Abstract
Certain β-lactam antibiotics induce the chromosomal ampC β-lactamase of many gram-negative bacteria. The natural inducer, though not yet unequivocally identified, is a cell wall breakdown product which enters the cell via the AmpG permease component of the murein recycling pathway. Surprisingly, it has been reported that β-lactamase is not induced by cefoxitin in the absence of FtsZ, which is required for cell division, or in the absence of penicillin-binding protein 2 (PBP2), which is required for cell elongation. Since these results remain unexplained, we examined an ftsZ mutant and other cell division mutants (ftsA, ftsQ, and ftsI) and a PBP2 mutant for induction of β-lactamase. In all mutants, β-lactamase was not induced by cefoxitin, which confirms the initial reports. The murein precursor, UDP-N-acetylmuramyl-l-Ala-γ-d-Glu-meso-diaminopimelic acid-d-Ala-d-Ala (UDP-MurNAc-pentapeptide), has been shown to serve as a corepressor with AmpR to repress β-lactamase expression in vitro. Our results suggest that β-lactamase is not induced because the fts mutants contain a greatly increased amount of corepressor which the inducer cannot displace. In the PBP2(Ts) mutant, in addition to accumulation of corepressor, cell wall turnover and recycling were greatly reduced so that little or no inducer was available. Hence, in both cases, a high ratio of repressor to inducer presumably prevents induction.
In many gram-negative organisms, class I β-lactamase is induced by certain β-lactam antibiotics, such as cefoxitin and imipenem, as well as some nonspecific inducers (9, 26, 30). Citrobacter freundii and Enterobacter cloacae have been the principal bacteria studied. However, the ampC β-lactamase of Escherichia coli is not inducible (11). Therefore, in the studies reporting noninducibility in fts and penicillin-binding protein 2 (PBP2) mutants, the C. freundii ampR-ampC inducible region present on plasmid pNU305 in various E. coli strains was studied (28, 29).
Induction is dependent upon recycling of murein degradation products (14, 18, 33). AmpD, AmpG, and NagZ, components of the murein recycling pathway, are involved in β-lactamase induction (6, 10, 14, 15, 17, 22, 39). Deletion of AmpD causes both high-level constitutive expression of ampC (19) and a massive accumulation of the murein breakdown prod-uct anhydro-N-acetylmuramyl-l-Ala-γ-d-Glu-meso-diamino-pimelic acid (DAP) (anhMurNAc-tripeptide) (14). AmpG is required for the uptake of GlcNAc-anhMurNAc-peptides (Cheng and Park, unpublished observations) and for the high-level constitutive expression, as well as the induction, of β-lactamase (17).
The expression of ampC is transcriptionally controlled by the divergently coded gene ampR (21). AmpR belongs to the LysR family of bacterial regulators. The proteins of this family have a helix-turn-helix DNA-binding motif at the N-terminal region and a cofactor-binding domain (26). Jacobs et al. (13), employing an in vitro transcription assay, showed that AmpR is an activator for ampC expression. However, in the presence of UDP-N-acetylmuramyl-l-Ala-γ-d-Glu-meso-DAP-d-Ala-d-Ala (UDP-MurNAc-pentapeptide), AmpR served as a repressor. AnhMurNAc-tripeptide, if present in sufficient amounts, was shown to displace the corepressor from AmpR, leading to the expression of ampC. Since UDP-MurNAc-pentapeptide is present at a high concentration in the cytoplasm (24), it has been assumed that ampC expression is normally repressed in vivo. In an ampD mutant, anhMurNAc-tripeptide is accumulated sufficiently to counteract UDP-MurNAc-pentapeptide and cause the constitutive expression of ampC. However, the induction of ampC by the addition of cefoxitin to a growing culture cannot be explained by anhMurNAc-tripeptide as an inducer since anhMurNAc-tripeptide is not accumulated under these conditions (14).
A decade ago, Ottolenghi and Ayala showed that in an E. coli temperature-sensitive ftsZ mutant, induction by cefoxitin of C. freundii ampC (present on a plasmid) was inhibited by about 80% whereas in ftsA and ftsQ mutants, induction, although somewhat delayed, did occur (29). Since FtsZ, FtsA, FtsQ, and FtsI are all essential cell division proteins required for formation of the septum (23), it seemed somewhat surprising that loss of FtsZ would affect β-lactamase induction and that the other proteins essential for the same process did not. Therefore, we reinvestigated the relationship of the fts genes to β-lactamase induction. PBP2, a murein transpeptidase required for lateral wall synthesis and cell shape (12, 37, 38), was also studied since it has been shown to be required for induction (28).
In this paper, we report the probable mechanism that links cell division and ampC induction and present evidence to explain the requirement for PBP2.
MATERIALS AND METHODS
Bacteria, plasmids, and culture conditions.
The E. coli strains and plasmids used in this work are listed in Table 1. Because E. coli does not have an inducible β-lactamase operon (11), we studied the C. freundii system. Thus, all strains carried plasmid pNU305, which expresses the ampR-ampC region of C. freundii. Bacteria were grown in L broth supplemented with lysine (100 μg/ml), threonine (100 μg/ml), and methionine (100 μg/ml). Tetracycline (10 μg/ml) or spectinomycin (25 μg/ml) was added to maintain the selection of cells carrying pNU305 or pBP58, respectively. Growth of culture was assayed by measuring the optimal density of the cultures in a Klett-Summerson colorimeter.
TABLE 1.
E. coli K-12 strains and plasmids used in this work
| Strain or plasmid | Genotype | Source and/or reference |
|---|---|---|
| E. coli strainsa | ||
| TP71 | F−lysA opp araD139 rpsL150 relA1 thi-1 deoC1 ptsF25 flbB5301 rbsR Δ(argF-lac) | 14 |
| TP71ftsZ | TP71 ftsZ84(Ts) leu+ | ftsZ84 allele from DRC13 (32) |
| TP72ftsZ | TP71 ftsZ84(Ts) ampG::kan | ampG::kan from TP72 (14) |
| TP71ftsA | TP71 ftsA6(Ts) leu+ | ftsA6 allele from BUG6 (4) |
| TP71ftsQ | TP71 ftsQ1(Ts) leu+ | ftsQ1 allele from TOE1 (1) |
| TP71ftsI | TP71 ftsI23(Ts) leu+ | ftsI23 allele from TOE23 (2) |
| TP71PBP2 | TP71 pbpA45(Ts) nag+ | pbpA45 allele from SP4500 (37) |
| TP72PBP2 | TP71 ampG::kan pbpA45(Ts) | ampG::kan from TP72 (14) |
| Plasmids | ||
| pNU305 | ampC-ampR cluster from C. freundii in pBR322, Tcr | 20 |
| pBS58 | ftsQ-ftsA-ftsZ operon from E. coli in pGB2, Spcr | 3 |
β-Lactamase induction assay.
Eighty milliliters of medium was inoculated with 400 μl of an overnight culture of the test strain and incubated at 30°C with aeration. When the culture reached a turbidity of 15 Klett units, the culture was divided into two aliquots (40 ml each). The aliquots were incubated at 42°C for 30 min. Cefoxitin was then added to one aliquot, and the other served as an uninduced control. Both aliquots were incubated further at 42°C. At the appropriate times, samples were taken from both aliquots, rapidly chilled to 0°C, collected by centrifugation, washed once with 0.1 M potassium phosphate buffer (pH 7.0), and frozen at −20°C. The cells were resuspended in 1 ml of 0.1 M potassium phosphate buffer (pH 7.0) and ruptured on ice with a sonifier (Branson Ultrasonics Corp., Danbury, Conn.), generating the cell extract for β-lactamase determination. β-Lactamase activity was measured with nitrocefin used as a substrate (27). The protein content of each sample was determined by the protein assay method (Bio-Rad Laboratories, Hercules, Calif.), with bovine serum albumin used as the standard.
Labeling with [3H]DAP.
All strains carry the lysA mutation. In lysA mutants, DAP cannot be converted into lysine and 3H remains exclusively in [3H]DAP. Twenty milliliters of medium supplemented with 20 μCi of [3H]DAP (20 Ci/mmol; Moravek Biochemicals, Inc., Brea, Calif.) was inoculated with 100 μl of an overnight culture of the test strain and incubated at 30°C with aeration. When the culture reached a turbidity of 15 Klett units, the culture was divided into two aliquots. The aliquots were then incubated at 42°C for 30 min. Cefoxitin was added (final concentration, 8 μg/ml) to one aliquot, and incubation of the two aliquots was continued for another 30 min. The aliquots were rapidly chilled to 0°C, collected by centrifugation, and washed once with 10 ml of cold water. The cells were suspended in 2.5 ml of water and heated at >95°C for 5 min, and the resulting suspension was chilled and centrifuged to remove cell debris. The supernatant (hot-water extract) was collected and lyophilized. Samples of hot-water extracts were analyzed by high-pressure liquid chromatography (HPLC) as described below.
HPLC analysis.
HPLC was performed with Rabbit high-pressure pumps and mixer equipment (Rainin Instrument Co., Woburn, Mass.). Samples were adjusted to a pH of ∼2.5 with trifluoroacetic acid, centrifuged to remove the particulate matter, adsorbed on a 150- by 4.6-mm XTerra RP18 column (Waters, Milford, Mass), and eluted at 0.5 ml/min with 0.05% trifluoroacetic acid for 10 min, followed by a gradient from 0.05% trifluoroacetic acid to 10% acetonitrile containing 0.035% trifluoroacetic acid (buffer ACN) over a period of 50 min. Thereafter, elution was continued with 10% buffer ACN over 20 min. Fractions of the column effluent of 0.25 ml were collected. The radioactivity of each fraction was determined.
RESULTS
β-Lactamase activity of E. coli cell division mutants in the presence of cefoxitin.
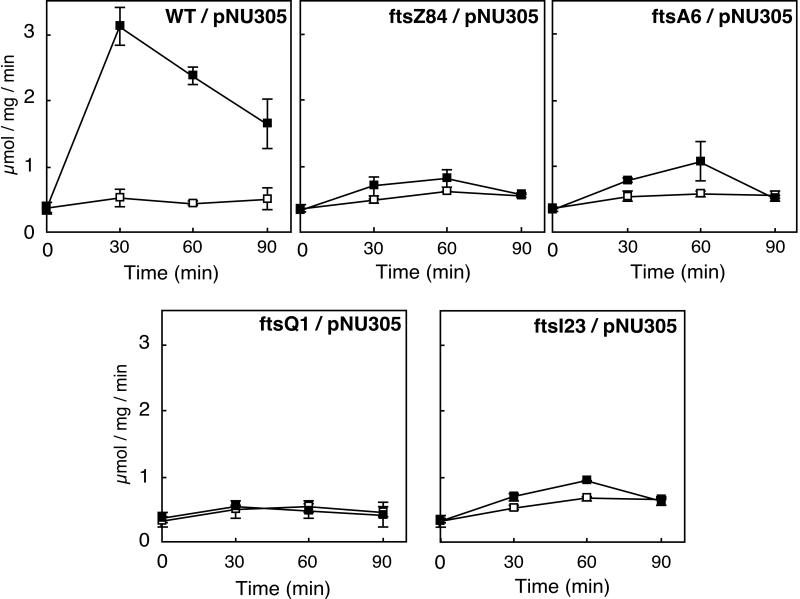
Figure 1 shows the changes in C. freundii ampC β-lactamase activity over time in wild-type and isogenic cell division mutants grown at 42°C following the addition of cefoxitin. The concentration of cefoxitin used (8 μg/ml) was the MIC at 42°C for the wild-type strain TP71/pNU305.
FIG. 1.
Time course of β-lactamase activity in cell division mutants of E. coli carrying ampR-ampC of C. freundii. Cells were grown at 30°C to 15 Klett units. Following incubation at 42°C for 30 min, cefoxitin (8 μg/ml) was added (▪) or not added (□) to the culture. Samples were taken at the times indicated and chilled. Extracts were prepared, and β-lactamase activity was measured as described in Materials and Methods. Symbols represent means of two independent experiments.
At the restrictive temperature, growth of wild-type cells appeared to slow after the addition of cefoxitin. This was the result of the lysis of some cells. On the other hand, cell division mutants grew steadily at the restrictive temperature whether cefoxitin was present or not. As shown in Fig. 1, β-lactamase activities in the cell division mutants increased only slightly following the addition of cefoxitin while in the wild-type strain, in spite of significant lysis, β-lactamase was induced about eightfold in 30 min. Note that the specific activity of β-lactamase in wild-type cells decreased after 30 min, suggesting loss of the inducer. When another 8 μg of cefoxitin/ml was added to the culture at 30 min, induction continued (data not shown), indicating that cessation of induction was caused by the breakdown of cefoxitin by the induced β-lactamase.
pBS58 is a low-copy plasmid expressing the ftsQ-ftsA-ftsZ operon of E. coli. It is compatible with pNU305. When β-lactamase activities were measured 30 min after the addition of cefoxitin in the presence or absence of pBS58 (Table 2), ftsZ, ftsA, ftsQ, and ftsI were all needed to produce full expression of C. freundii ampC β-lactamase. pBS58 complemented the temperature sensitivity of cell division mutants TP71ftsZ, TP71ftsA, and TP71ftsQ and restored induction of ampC β-lactamase in all of the cell division mutants. These results strongly suggest that the cell division process must be functioning in order for ampC induction to occur.
TABLE 2.
Influence of cell division mutations on β-lactamase inductiona
| Strainb | β-Lactamase activity (μmol/mg/min)c
|
|
|---|---|---|
| Noninduced | Induced | |
| WT | 0.5 | 3.1 |
| WT/pBS58 | 0.4 | 3.3 |
| ftsZ84 | 0.5 | 0.7 |
| ftsZ84/pBS58 | 0.5 | 2.6 |
| ftsA6 | 0.6 | 0.8 |
| ftsA6/pBS58 | 0.4 | 2.6 |
| ftsQ1 | 0.5 | 0.6 |
| ftsQ1/pBS58 | 0.4 | 3.0 |
| ftsI23 | 0.5 | 0.7 |
Antibiotic was added after the cells had grown at 42°C for 30 min following the temperature shift from 30 to 42°C. β-Lactamase activity was measured after a 30-min induction period. The concentration of each antibiotic was the MIC at 42°C.
All strains carry the plasmid pNU305, which contains the ampR-ampC region from C. freundii. WT, wild-type strain.
Micromoles of nitrocefin destroyed per minute per milligram of protein. Destruction of 10−4 mol of nitrocefin increases the A490 value by 1.5. Data are means from two individual experiments.
Why is cell division required? (i) Do nondividing cells fail to take up inducer?
One possible reason for the repression of ampC induction in cell division mutants is that the block of cell division causes a defect in murein turnover or recycling. Since AmpG is a permease essential for murein recycling, we compared the rates of murein peptide turnover in TP71ftsZ and the ampG ftsZ double mutant TP72ftsZ growing at 42°C. The turnover rate of TP71ftsZ (12% per generation) was much less than that of TP72ftsZ (32% per generation), indicating that the murein was being recycled by TP71ftsZ. Hence, the cell wall-derived inducer was able to enter TP71ftsZ cells.
(ii) Does the corepressor UDP-MurNAc-pentapeptide accumulate in the cell division mutants?
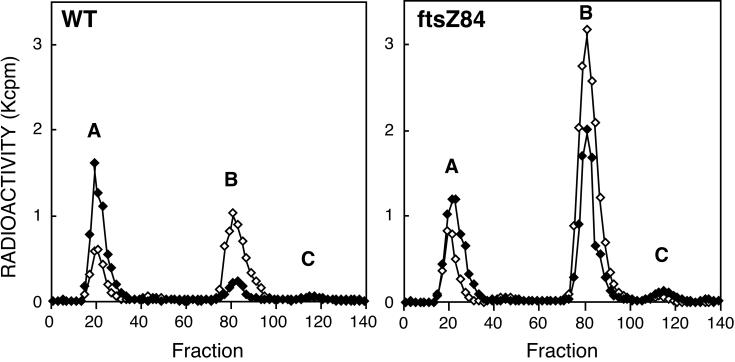
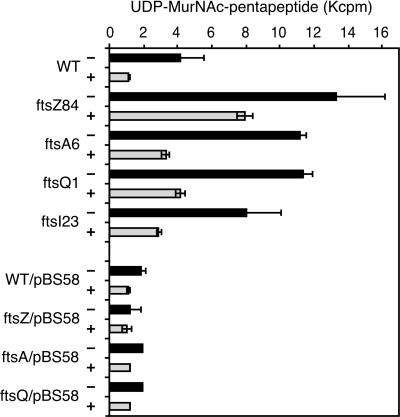
Since interrupting septal murein synthesis prevents β-lactamase induction, another possibility is that the concentration of the murein biosynthetic intermediates increases when cell division ceases in the mutants. This could offset the increased amount of inducer produced in the presence of cefoxitin. The four cell division mutants were compared with the wild type as follows. The wild type and mutants were continuously labeled with [3H]DAP and treated or not treated with 8 μg of cefoxitin/ml at 42°C as described in Materials and Methods. Hot-water extracts were analyzed by HPLC to compare their 3H-labeled components. Figure 2 shows the HPLC analysis of the wild-type strain and the ftsZ84 mutant with or without cefoxitin. Free DAP and murein peptides were eluted in fraction A, UDP-MurNAc-pentapeptide was eluted in fraction B, and anhMurNAc-peptides were eluted in fraction C. In the absence of cefoxitin, the amount of UDP-MurNAc-pentapeptide (peak B) in the ftsZ mutant was about three times larger than that in the wild type. Although cefoxitin treatment reduced the amount of UDP-MurNAc-pentapeptide in both the ftsZ mutant and the wild-type cells, the ftsZ mutant with cefoxitin contained much more UDP-MurNAc-pentapeptide than the wild-type strain with or without cefoxitin. Figure 3 compares the amount of UDP-MurNAc-pentapeptide in the wild type with that of the four cell division mutants and the mutants carrying a plasmid expressing ftsZ, ftsQ, and ftsA. The amount of UDP-MurNAc-pentapeptide was also large in the other three cell division mutants, being lowest in the ftsI mutant, which still contained about three times that present in the wild type. As expected, the mutants complemented by pBS58 contained an amount of UDP-MurNAc-pentapeptide comparable to that in the wild-type strain. These results indicate that the inhibition of cell division causes a large accumulation of UDP-MurNAc-pentapeptide. This may prevent the activation of AmpR that normally occurs in the presence of cefoxitin.
FIG. 2.
HPLC analysis of hot-water extracts of E. coli TP71 (wild type) and TP71ftsZ84. As described in Materials and Methods, cells were labeled with [3H]DAP at 30°C, shifted to 42°C for 30 min, and then incubated a further 30 min at 42°C with (♦) or without (⋄) 8 μg of cefoxitin/ml at 42°C. Values were corrected by the cell turbidity (100 Klett units). Fractions: A, a mixture containing DAP and free murein peptides; B, UDP-MurNAc-pentapeptide; C, GlcNAc-anhMurNAc-tripeptide and anhMurNAc-pentapeptide.
FIG. 3.
Comparison of amount of UDP-MurNAc-pentapeptide in wild-type, ftsZ, ftsA, ftsQ, and ftsI mutants and the four cell division mutants complemented by pBS58. The absence (−) or presence (+) of 8 μg of cefoxitin/ml is shown. Bars represent the means of two independent experiments except that the values for ftsA6/pBS58 and ftsQ1/pBS58 were measured from one experiment.
β-Lactamase induction and murein metabolism in the PBP2(Ts) mutant.
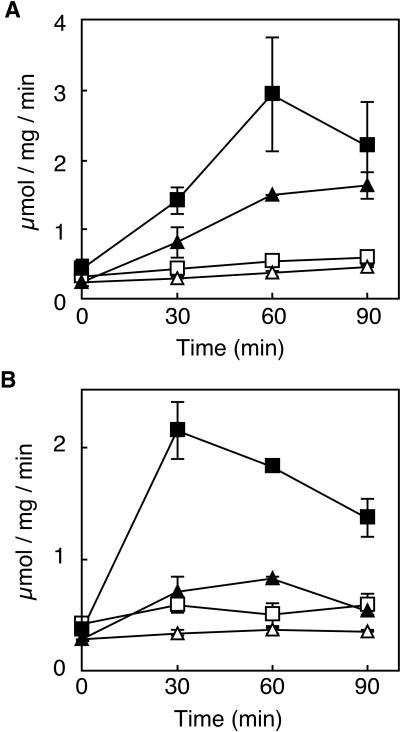
Figure 4 shows the change in β-lactamase activity over time in wild-type TP71 and the TP71PBP2(Ts) mutant with or without cefoxitin at 30 and 42°C. Cefoxitin (8 μg/ml) was used at 30°C, but it was necessary to use only 4 μg/ml at 42°C to avoid lysis. As shown in Fig. 4, β-lactamase was induced in both strains at 30°C, but at 42°C induction was much less in the mutant than in the wild type. These results confirm those of Oliva et al. (28).
FIG. 4.
Time course of β-lactamase activity in wild-type TP71 and the PBP2(Ts) mutant carrying pNU305. Cells were grown at 30°C (A) or 42°C (B) in the presence or absence of cefoxitin (8 μg/ml at 30°C, 4 μg/ml at 42°C). Results for TP71 with (▪) or without (□) cefoxitin and TP71PBP2(Ts) with (▴) or without (▵) cefoxitin are shown.
As mentioned earlier, there are three possible reasons for the lack of induction: no turnover, no recycling, or accumulation of the corepressor. As expected, accumulation of corepressor occurred comparable to that observed with the fts mutants (data not shown). The PBP2(Ts) mutant was also found to have greatly reduced turnover. Specifically, the turnover rate of TP72PBP2 (ampG pbpA double mutant) was only about 5% per generation, which is remarkably lower than the 30 or 40% turnover rate in TP72 (ampG pbpA+) (14). Since the inducer enters the cell via the recycling pathway and turnover provides the ligands to be recycled, this result suggests that much less inducer is available in the PBP2(Ts) mutant. Thus, either a reduced amount of inducer or an accumulation of corepressor is sufficient to explain the inability of the PBP2(Ts) mutant to induce β-lactamase.
DISCUSSION
Basis for noninducibility of fts mutants.
As we have demonstrated, ampC β-lactamase is not induced by cefoxitin in the four cell division mutants tested. All the cell division mutants, when grown at the restrictive temperature, accumulated a large amount of the nucleotide-bound murein precursor. Hence, the large amount of UDP-MurNAc-pentapeptide is likely responsible for the lack of induction of β-lactamase by cefoxitin. When cell division is inhibited by shifting an fts mutant to the restrictive temperature, the rate of murein synthesis is reduced by over 30% (4, 40). The continued synthesis of precursors at a near-normal rate accounts for the observed accumulation of UDP-MurNAc-pentapeptide.
Our results indicate that the model for β-lactamase induction proposed by Jacobs et al. (13) is operative in vivo. The concentration of corepressor (UDP-MurNAc-pentapeptide) present in the cell division mutants growing at the restrictive temperature was 2.5- to 6-fold higher than that in normal cells, and presumably this was sufficient to prevent the natural inducer, the levels of which were increased by cefoxitin, from converting AmpR into an activator. Thus, whatever level of natural inducer is produced in the presence of cefoxitin would have to be 2.5- to 6-fold higher in the cell division mutants to obtain the equivalent amount of ampC message. This may explain why our results are somewhat different from those of Ottolenghi and Ayala, who reported that β-lactamase induction requires FtsZ but not FtsA and FtsQ (29). Less UDP-MurNAc-pentapeptide is present in the ftsA and ftsQ cells than in the ftsZ cells (Fig. 3) so that a smaller amount of natural inducer would be required to induce the ftsA and ftsQ strains. The largest accumulation of UDP-MurNAc-pentapeptide is in the ftsZ mutant because it does not initiate septal murein synthesis at the restrictive temperature (8). ftsA, ftsQ, and ftsI mutants, on the other hand, do initiate septal murein synthesis (8), thus accounting for the small amount of UDP-MurNAc-pentapeptide accumulating in these mutants compared to that in ftsZ.
Basis for poor inducibility of the PBP2(Ts) mutant.
Murein synthesis is inhibited by more than 60% when elongation of the murein sacculus is interrupted by inactivation of PBP2 (34). Consistent with this reduced rate of synthesis, the PBP2(Ts) mutant, like the fts mutants, accumulated UDP-MurNAc-pentapeptide when growing at the restrictive temperature. PBP2 mutants appear to grow exclusively by the septation pathway that forms the cells' poles (37, 38). Cell poles, once formed, are stable (5, 7, 8, 16). This suggests that, unlike the sidewalls, the poles do not turn over. We have confirmed this by directly demonstrating lack of turnover by an ampG PBP2(Ts) double mutant growing at the restrictive temperature. Without turnover, therre can be no recycling and hence there is no source of cell wall-derived inducer. Thus, for two reasons, the ratio of corepressor to inducer remains high and there is little induction of β-lactamase.
What is the true inducer of β-lactamase?
The natural inducer of β-lactamase has not yet been unambiguously identified. Jacobs et al. (14) demonstrated that in ampD cells, which constitutively express ampC due to the lack of the AmpD amidase that cleaves anhMurNAc-tripeptide (10, 15), large amounts of anhMurNAc-tripeptide accumulate. Hence it was postulated that anhMurNAc-tripeptide serves as the natural inducer. Jacobs et al. later demonstrated that indeed very large amounts of anhMurNAc-tripeptide could overcome repression by AmpR:UDP-MurNAc-pentapeptide and induce the production of ampC message in a cell-free expression system (13). However, it is clear from the results shown in Fig. 5D of reference 14 that very little, and certainly insufficient, anhMurNAc-tripeptide accumulates in cefoxitin-treated cells to cause induction. The cefoxitin-treated cells do show a significant accumulation of DAP-containing peptides, however.
Dietz et al. have presented strong evidence that the effectiveness of an antibiotic as an inducer correlates with its ability to cause the accumulation of GlcNAc-anhMurNAc-pentapeptide in the periplasm and suggest that anhMurNAc-pentapeptide may be the true inducer (9). Imipenem and cefoxitin were by far the best accumulators and inducers in their report. Cefoxitin and imipenem inhibit d,d-carboxypeptidases (PBP4, PBP5, and PBP6) (36), thus causing more pentapeptides to be present in the murein. The normal recycling pathway degrades over 50% of the sidewall each generation and would transport the increased amount of GlcNAc-anhMurNAc-pentapeptide formed in response to cefoxitin into the cytoplasm, where it would be further degraded (14, 31, 33). It seems logical that the natural inducer should contain pentapeptide. Cefoxitin causes a marked increase in the pentapeptide content of the murein, and the inducer must compete with UDP-MurNAc-pentapeptide to activate ampC expression. In fact, an E. coli strain lacking PBP4, PBP5, PBP6a, and PBP6b has been reported to express E. cloacae β-lactamase constitutively (35). However, we observed very little accumulation of anhMurNAc-peptides in cefoxitin-treated cells (Fig. 2, fraction C) (14). In the same experiments, we did see a significant increase in fractions A, which contained free tripeptide and pentapeptide as well as DAP. Thus, it is possible that free pentapeptide may be the true inducer of β-lactamase. Further work is necessary in order to test this hypothesis.
Acknowledgments
This work was supported in part by Public Health Service grant GM51610 from the National Institute of General Medical Sciences.
We thank Miguel A. de Pedro for E. coli strain SP4500.
REFERENCES
- 1.Begg, K. J., G. F. Hatfull, and W. D. Donachie. 1980. Identification of new genes in a cell envelope-cell division gene cluster of Escherichia coli: cell division gene ftsQ. J. Bacteriol. 144:435-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begg, K. J., B. G. Spratt, and W. D. Donachie. 1986. Interaction between membrane proteins PBP3 and RodA is required for normal cell shape and division in Escherichia coli. J. Bacteriol. 167:1004-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bi, E., and J. Lutkenhaus. 1990. FtsZ regulates frequency of cell division in Escherichia coli. J. Bacteriol. 172:2765-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Botta, G. A., and J. T. Park. 1981. Evidence for involvement of penicillin-binding protein 3 in murein synthesis during septation but not during cell elongation. J. Bacteriol. 145:333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burman, L. G., J. Raichler, and J. T. Park. 1983. Evidence for diffuse growth of the cylindrical portion of the Escherichia coli murein sacculus. J. Bacteriol. 155:983-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng, Q., H. Li, K. Merdek, and J. T. Park. 2000. Molecular characterization of the β-N-acetylglucosaminidase of Escherichia coli and its role in cell wall recycling. J. Bacteriol. 182:4836-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Pedro, M. A., W. D. Donachie, J. V. Holtje, and H. Schwarz. 2001. Constitutive septal murein synthesis in Escherichia coli with impaired activity of the morphogenetic proteins RodA and penicillin-binding protein 2. J. Bacteriol. 183:4115-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Pedro, M. A., J. C. Quintela, J. V. Holtje, and H. Schwarz. 1997. Murein segregation in Escherichia coli. J. Bacteriol. 179:2823-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dietz, H., D. Pfeifle, and B. Wiedemann. 1997. The signal molecule for β-lactamase induction in Enterobacter cloacae is the anhydromuramyl-pentapeptide. Antimicrob. Agents Chemother. 41:2113-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holtje, J. V., U. Kopp, A. Ursinus, and B. Wiedemann. 1994. The negative regulator of β-lactamase induction AmpD is a N-acetyl-anhydromuramyl-L-alanine amidase. FEMS Microbiol. Lett. 122:159-164. [DOI] [PubMed] [Google Scholar]
- 11.Honore, N., M. H. Nicolas, and S. T. Cole. 1986. Inducible cephalosporinase production in clinical isolates of Enterobacter cloacae is controlled by a regulatory gene that has been deleted from Escherichia coli. EMBO J. 5:3709-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishino, F., S. Tamaki, B. G. Spratt, and M. Matsuhashi. 1982. A mecillinam-sensitive peptidoglycan crosslinking reaction in Escherichia coli. Biochem. Biophys. Res. Commun. 109:689-696. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs, C., J. M. Frere, and S. Normark. 1997. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in gram-negative bacteria. Cell 88:823-832. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs, C., L. J. Huang, E. Bartowsky, S. Normark, and J. T. Park. 1994. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 13:4684-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, C., B. Joris, M. Jamin, K. Klarsov, B. J. Van Beeumen, D. Mengin-Lecreulx, J. van Heijenoort, J. T. Park, S. Normark, and J. M. Frere. 1995. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-L-alanine amidase. Mol. Microbiol. 15:553-559. [DOI] [PubMed] [Google Scholar]
- 16.Koch, A. L., and C. L. Woldtingh. 1994. The metabolic inertness of the pole wall of a gram-negative rod. J. Theor. Biol. 171:415-425. [Google Scholar]
- 17.Korfmann, G., and C. C. Sanders. 1989. ampG is essential for high-level expression of AmpC β-lactamase in Enterobacter cloacae. Antimicrob. Agents Chemother. 33:1946-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraft, A. R., J. Prabhu, A. Ursinus, and J. V. Holtje. 1999. Interference with murein turnover has no effect on growth but reduces β-lactamase induction in Escherichia coli. J. Bacteriol. 181:7192-7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindberg, F., S. Lindquist, and S. Normark. 1987. Inactivation of the ampD gene causes semiconstitutive overproduction of the inducible Citrobacter freundii β-lactamase. J. Bacteriol. 169:1923-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindberg, F., L. Westman, and S. Normark. 1985. Regulatory components in Citrobacter freundii ampC β-lactamase induction. Proc. Natl. Acad. Sci. USA 82:4620-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindquist, S., F. Lindberg, and S. Normark. 1989. Binding of the Citrobacter freundii AmpR regulator to a single DNA site provides both autoregulation and activation of the inducible ampC β-lactamase gene. J. Bacteriol. 171:3746-3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindquist, S., H. K. Weston, H. Schmidt, C. Pul, G. Korfmann, J. Erickson, C. Sanders, H. H. Martin, and S. Normark. 1993. AmpG, a signal transducer in chromosomal β-lactamase induction. Mol. Microbiol. 9:703-715. [DOI] [PubMed] [Google Scholar]
- 23.Lutkenhaus, J., and A. Mukherjee. 1996. Cell division, p. 1615-1626. In F. C. Neidhardt, R. Curtis III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed., vol. 2. American Society for Microbiology, Washington, D.C.
- 24.Mengin-LeCreulx, D., B. Flouret, and H. J. van Heijenoort. 1982. Cytoplasmic steps of peptidoglycan synthesis in Escherichia coli. J. Bacteriol. 151:1109-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual, p. 268-274. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Normark, S., E. Bartowsky, J. Erickson, C. Jacobs, F. Lindberg, S. Lindquist, K. Weston-Hafer, and M. Wikstrom. 1994. Mechanisms of chromosomal β-lactamase induction in gram-negative bacteria, p. 485-503. In J.-M. Ghuysen and R. Hakenbeck (ed.), Bacterial cell wall. New comprehensive biochemistry, vol. 27. Elsevier Science B. V., Amsterdam, The Netherlands.
- 27.O'Callaghan, C. H., A. Morris, S. M. Kirby, and A. H. Shingler. 1972. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob. Agents Chemother. 1:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliva, B., P. M. Bennett, and I. Chopra. 1989. Penicillin-binding protein 2 is required for induction of the Citrobacter freundii class I chromosomal β-lactamase in Escherichia coli. Antimicrob. Agents Chemother. 33:1116-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottolenghi, A. C., and J. A. Ayala. 1991. Induction of a class I β-lactamase from Citrobacter freundii in Escherichia coli requires active ftsZ but not ftsA or ftsQ products. Antimicrob. Agents Chemother. 35:2359-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ottolenghi, A. C., M. Caparrós, and M. A. de Pedro. 1993. Peptidoglycan tripeptide content and cross-linking are altered in Enterobacter cloacae induced to produce AmpC β-lactamase by glycine and d-amino acids. J. Bacteriol. 175:1537-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, J. T. 2001. Identification of a dedicated recycling pathway for anhydro-N-acetylmuramic acid and N-acetylglucosamine derived from Escherichia coli cell wall murein. J. Bacteriol. 183:3842-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park, J. T. 1993. Turnover and recycling of the murein sacculus in oligopeptide permease-negative strains of Escherichia coli: indirect evidence for an alternative permease system and for a monolayered sacculus. J. Bacteriol. 175:7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park, J. T. 1995. Why does Escherichia coli recycle its cell wall peptides? Mol. Microbiol. 17:421-426. [DOI] [PubMed] [Google Scholar]
- 34.Park, J. T., and L. Burman. 1973. FL-1060: a new penicillin with a unique mode of action. Biochem. Biophys. Res. Commun. 51:863-868. [DOI] [PubMed] [Google Scholar]
- 35.Pfeifle, D., E. Janas, and B. Wiedemann. 2000. Role of penicillin-binding proteins in the initiation of the AmpC beta-lactamase expression in Enterobacter cloacae. Antimicrob. Agents Chemother. 44:169-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders, C. C., P. A. Bradford, A. F. Ehrhardt, K. Bush, K. D. Young, T. A. Henderson, and W. J. Sanders. 1997. Penicillin-binding proteins and induction of AmpC β-lactamase. Antimicrob. Agents Chemother. 41:2013-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spratt, B. G. 1975. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc. Natl. Acad. Sci. USA 72:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spratt, B. G., and A. B. Pardee. 1975. Penicillin-binding proteins and cell shape in E. coli. Nature (London) 254:516-517. [DOI] [PubMed] [Google Scholar]
- 39.Votsch, W., and M. F. Templin. 2000. Characterization of a β-N-acetylglucosaminidase of Escherichia coli and elucidation of its role in muropeptide recycling and β-lactamase induction. J. Biol. Chem. 275:39032-39038. [DOI] [PubMed] [Google Scholar]
- 40.Wientjes, F. B., and N. Nanninga. 1991. On the role of the high molecular weight penicillin-binding proteins in the cell cycle of Escherichia coli. Res. Microbiol. 142:333-344. [DOI] [PubMed] [Google Scholar]