected. One of these clones (K14) appears to have produced lysyl oxidase activity principally from one of the relatives of lysyl oxidase, as lower levels of lysyl oxidase mRNA expression and protein and relatively high lysyl oxidase activity were found. Remaining clones produced little type I collagen and did not deposit collagen to any significant degree. The most important finding of this study is that one or more lysyl oxidase like genes could potentially contribute to collagen deposition by osteoblastic cells [Uzel et al., 2000]. A complication of this study is that cells used were osteosarcoma-derived cell lines, and lysyl oxidase itself is known to be down-regulated in many tumor cells and cell lines [Contente et al., 1990; Kenyon et al., 1991; Kuivaniemi et al., 1986]. Thus, low lysyl oxidase expression in some of these cell lines may well have been due to the transformed cell phenotype, rather than being reflective of gene expression patterns in normal osteoblasts.
Phenotypically normal differentiating osteoblasts were, therefore, then studied to assess the regulation of BMP-1 mRNA levels, lysyl oxidase activity and mRNA levels, and collagen protein deposition and mRNA levels [Hong et al., 2004]. The objective of this study was to investigate regulation of these genes and proteins as a function of time in culture as cells progressed through the well known developmental pattern of normal osteoblast development in vitro. Data showed that BMP-1 mRNA levels were constant throughout the entire experimental period of 18 days, whereas lysyl oxidase mRNA levels increased as cells differentiated. Collagen mRNA levels were high early, and then dramatically decreased, while collagen protein deposition increased with time. Most important, the greatest increase in lysyl oxidase enzyme activity immediately preceded the increased collagen deposition, suggesting that lysyl oxidase regulation contributes to collagen deposition. Inhibition of lysyl oxidase enzyme activity with BAPN resulted in accumulation of abnormal collagen fibrils with increased and less uniform diameter. Thus, lysyl oxidase regulation is important for normal collagen deposition by osteoblast cultures, and BMP-1 was found to be constitutively expressed and not regulated as these osteoblasts progressed through their well known differentiation program [Hong et al., 2004].
As noted, TNF-α decreases collagen deposition by osteoblasts by a mechanism that does not include inhibition of collagen synthesis. A role for lysyl oxidase as a target of this inflammatory cytokine was recently investigated. TNF-α treatment of phenotypically normal osteoblast cultures resulted in no change in collagen mRNA levels or collagen synthesis, but collagen cross-linking and lysyl oxidase mRNA, protein, and enzyme activity were all decreased [Pischon et al., 2004]. BMP-1 and PCPE were not regulated by TNF-α.
Taken together, these data summarized above support the idea that lysyl oxidase is regulated as osteoblasts differentiate, and that this regulation contributes to normal collagen deposition [Hong et al., 2004]. In addition, inhibition of lysyl oxidase production by the pro-inflammatory cytokine TNF-α inhibits normal collagen deposition and cross-linking without inhibiting collagen synthesis [Pischon et al., 2004]. It is interesting that BMP-1 was not regulated in untreated differentiating osteoblasts or by TNF-α treated cultures. TNF-α is elevated in osteoporosis and in diabetes, and both pathologies result in osteopenic bone [Pischon et al., 2004]. Thus, down-regulation of lysyl oxidase by TNF-α in osteoblasts, if verified in vivo, could be physiologically significant and related to these pathologies.
Procollagen C-proteinase acts to process both lysyl oxidase and pro-collagens. In order to determine whether inhibition of procollagen C-proteinases with a small molecule inhibitor could result in diminished lysyl oxidase activation, and collagen processing and deposition, we treated differentiating osteoblast cultures with a small molecule BMP-1 inhibitor [Pischon et al., 2005]. This inhibitor was effective in reducing lysyl oxidase processing and activation by about 50%. pC-α 1 collagen was detected by Western blots in media samples from inhibitor treated cultures, indicating that pro-collagen processing was inhibited. Accumulation of mature collagen cross-links was not affected, however, but a small increase and non-uniformity of collagen fibril diameter was observed in inhibitor treated cultures [Pischon et al., 2005]. Thus, BMP-1 inhibitor was partially effective in perturbing lysyl oxidase activation and collagen deposition, but effects were surprisingly modest. We speculate that inhibitors may not have full access to procollagen C-proteinases because these enzymes may be sequestered and inaccessible. These observations may be consistent with the finding of partially processed collagens in vesicles near the cell surface, suggesting that some proteolytic propeptide processing could occur in a protected environment before full extracellular release of procollagens [Canty and Kadler, 2005]. This possibility raises interesting questions regarding whether procollagen and its processing enzymes share the same secretory pathways and vesicles, and where initial processing events occur and how they are controlled. Recent EM studies of tendon cells that deposit collagen fibrils have shown some fibrils sequestered in membranes near the cell surface. A cell surface “fibropositor” structure has been proposed that may coordinate fibril formation in a partially protected environment allowing for cellular control of collagen deposition that may sometimes also include procollagen C-proteinase processing of procollagens [Canty and Kadler, 2005].
The apparent constitutive expression of BMP-1 in developing osteoblast cultures is interesting and was initially somewhat surprising. Because procollagen C-proteinases process both procollagens and lysyl oxidase, it seemed to be ideally suited as a biological control point for the regulation of extracellular collagen deposition and the presence of a generally fibrogenic or generally resorptive phenotype [Prockop and Kivirikko, 1995]. However, and as noted above, it has become clear that that in addition to collagen types I, II, and III, BMP-1 processes a wide variety of extracellular matrix pro-proteins, most notably lysyl oxidase [Panchenko et al., 1996; Uzel et al., 2001], type V collagen [Imamura et al., 1998; Kessler et al., 2001], laminin 5 [Amano et al., 2000], probiglycan [Scott et al., 2000], type VII collagen [Colombo et al., 2003], and dentin matrix protein [Steiglitz et al., 2004]. We speculate that because BMP-1 has such a wide portfolio if important substrates, that control of processing and maturation of extracellular matrix elements is better controlled by regulating each component individually. This permits context-specific regulation of a wide variety of extracellular matrix elements that would not be possible if BMP-1 were a primary control point [Hong et al., 2004].
Biological Functions of Released Pro-peptides
It has been appreciated for some time that released collagen pro-peptides have biological functions. As proteins, these “peptides” are significant in size and have interesting structural properties; and therefore it should be not be a surprise that they have functions. For example, the carboxy-propeptides of type I collagen are cross-linked via disulfide bonds, and once released by procollagen C-proteinase activity the released entity is a non-helical 120 kDa protein made up of three polypeptide chains. This product is a prominent protein in osteoblastic culture media, and has shown to be chemotactic for endothelial cells [Palmieri et al., 2000]. This is likely to be a biologically significant activity, as endochondral bone formation requires vascularization of cartilage to initiate bone formation. Similarly, this released pro-peptide promotes osteoblast cell attachment [Mizuno et al., 1996], inhibits pro-collagen synthesis [Mizuno et al., 2000b], but stimulates the effect of TGF-β on MC3T3-E1 osteoblasts [Mizuno et al., 2000a]. The C-propeptide of type II collagen was found to be the same protein as chodrocalcin [Van der Rest et al., 1986], a basic calcium binding cartilage protein that stimulates mineralization of calcifying cartilage [Poole et al., 1984]. Chondrocalcin binds to other extracellular matrix proteins, suggesting that it may have additional functions in the extracellular matrix [Kirsch and Pfaffle, 1992]. Another well known collagen-derived fragment is endostatin released by elastase or cathepsin L from the C-terminal non-collagenous domain (NC1 domain) of type VXIII collagen [O’Reilly et al., 1997; Zatterstrom et al., 2000]. This and other collagen fragments are angiogenesis inhibitors [Marneros and Olsen, 2001] including the C-terminal non-collagenous region of type XV collagen [Sasaki et al., 2000]. Both products are downstream of a non-collagenous trimerization domain, followed by a non-helical protease sensitive domain, and are released following proteolysis that occurs in the sensitive domain [Kuo et al., 2001; Sasaki et al., 2000; Zatterstrom et al., 2000]. Similarly, the released NC1 domain of α 3 type IV collagen inhibits angiogenesis and is known as tumistatin [Sudhakar et al., 2003].
Recently our laboratory has reported that the N-terminal pro-peptide of lysyl oxidase, released from pro-lysyl oxidase by procollagen C-proteinase, promotes the normal phenotype of cells [Palamakumbura et al., 2004], and may be largely responsible for the tumor suppressor activity of lysyl oxidase [Contente et al., 1990; Kenyon et al., 1991]. This peptide is an arginine-rich but lysine-free 18 kDa protein and is unusually basic with an isoelectric point of 12. The tumor suppressor activity of lysyl oxidase originally identified by Friedman and co-workers [Contente et al., 1990] appears to be largely explained by the ability of the pro-peptide region to inhibit NF-κ B activation that is elevated in tumor cells in which Ras is elevated [Jeay et al., 2003; Palamakumbura et al., 2004]. The mechanisms by which the lysyl oxidase pro-peptide inhibits the ras-dependent activation of NF- κB are actively under investigation. Additional ongoing studies in our laboratory indicate that the lysyl oxidase propeptide helps to control the phenotype of normal osteoblasts as well. The propeptide appears to inhibit osteoblast proliferation and delays differentiation.
PCPE has been found to have tumor suppressor activity. Disruption of the PCPE gene in Rat2 cells resulted in transformation, whereas reconstitution of the functional PCPE gene restored a normal phenotype [Matsuda et al., 1992]. The mechanism by which PCPE inhibits cell proliferation and promotes a normal phenotype is not known, though it is has been suggested that PCPE may be an RNA binding protein that stabilizes collagen and non-collagen mRNA’s [Matsui et al., 2002]. This idea predicts that PCPE can be an intracellular RNA binding protein.
PCPE undergoes proteolytic processing to release the C-terminal domain known as the netrin (NTR) like fragment fragment [Mott et al., 2000]. The NTR peptide was thought to inhibit MMP’s due to its similarity in structure to TIMP’s, but based on more detailed structural studies it now seems more likely that the NTR fragment could be a serine protease inhibitor, or have still to be discovered functions [Liepinsh et al., 2003].
Progress has been made in developing an integrated understanding of extracellular matrix biosynthesis and deposition that includes analyses of extracellular enzymatic and non-enzymatically driven processes. In the context of understanding the regulation of collagen deposition, additional information is still needed regarding the role of varying efficiencies of C- and N-terminal propeptide removal and the regulation of pro-collagen C- and N-proteinases in different tissues and biological contexts. In addition, further studies are needed regarding cellular control of collagen deposition, and the identity and roles of non-collagenous proteins that control collagen fibril formation and maintenance in different tissues, and perturbations of these interactions in diseases. Further insights into the fate and functions of pro-peptides and other released proteolytic fragments derived from extracellular matrix proteins formed in the course of extracellular matrix biosynthesis promise to further enlighten our understanding of the intimate interactions that occur between cells, and the dynamic processes of extracellular matrix biosynthesis.
References
- Adar R, Kessler E, Goldberg B. Evidence for a protein that enhances the activity of type I procollagen C-proteinase. Collagen and Related Research. 1986;6:267 – 277. doi: 10.1016/s0174-173x(86)80011-5. [DOI] [PubMed] [Google Scholar]
- Amano S, Scott IC, Takahara K, Koch M, Champliaud MF, Gerecke DR, Keene DR, Hudson DL, Nishiyama T, Lee S, Greenspan DS, Burgeson RE. Bone morphogenetic protein 1 is an extracellular processing enzyme of the laminin 5 gamma 2 chain. J Biol Chem. 2000;275:22728–35. doi: 10.1074/jbc.M002345200. [DOI] [PubMed] [Google Scholar]
- Borel A, Eichenberger D, Farjanel J, Kessler E, Gleyzal C, Hulmes DJ, Sommer P, Font B. Lysyl oxidase-like protein from bovine aorta. Isolation and maturation to an active form by bone morphogenetic protein-1. J Biol Chem. 2001;276:48944–9. doi: 10.1074/jbc.M109499200. [DOI] [PubMed] [Google Scholar]
- Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–53. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- Centrella M, Casinghino S, Ignotz R, McCarthy TL. Multiple regulatory effects by transforming growth factor-β on type I collagen from fetal rat bone. Endocrinology. 1992;131:2863 –2872. doi: 10.1210/endo.131.6.1446624. [DOI] [PubMed] [Google Scholar]
- Colige A, Sieron AL, Li SW, Schwarze U, Petty E, Wertelecki W, Wilcox W, Krakow D, Cohn DH, Reardon W, Byers PH, Lapiere CM, Prockop DJ, Nusgens BV. Human Ehlers-Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am J Hum Genet. 1999;65:308–17. doi: 10.1086/302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colige A, Vandenberghe I, Thiry M, Lambert CA, Van Beeumen J, Li SW, Prockop DJ, Lapiere CM, Nusgens BV. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J Biol Chem. 2002;277:5756–66. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- Colombo M, Brittingham RJ, Klement JF, Majsterek I, Birk DE, Uitto J, Fertala A. Procollagen VII self-assembly depends on site-specific interactions and is promoted by cleavage of the NC2 domain with procollagen C-proteinase. Biochemistry. 2003;42:11434–42. doi: 10.1021/bi034925d. [DOI] [PubMed] [Google Scholar]
- Contente S, Kaylene K, Rimoldi D, Friedman RM. Expression of gene rrg is associated with reversion of NIH 3T3 cells transformed by LTR -c-H-ras. Science. 1990;249:796 – 798. doi: 10.1126/science.1697103. [DOI] [PubMed] [Google Scholar]
- Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–9. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- Cronshaw AD, Fothergill-Gilmore LA, Hulmes DJS. The proteolytic processing site of the precursor of lysyl oxidase. Biochem J. 1995;306:279 – 284. doi: 10.1042/bj3060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronshaw AD, MacBeath JR, Shackleton DR, Collins JF, Fothergill-Gilmore LA, Hulmes DJ. TRAMP (tyrosine rich acidic matrix protein), a protein that co-purifies with lysyl oxidase from porcine skin. Identification of TRAMP as the dermatan sulphate proteoglycan-associated 22K extracellular matrix protein. Matrix. 1993;13:255–66. doi: 10.1016/s0934-8832(11)80009-0. [DOI] [PubMed] [Google Scholar]
- Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- Di Donato A, Lacal JC, Di Duca M, Giampuzzi M, Ghiggeri G, Gusmano R. Micro-injection of recombinant lysyl oxidase blocks oncogenic p21-Ha-Ras and progesterone effects on Xenopus laevis oocyte maturation. FEBS Lett. 1997;419:63–8. doi: 10.1016/s0014-5793(97)01420-8. [DOI] [PubMed] [Google Scholar]
- Fernandes RJ, Hirohata S, Engle JM, Colige A, Cohn DH, Eyre DR, Apte SS. Procollagen II amino propeptide processing by ADAMTS-3. Insights on dermatosparaxis. J Biol Chem. 2001;276:31502–9. doi: 10.1074/jbc.M103466200. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Perlish JS, Olsen BR. Amino and carboxyl propeptides in bone collagen fibrils during embryogenesis. Cell Tissue Res. 1987;247:105–9. doi: 10.1007/BF00216552. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R, Perlish JS, Timpl R, Olsen BR. Procollagen intermediates during tendon fibrillogenesis. J Histochem Cytochem. 1988;36:1425–32. doi: 10.1177/36.11.3049791. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Iyer B. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Min Res. 1992;7:235 – 246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- Franceschi RT, Iyer BS, Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in MC3T3-E1 cells. J Bone Min Res. 1994;9:843 – 854. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- Geiger BJ, Steenbock H, Parsons HT. Lathyrism in the rat. J Nutr. 1933;6:427–42. doi: 10.1111/j.1753-4887.1976.tb05778.x. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld LC, Chipman SD, Kelly CM, Hodgens KJ, Lee DD, Landis WJ. Collagen expression, ultrastructural assembly, and mineralization in cultures of chicken embryo osteoblasts. J Cell Biol. 1988;106:979 – 989. doi: 10.1083/jcb.106.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen J, Risteli M, Wang C, Latvala J, Rossi M, Valtavaara M, Myllyla R. Lysyl hydroxylase 3 is a multifunctional protein possessing collagen glucosyltransferase activity. J Biol Chem. 2000;275:36158–63. doi: 10.1074/jbc.M006203200. [DOI] [PubMed] [Google Scholar]
- Hong HH, Pischon N, Santana RB, Palamakumbura AH, Chase HB, Gantz D, Guo Y, Uzel MI, Ma D, Trackman PC. A role for lysyl oxidase regulation in the control of normal collagen deposition in differentiating osteoblast cultures. J Cell Physiol. 2004;200:53–62. doi: 10.1002/jcp.10476. [DOI] [PubMed] [Google Scholar]
- Hong HH, Uzel MI, Duan C, Sheff MC, Trackman PC. Regulation of lysyl oxidase, collagen, and connective tissue growth factor by TGF-beta1 and detection in human gingiva. Lab Invest. 1999;79:1655–67. [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278:14387–93. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Steiglitz BM, Greenspan DS. Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-alpha1(V) collagen. J Biol Chem. 1998;273:27511–7. doi: 10.1074/jbc.273.42.27511. [DOI] [PubMed] [Google Scholar]
- Ito H, Akiyama H, Iguchi H, Iyama K, Miyamoto M, Ohsawa K, Nakamura T. Molecular cloning and biological activity of a novel lysyl oxidase-related gene expressed in cartilage. J Biol Chem. 2001;276:24023–9. doi: 10.1074/jbc.M100861200. [DOI] [PubMed] [Google Scholar]
- Jeay S, Pianetti S, Kagan HM, Sonenshein GE. Lysyl oxidase inhibits ras-mediated transformation by preventing activation of NF-kappa B. Mol Cell Biol. 2003;23:2251–63. doi: 10.1128/MCB.23.7.2251-2263.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan-Le Saux C, Tronecker H, Bogic L, Bryant-Greenwood GD, Boyd CD, Csiszar K. The LOXL2 gene encodes a new lysyl oxidase-like protein and is expressed at high levels in reproductive tissues. J Biol Chem. 1999;274:12939–44. doi: 10.1074/jbc.274.18.12939. [DOI] [PubMed] [Google Scholar]
- Jung ST, Kim MS, Seo JY, Kim HC, Kim Y. Purification of enzymatically active human lysyl oxidase and lysyl oxidase-like protein from Escherichia coli inclusion bodies. Protein Expr Purif. 2003;31:240–6. doi: 10.1016/s1046-5928(03)00217-1. [DOI] [PubMed] [Google Scholar]
- Kadler KE, Hojima Y, Prockop DJ. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J Biol Chem. 1987;262:15696–701. [PubMed] [Google Scholar]
- Kagan H (1986): Characterization and regulation of lysyl oxidase. In Mecham RP (ed): “Biology and Regulation of Extracellular Matrix: A Series. Regulation of Matrix Accumulation.” Orlando: Academic Press, pp 321 – 398.
- Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Resp Cell Mol Biol. 1991;5:206 – 210. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- Kenyon K, Contente S, Trackman PC, Tang J, Kagan HM, Friedman RF. Lysyl oxidase and rrg messenger. RNA Science. 1991;253:802. doi: 10.1126/science.1678898. [DOI] [PubMed] [Google Scholar]
- Kenyon K, Modi WS, Contente S, Friedman RM. A novel human cDNA with a predicted protein similar to lysyl oxidase maps to chromosome 15q24-q25. J Biol Chem. 1993;268:18435–7. [PubMed] [Google Scholar]
- Kessler E, Fichard A, Chanut-Delalande H, Brusel M, Ruggiero F. Bone morphogenetic protein-1 (BMP-1) mediates C-terminal processing of procollagen V homotrimer. J Biol Chem. 2001;276:27051–7. doi: 10.1074/jbc.M102921200. [DOI] [PubMed] [Google Scholar]
- Kessler E, Goldberg B. A method for assaying the activity of the endopeptidase which excises the nonhelical carboxyterminal extensions from type I procollagen. Anal Biochem. 1978;86:463 – 469. doi: 10.1016/0003-2697(78)90770-4. [DOI] [PubMed] [Google Scholar]
- Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenic protein-1: the type I procollagen C-proteinase. Science. 1996;271:360 – 362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim SS, Jung ST, Park JY, Yoo HW, Ko J, Csiszar K, Choi SY, Kim Y. Expression and purification of enzymatically active forms of the human lysyl oxidase-like protein 4. J Biol Chem. 2003;278:52071–4. doi: 10.1074/jbc.M308856200. [DOI] [PubMed] [Google Scholar]
- Kim Y, Boyd CD, Csiszar K. A new gene with sequence and structural similarity to the gene encoding human lysyl oxidase. J Biol Chem. 1995;270:7176–82. doi: 10.1074/jbc.270.13.7176. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Pfaffle M. Selective binding of anchorin CII (annexin V) to type II and X collagen and to chondrocalcin (C-propeptide of type II collagen). Implications for anchoring function between matrix vesicles and matrix proteins. FEBS Lett. 1992;310:143–7. doi: 10.1016/0014-5793(92)81316-e. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H, Korhonen R-M, Vaheri A, Kivirikko KI. Deficient production of lysyl oxidase in cultures of malignantly transformed human cells. FEBS Let. 1986;195:261 –264. doi: 10.1016/0014-5793(86)80172-7. [DOI] [PubMed] [Google Scholar]
- Kuo CJ, LaMontagne KR, Jr, Garcia-Cardena G, Ackley BD, Kalman D, Park S, Christofferson R, Kamihara J, Ding YH, Lo KM, Gillies S, Folkman J, Mulligan RC, Javaherian K. Oligomerization-dependent regulation of motility and morphogenesis by the collagen XVIII NC1/endostatin domain. J Cell Biol. 2001;152:1233–46. doi: 10.1083/jcb.152.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton M, Kadler KE. Paired basic/Furin-like proprotein convertase cleavage of Pro-BMP-1 in the trans-Golgi network. J Biol Chem. 2003;278:18478–84. doi: 10.1074/jbc.M213021200. [DOI] [PubMed] [Google Scholar]
- Li SW, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ. The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc Natl Acad Sci U S A. 1996;93:5127–30. doi: 10.1073/pnas.93.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepinsh E, Banyai L, Pintacuda G, Trexler M, Patthy L, Otting G. NMR structure of the netrin-like domain (NTR) of human type I procollagen C-proteinase enhancer defines structural consensus of NTR domains and assesses potential proteinase inhibitory activity and ligand binding. J Biol Chem. 2003;278:25982–9. doi: 10.1074/jbc.M302734200. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Maki JM, Kivirikko KI. Cloning and characterization of a fourth human lysyl oxidase isoenzyme. Biochem J. 2001;355:381–7. doi: 10.1042/0264-6021:3550381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–9. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Maki JM, Tikkanen H, Kivirikko KI. Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol. 2001;20:493–6. doi: 10.1016/s0945-053x(01)00157-3. [DOI] [PubMed] [Google Scholar]
- Malfait F, De Coster P, Hausser I, van Essen AJ, Franck P, Colige A, Nusgens B, Martens L, De Paepe A. The natural history, including orofacial features of three patients with Ehlers-Danlos syndrome, dermatosparaxis type (EDS type VIIC) Am J Med Genet A. 2004;131:18–28. doi: 10.1002/ajmg.a.30299. [DOI] [PubMed] [Google Scholar]
- Marneros AG, Olsen BR. The role of collagen-derived proteolytic fragments in angiogenesis. Matrix Biol. 2001;20:337–45. doi: 10.1016/s0945-053x(01)00151-2. [DOI] [PubMed] [Google Scholar]
- Martin GR, Gross J, Piez KA, Lewis MS. On the intramolecular cross-linking of collagen in lathyritic rats. Biochim Biophys Acta. 1961;53:599–601. doi: 10.1016/0006-3002(61)90227-x. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Lin W, Kumar N, Cho M, Genco R. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J Periodontol. 1992;63:515–525. doi: 10.1902/jop.1992.63.6.515. [DOI] [PubMed] [Google Scholar]
- Matsui A, Yanase M, Tomiya T, Ikeda H, Fujiwara K, Ogata I. Stabilization of RNA strands in protein synthesis by type I procollagen C-proteinase enhancer protein, a potential RNA-binding protein, in hepatic stellate cells. Biochem Biophys Res Commun. 2002;290:898–902. doi: 10.1006/bbrc.2001.6287. [DOI] [PubMed] [Google Scholar]
- McKay GF, Lalich JJ, Schilling ED, Strong FM. A crystalline “lathyrus factor” from Lathyrus odoratus. Arch Biochem. 1954;52:313–22. doi: 10.1016/0003-9861(54)90129-0. [DOI] [PubMed] [Google Scholar]
- Mercer DK, Nicol PF, Kimbembe C, Robins SP. Identification, expression, and tissue distribution of the three rat lysyl hydroxylase isoforms. Biochem Biophys Res Commun. 2003;307:803–9. doi: 10.1016/s0006-291x(03)01262-2. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Fujisawa R, Kuboki Y. Carboxyl-terminal propeptide of type I collagen (c-propeptide) modulates the action of TGF-beta on MC3T3-E1 osteoblastic cells. FEBS Lett. 2000a;479:123–6. doi: 10.1016/s0014-5793(00)01900-1. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Fujisawa R, Kuboki Y. The effect of carboxyl-terminal propeptide of type I collagen (c-propeptide) on collagen synthesis of preosteoblasts and osteoblasts. Calcif Tissue Int. 2000b;67:391–9. doi: 10.1007/s002230001150. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Kitafima T, Tomita M, Kuboki Y. The osteoblastic MC3T3-E1 cells synthesized C-terminal propeptide of type I collagen, which promoted cell-attachment of osteoblasts. Biochim Biophys Acta. 1996;1310:97–102. doi: 10.1016/0167-4889(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Mott J, Thomas C, Rosenbach M, Takahara K, Greenspan D, Banda M. Post-translational proteolytic processing of procollagen C-terminal proteinase enhancer releases a metalloproteinase inhibitor. J Biol CHem. 2000;275:1384–1390. doi: 10.1074/jbc.275.2.1384. [DOI] [PubMed] [Google Scholar]
- Mould AP, Hulmes DJS, Holmes DF, Cummings C, Sear CHJ, Chapman JA. D-periodic assemblies of type I procollagen. J Mol Biol. 1990;211:581–594. doi: 10.1016/0022-2836(90)90267-P. [DOI] [PubMed] [Google Scholar]
- Narouz-Ott L, Maurer P, Nitsche DP, Smyth N, Paulsson M. Thrombospondin-4 binds specifically to both collagenous and non-collagenous extracellular matrix proteins via its C-terminal domains. J Biol Chem. 2000;275:37110–7. doi: 10.1074/jbc.M007223200. [DOI] [PubMed] [Google Scholar]
- Noiva R, Lennarz WJ. Protein disulfide isomerase. A multifunctional protein resident in the lumen of the endoplasmic reticulum. J Biol Chem. 1992;267:3553–6. [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Ouzzine M, Boyd A, Hulmes DJ. Expression of active, human lysyl oxidase in Escherichia coli. FEBS Lett. 1996;399:215–9. doi: 10.1016/s0014-5793(96)01323-3. [DOI] [PubMed] [Google Scholar]
- Palamakumbura AH, Jeay S, Guo Y, Pischon N, Sommer P, Sonenshein GE, Trackman PC. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279:40593–600. doi: 10.1074/jbc.M406639200. [DOI] [PubMed] [Google Scholar]
- Palmieri D, Camardella L, Ulivi V, Guasco G, Manduca P. Trimer carboxyl propeptide of collagen I produced by mature osteoblasts is chemotactic for endothelial cells. J Biol Chem. 2000;275:32658–63. doi: 10.1074/jbc.M002698200. [DOI] [PubMed] [Google Scholar]
- Panagakos FS, Hinojosa LP, Kumar S. Formation and mineralization of extracellular matrix secreted by an immortal human osteoblastic cell line: modulation by tumor necrosis factor-α. Inflammation. 1994;18:267 – 284. doi: 10.1007/BF01534268. [DOI] [PubMed] [Google Scholar]
- Panchenko MV, Stetler-Stevenson WG, Trubetskoy OV, Gacheru SN, Kagan HM. Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. J Biol Chem. 1996;271:7113 –7119. doi: 10.1074/jbc.271.12.7113. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T, Helaakoski T, Tasanen K, Myllyla R, Huhtala ML, Koivu J, Kivirikko KI. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987;6:643–9. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnell SA, Martin GR. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-amino adipic-delta-semialdehyde (allysine) by an extract from bone. Proc of the Natl Acad Sci, USA. 1968;61:708 – 716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischon N, Babakhanlou-Chase H, Darbois L, Ho WB, Brenner MC, Kessler E, Palamakumbura AH, Trackman PC. A procollagen C-proteinase inhibitor diminishes collagen and lysyl oxidase processing but not collagen cross-linking in osteoblastic cultures. J Cell Physiol. 2005;203:111–7. doi: 10.1002/jcp.20206. [DOI] [PubMed] [Google Scholar]
- Pischon N, Darbois LM, Palamakumbura AH, Kessler E, Trackman PC. Regulation of collagen deposition and lysyl oxidase by tumor necrosis factor-alpha in osteoblasts. J Biol Chem. 2004;279:30060–5. doi: 10.1074/jbc.M404208200. [DOI] [PubMed] [Google Scholar]
- Poole AR, Pidoux I, Reiner A, Choi H, Rosenberg LC. Association of an extracellular protein (chondrocalcin) with the calcification of cartilage in endochondral bone formation. J Cell Biol. 1984;98:54–65. doi: 10.1083/jcb.98.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornprasertsuk S, Duarte WR, Mochida Y, Yamauchi M. Lysyl hydroxylase-2b directs collagen cross-linking pathways in MC3T3-E1 cells. J Bone Miner Res. 2004;19:1349–55. doi: 10.1359/JBMR.040323. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Ann Rev Biochem. 1995;64:403 – 434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Sieron AL, Li SW. Procollagen N-proteinase and procollagen C-proteinase. Two unusual metalloproteinases that are essential for procollagen processing probably have important roles in development and cell signaling. Matrix Biol. 1998;16:399–408. doi: 10.1016/s0945-053x(98)90013-0. [DOI] [PubMed] [Google Scholar]
- Quarles L, Yohay D, Lever L, Caton R, Wenstrup R. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone MIneral Res. 1992;7:683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S, Bernocco S, Font B, Moali C, Eichenberger D, Farjanel J, Burchardt E, van der Rest M, Kessler E, Hulmes D. Interaction properties of the procollagen C-proteinase enhancer protein shed light on the mechanism of stimulation of BMP-1. J Biol Chem. 2002;277:33864–33869. doi: 10.1074/jbc.M205018200. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Larsson H, Tisi D, Claesson-Welsh L, Hohenester E, Timpl R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution and anti-angiogenic activity. J Mol Biol. 2000;301:1179–90. doi: 10.1006/jmbi.2000.3996. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KW, Greenspan DS. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- Scott IC, Imamura Y, Pappano WN, Troedel JM, Recklies AD, Roughley PJ, Greenspan DS. Bone morphogenetic protein-1 processes probiglycan. J Biol Chem. 2000;275:30504–11. doi: 10.1074/jbc.M004846200. [DOI] [PubMed] [Google Scholar]
- Siegel RC. Lysyl Oxidase. International Review of Connective Tissue Research. 1979;8:73 – 118. doi: 10.1016/b978-0-12-363708-6.50009-6. [DOI] [PubMed] [Google Scholar]
- Siegel RC, Martin GR. Collagen cross-linking. Enzymatic synthesis of lysine-derived aldehydes and the production of cross-linked components. J Biol Chem. 1970;245:1653–8. [PubMed] [Google Scholar]
- Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 2003;36:1529–53. doi: 10.1016/s0021-9290(03)00135-0. [DOI] [PubMed] [Google Scholar]
- Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279:980–6. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- Steiglitz BM, Keene DR, Greenspan DS. PCOLCE2 encodes a functional procollagen C-proteinase enhancer (PCPE2) that is a collagen-binding protein differing in distribution of expression and post-translational modification from the previously described PCPE1. J Biol Chem. 2002;277:49820–30. doi: 10.1074/jbc.M209891200. [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Stein JL, Van Wijnen J, Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol Rev. 1996;76:593 – 629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by alpha v beta 3 and alpha 5 beta 1 integrins. Proc Natl Acad Sci U S A. 2003;100:4766–71. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Takahara K, Kessler E, Biniaminov L, Brusel M, Eddy RL, Jani-Sait S, Shows TB, Greenspan DS. Type I procollagen COOH-terminal proteinase enhancer protein: identification, primary structure, and chromosomal localization of the cognate human gene (PCOLCE) J Biol Chem. 1994;269:26280–5. [PubMed] [Google Scholar]
- Trackman PC, Tang J, Bedell-Hogan D, Kagan HM. Posttranslational glycosylation and proteolytic processing of a lysyl oxidase precursor. J Biol Chem. 1992;267:8666 –8671. [PubMed] [Google Scholar]
- Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, Hong HH, Greenspan DS, Trackman PC. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem. 2001;276:22537–43. doi: 10.1074/jbc.M102352200. [DOI] [PubMed] [Google Scholar]
- Uzel MI, Shih SD, Gross H, Kessler E, Gerstenfeld LC, Trackman PC. Molecular events that contribute to lysyl oxidase enzyme activity and insoluble collagen accumulation in osteosarcoma cell clones. J Bone Miner Res. 2000;15:1189–97. doi: 10.1359/jbmr.2000.15.6.1189. [DOI] [PubMed] [Google Scholar]
- Van der Rest M, Rosenberg L, Olsen B, Poole A. Chondrocalcin is identical with the C-propeptide of type II procollagen. J Biochem. 1986;237:923–925. doi: 10.1042/bj2370923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Slot AJ, Zuurmond AM, Bardoel AF, Wijmenga C, Pruijs HE, Sillence DO, Brinckmann J, Abraham DJ, Black CM, Verzijl N, DeGroot J, Hanemaaijer R, TeKoppele JM, Huizinga TW, Bank RA. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem. 2003;278:40967–72. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- Velling T, Risteli J, Wennerberg K, Mosher DF, Johansson S. Polymerization of type I and III collagens is dependent on fibronectin and enhanced by integrins alpha 11beta 1 and alpha 2beta 1. J Biol Chem. 2002;277:37377–81. doi: 10.1074/jbc.M206286200. [DOI] [PubMed] [Google Scholar]
- Walker LC, Overstreet MA, Yeowell HN. Tissue-specific expression and regulation of the alternatively-spliced forms of lysyl hydroxylase 2 (LH2) in human kidney cells and skin fibroblasts. Matrix Biol. 2005;23:515–23. doi: 10.1016/j.matbio.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Wang C, Luosujarvi H, Heikkinen J, Risteli M, Uitto L, Myllyla R. The third activity for lysyl hydroxylase 3: galactosylation of hydroxylysyl residues in collagens in vitro. Matrix Biol. 2002a;21:559–66. doi: 10.1016/s0945-053x(02)00071-9. [DOI] [PubMed] [Google Scholar]
- Wang C, Risteli M, Heikkinen J, Hussa AK, Uitto L, Myllyla R. Identification of amino acids important for the catalytic activity of the collagen glucosyltransferase associated with the multifunctional lysyl hydroxylase 3 (LH3) J Biol Chem. 2002b;277:18568–73. doi: 10.1074/jbc.M201389200. [DOI] [PubMed] [Google Scholar]
- Wang WM, Lee S, Steiglitz BM, Scott IC, Lebares CC, Allen ML, Brenner MC, Takahara K, Greenspan DS. Transforming growth factor-beta induces secretion of activated ADAMTS-2. A procollagen III N-proteinase. J Biol Chem. 2003;278:19549–57. doi: 10.1074/jbc.M300767200. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Florer JB, Cole WG, Willing MC, Birk DE. Reduced type I collagen utilization: a pathogenic mechanism in COL5A1 haplo-insufficient Ehlers-Danlos syndrome. J Cell Biochem. 2004;92:113–24. doi: 10.1002/jcb.20024. [DOI] [PubMed] [Google Scholar]
- Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci U S A. 2003;100:15842–6. doi: 10.1073/pnas.2534946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatterstrom UK, Felbor U, Fukai N, Olsen BR. Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell Struct Funct. 2000;25:97–101. doi: 10.1247/csf.25.97. [DOI] [PubMed] [Google Scholar]


