Abstract
To help assess the potential for aflatoxin production by Aspergillus oryzae, the structure of an aflatoxin biosynthesis gene homolog cluster in A. oryzae RIB 40 was analyzed. Although most genes in the corresponding cluster exhibited from 97 to 99% similarity to those of Aspergillus flavus, three genes shared 93% similarity or less. A 257-bp deletion in the aflT region, a frameshift mutation in norA, and a base pair substitution in verA were found in A. oryzae RIB 40. In the aflR promoter, two substitutions were found in one of the three putative AreA binding sites and in the FacB binding site. PCR primers were designed to amplify homologs of aflT, nor-1, aflR, norA, avnA, verB, and vbs and were used to detect these genes in 210 A. oryzae strains. Based on the PCR results, the A. oryzae RIB strains were classified into three groups, although most of them fell into two of the groups. Group 1, in which amplification of all seven genes was confirmed, contained 122 RIB strains (58.1% of examined strains), including RIB 40. Seventy-seven strains (36.7%) belonged to group 2, characterized by having only vbs, verB, and avnA in half of the cluster. Although slight expression of aflR was detected by reverse transcription-PCR in some group 1 strains, including RIB 40, other genes (avnA, vbs, verB, and omtA) related to aflatoxin production were not detected. aflR was not detected in group 2 strains by Southern analysis.
Koji molds, Aspergillus oryzae and Aspergillus sojae, have traditionally been used in the brewing industry for the production of sake, miso, and soy sauce. A history of safety (1) and nonproductivity of aflatoxin is well established for industrial strains, and A. oryzae is considered “generally recognized as safe” by the U.S. Food and Drug Administration (41). These fungi belong to the Aspergillus section Flavi, which includes Aspergillus flavus and Aspergillus parasiticus, some of which produce the procarcinogen aflatoxin. It is thought that A. oryzae and A. sojae are taxonomically differentiated from A. flavus and A. parasiticus, respectively (20, 21, 23, 35, 37). It has been demonstrated that most of the 25 identified genes clustered within a specific 70-kb region of the fungal genome are involved in aflatoxin biosynthesis (reviewed in references 3, 13, 22, 40, 45, 49, and 54). Among them, the aflR gene is known to encode a major transcriptional regulator of aflatoxin biosynthesis genes (6, 9, 19, 28, 47, 55). AflR binds to the consensus sequence 5′-TCGN5CGR-3′ (16) found in the promoters of most of the aflatoxin biosynthesis genes (54), including aflR (14, 17). Putative binding sites (14, 17) for the transcription factors AreA (10, 34, 36), PacC (18), and FacB (43) have been identified in the aflR promoter.
For A. sojae, several studies (30, 31, 42) have suggested an inability to produce aflatoxin, because mutations have been found in the aflR homolog (46). On the other hand, Kusumoto et al. (25) reported that 39 strains of A. oryzae could be classified into three groups based on fragment analysis by a long PCR method targeting the aflatoxin biosynthesis gene homologs. Strains which belonged to groups 2 and 3 harbored deletions in the gene cluster. However, it is thought that group 1 strains have a nearly intact gene cluster, including an almost complete aflR gene.
Aflatoxin has not been detected in any A. oryzae cultures (24, 29, 33, 51). Therefore, it is thought that the aflatoxin gene homolog cluster in A. oryzae is not functional. It is important to prove at the molecular level that A. oryzae is incapable of producing aflatoxin in order to continue to use strains of this species with confidence in the food-processing industry.
In the present study, the complete sequence of the homologous aflatoxin biosynthesis cluster in A. oryzae RIB 40 was determined, and expression of cluster genes in A. oryzae strains was investigated. PCR primers were designed to examine the structure of the gene cluster in 210 A. oryzae strains, and these strains were classified based on amplification results.
MATERIALS AND METHODS
Fungal strains.
Two hundred and ten A. oryzae RIB strains from the National Research Institute of Brewing (NRIB) (Higashi-Hiroshima, Japan) culture collection were used in this study. Information about the strains, including the isolation source and 20 mycological characteristics examined by Murakami (32), are available at the NRIB website (http://www.nrib.go.jp/ken/asp/strain.html).
Preparation of fungal genomic DNA and RNA.
All fungal strains were grown in DP medium, consisting of 1% peptone, 2% dextrin, 0.5% KH2PO4, and MgSO4 · 7H2O, for 3 days at 30°C. Genomic DNA was prepared from wet mycelia according to the method of Lee et al. (27). A. oryzae RIB 40, 81, 128, 176, 210, 515, 920, 1031, 1039, and 1401 and A. parasiticus NFRI-95, a UV-irradiated mutant of A. parasiticus SYS-4 (NRRL2999) (50), were grown on YES (2% yeast extract and 20% sucrose) liquid culture medium for secondary-metabolite production at 30°C for 2 days with shaking. Total RNA was prepared from harvested mycelia with ISOGEN (Nippon Gene Co., Toyama, Japan) according to the manufacturer’s instructions.
Primers, plaque hybridizations, and Southern hybridization analysis.
PCR was performed in a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.). The oligonucleotides used for PCR as probes for screening and for Southern analysis, for subcloning, and as primers for reverse transcription-PCR (RT-PCR) and real-time quantitative PCR (Q-PCR) and PCR amplification analysis of A. oryzae RIB strains are shown in Table 1. The amplified DNA fragments were labeled with digoxigenin-11-dUTP by using a PCR digoxigenin probe synthesis kit (Roche Diagnostics, Mannheim, Germany). Plaque, and Southern hybridization procedures were carried out according to the manufacturer’s instructions.
TABLE 1.
Primers
| Primera | Sequence | Use |
|---|---|---|
| aflR F | 5′-TCAGTGTTTGTAGTGCTAGCG-3′ | Probe for plaque hybridization |
| aflR R | 5′-TCCTCAATCGAATCAACCACC-3′ | |
| nor-1 F | 5′-CGGACGAGGTCTCATTGAAGCTTT-3′ | Probe for plaque hybridization and PCR amplification |
| nor-1 R | 5′-ATCGATGATGAAGGCCGTGA-3′ | |
| verB F | 5′-GATGCACCATGACCTCATGCGTTA-3′ | Probe for plaque hybridization, PCR amplification, and RT-PCR |
| verB R | 5′-CACGGCAGCGTTATTGATCATCTC-3′ | |
| aflR F2 | 5′-CCGGCGCATAACACGTACTC-3′ | Probe for Southern hybridization and PCR amplification |
| aflR R2 | 5′-GGCGCTTGGCCAATAGGTTC-3′ | |
| norB F | 5′-AGTTGCGATCTGTAACACTGCTGA-3′ | Subcloning for norB and cypA regions |
| cypA R | 5′-GGAACGGGGTCAAGGATATAAGGG-3′ | |
| aflT F | 5′-GCACCAAATGGGTCTTTCTCGT-3′ | PCR amplification |
| aflT R | 5′-ATCCACGGTGAAGAGGGTAAGG-3′ | |
| norA F | 5′-GGCTGGAAAGGGGTAATGGG-3′ | PCR amplification |
| norA R | 5′-TCTTGCGACCCTCACGAGAA-3′ | |
| avnA F | 5′-AATCGCACCCAATGAGCTGTCT-3′ | PCR amplification and RT-PCR |
| avnA R | 5′-ATGGCCCGGGTTCTTTAGCAAC-3′ | |
| vbs F | 5′-TGCGAATGCTACGGCTCTCA-3′ | PCR amplification and RT-PCR |
| vbs R | 5′-CAACCGCCATCTCCTGGTCT-3′ | |
| omtA F | 5′-TATCTGGCCACGGCAGGTGA-3′ | RT-PCR |
| omtA R | 5′-GGGGCGACGAATGTCATGCT-3′ | |
| aflR F3 | 5′-CAACCTGATGACGACTGATA-3′ | RT-PCR and real-time Q-PCR |
| aflR R3 | 5′-ACAATCCTCGCCCACCATAC-3′ | |
| β-tubulin F | 5′-CCAAGAACATGATGGCTGCT-3′ | RT-PCR and real-time Q-PCR |
| β-tubulin R | 5′-CTTGAAGAGCTCCTGGATGG-3′ |
F and R indicate forward and reverse primers, respectively.
Genomic-library construction and screening.
A. oryzae RIB 40 was used for structure and sequence analysis of the aflatoxin biosynthesis gene homolog cluster. Genomic DNA from an A. oryzae RIB 40 genomic library was partially digested with SauIIIAI and then ligated to a λ fixII (XhoI-cut) partially filled-in vector (Stratagene, La Jolla, Calif.). A. oryzae RIB 40 in the λ vector was then packaged using a Gigapack III XL packaging extract (Stratagene). Screening was performed using PCR products as probes. The probes were regions of the aflR, nor-1, and verB open reading frames (ORFs) (Table 1). Escherichia coli P2392 was transfected with the phage mixture. Several positive genomic clones were isolated and digested with NotI in order to be sublconed into pBluescript. Sequence analysis of clones containing the largest genomic inserts was performed using the GPS-1 Genome Priming System (New England Biolabs, Inc.). Nucleotide sequence analysis of genomic DNA was performed using an ABI PRISM 310 or 3100 Avant Genetic Analyzer (Applied Biosystems Japan Ltd.).
Nucleotide sequence analysis.
Sequence data were assembled using ATGC software (Genetyx Co., Tokyo, Japan). In the cases of the norB and cypA genes, PCR products generated with primers norB F and cypA R (Table 1) were cloned directly using a Zero Blunt PCR Cloning kit (Invitrogen Corp., San Diego, Calif.). The aflatoxin biosynthesis gene homologs from A. oryzae RIB 40 and their deduced amino acid sequences were compared with those from A. flavus or A. parasiticus available in the NCBI nucleotide database. The accession numbers for the nucleotides used in the analysis are AY 510453 (complete sequence of the aflatoxin biosynthesis gene cluster from A. flavus AF70) (12, 15) and AY371490 (complete sequence of the aflatoxin biosynthesis gene cluster from A. parasiticus SU-1 [ATCC 56775]) (2, 54). Both strains are aflatoxin-producing fungi.
PCR analysis of the seven aflatoxin biosynthesis gene homologs.
Amplification of the aflatoxin biosynthesis gene homologs was performed using Insert Check-Ready-Blue (Toyobo Co. Ltd., Osaka, Japan). PCR mixtures containing genomic DNA and primer mixtures were heated at 94°C for 4 min and then subjected to 30 cycles consisting of denaturation at 94°C for 30 s, annealing at 55 ± 1°C (adjusted according to the primer’s melting temperature) for 2 s, and extension at 74°C for 30 s. A 5-μl portion of each PCR product was electrophoresed in 1.0% agarose (Sigma-Aldrich, Inc., St. Louis, Mo.) in TAE buffer. The PCR products were visualized with UV light after the gels had been stained with ethidium bromide.
RT-PCR and real-time Q-PCR.
cDNAs of all the samples were prepared by reverse transcription of 50 ng/μl of total RNA with 1 μl (0.5 μg/μl) of an oligo(dT) primer using SuperScript II RNase H reverse transcriptase (Invitrogen) following the manufacture’s instructions. Primer pairs for aflR, avnA, vbs, verB, omtA, and β-tubulin (Table 1) were used for RT-PCR. The real time Q-PCR was performed using a GeneAmp 5700 sequence detection system (Applied Biosystems) and QuantiTest SYBR Green PCR kit (QIAGEN) as described in the manufacturers’ manuals. The real-time Q-PCR mixtures containing cDNA and each primer were heated at 95°C for 15 min and then subjected to 40 cycles consisting of denaturation at 95°C for 15 s, annealing at 54°C for 20 s, and extension at 72°C for 20 s. The primer pairs were designed for the specific amplification of the aflR gene. The housekeeping gene tub1, encoding β-tubulin, was chosen as a system control for reverse transcription. The set of primer sequences is shown in Table 1. Standard DNA for the calibration curve of the real-time Q-PCR was prepared using A. oryzae RIB 40 genomic DNA as a template. The real-time Q-PCR was carried out at three replicates per prepared cDNA sample, and the average data were normalized by mRNA accumulation of β-tubulin.
Nucleotide sequence accession numbers.
The DDBJ accession numbers registered in this study are AB071288 (aflR, aflJ, norA, and ver-1), AB076803 (aflT, pksA, and nor-1), AB076804 (avnA, verB, avfA, and omtB), AB182368 (norB-cypA region), and AB196490 (complete sequences of the A. oryzae RIB 40 homologous aflatoxin biosynthesis genes).
RESULTS
Sequence analysis of the aflatoxin biosynthesis gene homolog cluster in A. oryzae RIB 40.
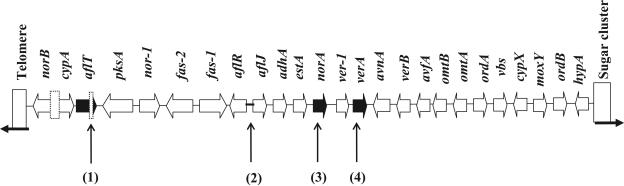
The aflatoxin biosynthesis gene homolog cluster in A. oryzae RIB 40 was cloned by plaque hybridization, and about 46 kb of sequence was determined. Recently, the complete A. oryzae RIB 40 genome sequence was determined by the Japanese A. oryzae genome consortium. Our sequence results were identical to those of the consortium. The structure of the aflatoxin biosynthesis gene homolog cluster in A. oryzae RIB 40 and the similarity of the deduced amino acid sequence for each gene were compared to those of A. flavus and A. parasiticus (Fig. 1 and Table 2). The distribution of the genes within the cluster in A. oryzae RIB 40 and the directional alignment of each are the same as in A. flavus (15) and A. parasiticus (52). While most of the amino acid sequences in the corresponding cluster genes from A. oryzae RIB 40 exhibited from 97 to 99% similarity to those from A. flavus, three genes (aflT, norA, and verA) shared 93% similarity or less.
FIG. 1.
Structure of the aflatoxin biosynthesis gene homolog cluster in Aspergillus oryzae RIB 40. Boldface black arrows indicate genes encoding deduced polypeptides with less than 93% amino acid similarity to those of A. flavus (arrow length is not proportional to gene size). Dotted boxes indicate deletions. The boldface line indicates mutations in a recognized consensus sequence within a promoter region. The numbered vertical arrows indicate specific mutations: 1, a 257-bp deletion resulting in loss of 1 of 14 putative transmembrane regions; 2, base substitutions in consensus sequences for putative AreA and FacB binding sites; 3, a frameshift mutation resulting in a truncation; 4, amino acid substitutions.
TABLE 2.
Alignment analysis of predicted polypeptides encoded by aflatoxin biosynthesis gene homologs in A. oryzae RIB 40 (% amino acid identitya)
| Gene | % Amino acid identitya
|
|
|---|---|---|
| A. flavus | A. parasiticus | |
| norB | Not possible | Not possibleb |
| cypA | Not possible | Not possible |
| aflT | 87 | 88 |
| pksA | 99 | 99 |
| nor-1 | 98 | 96 |
| fas-2 | 99 | 98 |
| fas-1 | 99 | 98 |
| aflR | 99 | 97 |
| aflJ | 99 | 98 |
| adhA | 98 | 98 |
| estA | 99 | 96 |
| norA | 93 | 93 |
| ver-1 | 99 | 99 |
| verA | 93 | 94 |
| avnA | 98 | 95 |
| verB | 97 | 96 |
| avfA | 98 | 86 |
| omtB | 98 | 94 |
| omtA | 98 | 97 |
| ordA | 98 | 96 |
| vbs | 99 | 98 |
| cypX | 97 | 97 |
| moxY | 99 | 94 |
| ordB | 99 | 92 |
| hypA | 97 | 93 |
Amino acid sequence data for A. flavus AF 70 and A. parasiticus SU-1 (ATCC 56775) were obtained from the NCBI nucleotide database.
Because the putative start codon was not found in A. oryzae RIB 40 and A. flavus AF 70, an alignment analysis could not be performed.
The aflR gene encodes a major transcriptional regulator of the aflatoxin biosynthesis genes (6, 9, 19, 28, 47, 55). At least one copy of the AflR binding motif (5′-TCGN5CGR-3′) (16) is present in the promoters of the A. parasiticus aflatoxin biosynthesis genes, including aflR itself (54). AflR binding motifs in A. oryzae RIB 40 were conserved almost completely, except in the norB and cypA intergenic region, in which a deletion was recognized relative to A. parasiticus (15). Five distinct types of protein binding sites are predicted in the aflR promoter: three copies of the AflR motif itself, two PacC sites, three AreA sites, one AbaA site, and one FacB site (14, 17). The A. oryzae RIB 40 aflR promoter was compared to that of A. flavus. Two substitutions (italicized) were found in one of the three putative AreA binding sites (HGATAR → AGATGG) which regulate nitrogen source utilization (10, 34, 36) and in the FacB binding site (GCAACGAAAAGGGC → GCAACGAAAAGGGT). facB is a positively acting regulatory gene involved in acetate induction (43).
In RIB 40, a 257-bp deletion at the C-terminal coding region of the aflT gene, which encodes a major facilitator superfamily transporter in A. parasiticus and A. flavus (11), was found, and this deletion caused a lack of the last 1 of 14 putative transmembrane regions. A frameshift mutation was found in a C-terminal coding region, resulting in a stop codon. The norA gene may be involved in the conversion of norsolorinic acid (NA) to averantin (7). The deduced polypeptide encoded by the predicted A. oryzae RIB 40 norA was 73 amino acids shorter than in A. flavus or A. parasiticus. Amino acid substitutions were found only in the verA gene product, which may encode a monooxygenase (52). Thirty of 453 amino acids (compared to the A. flavus verA gene product) were identified as substitutions, although no deletions or frameshift or nonsense mutations were found in the nucleic acid sequences of the verA gene.
A 1.5-kb deletion of nucleic acid sequences was observed between norB and cypA relative to these genes in A. parasiticus, which may encode an arylalcohol dehydrogenase and a cytochrome P450-type monooxygenase, respectively, as reported by Ehrlich et al. (15). This deletion is thought to result in loss of the start codons in both genes and an intergenic region.
Classification of A. oryzae RIB strains by PCR amplification.
Based on the A. oryzae RIB 40 sequence, we examined aflatoxin gene homolog clusters in 210 A. oryzae RIB strains. Seven homologous aflatoxin biosynthesis genes (vbs, verB, avnA, norA, aflR, nor-1, and aflT) were selected to cover sequences throughout the cluster. PCR primers which specifically amplified these genes were designed and used to detect their presence.
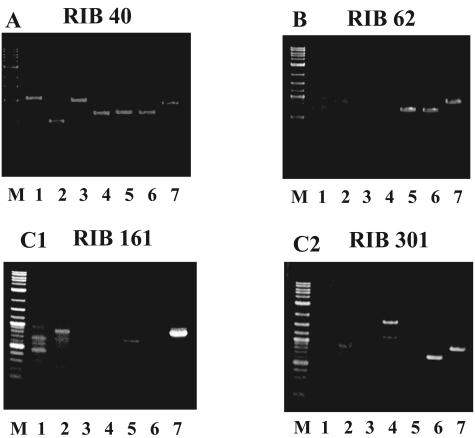
As shown in Table 3, 210 strains were classified into groups 1, 2, and 3 and others. The PCR amplification patterns of each group are shown in Fig. 2. (The grouping list is shown in Table S1 in the supplemental material, and the amplification patterns of all examined strains are listed in Fig. S1A to D in the supplemental material). Group 1, in which amplification of all seven genes was confirmed, contains 122 RIB strains (58.1% of examined strains), including RIB 40 (Fig. 2A). Seventy-seven strains (36.7%) belonged to group 2, characterized by the amplification of only three genes, vbs, verB, and avnA, found in half of the cluster (Fig. 2B). It is possible that the breakpoint within the cluster of group 2 strains would be near the ver-1 gene, as described by Kusumoto et al. (25). Nine strains (4.3%) in which at least vbs was amplified were classified into group 3 (Fig. 2C1 and C2). Two strains (0.9%) that could not be classified into group 1, 2, or 3 were called “others.” Most RIB strains (94.8%) were classified into groups 1 and 2.
TABLE 3.
Classification of A. oryzae RIB strains based on PCR amplification patterns of aflatoxin biosynthesis gene homologs
| Classification | Aflatoxin biosynthesis genes amplified by PCRa | No. of strainsb | Ratio (%) |
|---|---|---|---|
| Group 1 | aflT, nor-1, aflR, norA, avnA, verB, vbs | 122 | 58.1 |
| Group 2 | avnA, verB, vbs | 77 | 36.7 |
| Group 3 | verB, vbs or vbs | 9 | 4.3 |
| Others | No shared patterns | 2 | 0.9 |
Amplification of seven homologous aflatoxin biosynthesis genes was examined.
A total of 210 A. oryzae RIB strains were used in this study, details of which are available at the NRIB website described in Materials and Methods.
FIG. 2.
PCR amplification patterns of aflatoxin biosynthesis genes in A. oryzae RIB strains. Two hundred and ten A. oryzae RIB strains were classified into four groups. (A) Group 1; amplification of all seven genes was confirmed. (B) Group 2; amplification of only avnA, verB, and vbs was confirmed. (C1) Group 3; amplification of only vbs was confirmed. (C2) Group 3; amplification of vbs and verB was confirmed. Lanes: M, marker; 1, aflT; 2, nor-1; 3, aflR; 4, norA; 5, avnA; 6, verB; 7, vbs.
Furthermore, the norB-cypA regions, in which a 1.5-kb deletion has been recognized in A. oryzae RIB 40, of all group 1 strains were examined. Among other group 1 strains, 97 strains had the same 1.5-kb deletion as observed in A. oryzae RIB 40, 5 strains had a 0.8-kb deletion, and 19 strains had no deletion (A. parasiticus type) (the deletion sizes of the norB-cypA regions in group 1 strains are shown in Table S1 in the supplemental material). We also examined other Aspergillus strains. Among four A. flavus strains, two strains had 1.5-kb deletions and two others had 0.8-kb deletions. No deletions were found in 4 A. parasiticus or in 22 A. sojae strains. In 19 A. oryzae strains which had complete norB-cypA regions, aflR was sequenced, because these strains were thought to be strains of A. sojae. The sequences in all 19 strains were found to be consistent with those of A. sojae (30, 31, 42, 46, and data not shown).
RT-PCR and real-time Q-PCR of the aflatoxin biosynthesis pathway gene homologs in group 1 strains.
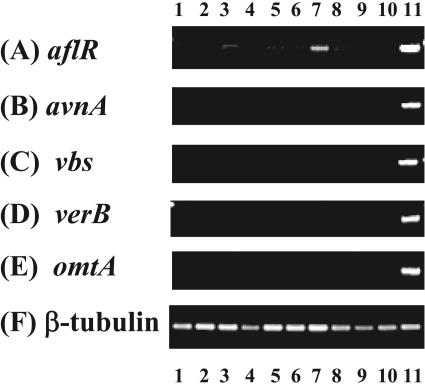
All 210 A. oryzae strains used in this study were proved by Murakami (32, 33) not to produce aflatoxin. Kusumoto et al. (26) reported that aflR, a gene involved in regulation of aflatoxin synthesis in A. flavus and A. parasiticus, was not expressed in A. oryzae strains. In order to determine whether the aflatoxin biosynthesis pathway gene homolog cluster was functional in A. oryzae RIB 40 and another nine group 1 strains selected at random, expression of aflR, avnA, vbs, verB, and omtA was tested by RT-PCR in cultures grown in YES medium. As a positive control, A. parasiticus NFRI-95 was used. This strain is an NA-accumulating mutant (50) that produces an orange pigment, so that its presence serves as a marker for aflatoxin production. Expression of β-tubulin was examined as a positive control for the genes.
Slight expression of aflR was detected in some group 1 strains, including RIB 40 (Fig. 3A). All avnA (53), vbs (39), verB (3, 48), and omtA (4) genes related to aflatoxin production were not detected in group 1 strains examined in this experiment (Fig. 3B to E). On the other hand, expression of all examined genes was confirmed in A. parasiticus NFRI-95. Further, real-time Q-PCR was performed in order to compare the transcription levels of aflR between those group 1 strains and A. parasiticus NFRI-95 quantitatively. Assuming that the transcription level in A. parasiticus NRFI-95 was 100, that in A. oryzae RIB 40 was 11.6. Almost the same transcription levels were confirmed in the other nine group 1 strains (data not shown).
FIG. 3.
Gel electrophoretic analysis of RT-PCR products using primers for the indicated genes. Strains were grown in YES medium for 2 days at 30°C. Lanes: 1, A. oryzae RIB 40; 2, RIB 81; 3, RIB 128; 4, RIB 176; 5, RIB 210; 6, RIB 515; 7, RIB 920; 8, RIB 1031; 9, RIB 1039; 10, RIB 1401; 11, A. parasiticus NFRI-95.
Southern analysis of the aflR gene in group 2 strains.
The presence of the aflR gene region in group 2 isolates could not be detected by PCR amplification of genomic DNA. The presence of the aflR gene in strains of this group was examined instead by Southern analysis using the aflR ORF as a probe, because of the possibility of mutations in the annealing sites of the aflR primers (Fig. 4). In A. oryzae RIB 40, the aflR gene was detected as the predicted 5-kb band. On the other hand, the aflR gene was not detected in any of the 77 strains of group 2 (Fig. 4; other data not shown). This result demonstrates that the group 2 A. oryzae RIB strains lack the aflR gene.
FIG. 4.
Southern blot analysis of group 2 A. oryzae RIB strains using an aflR ORF probe. Lane numbers correspond to RIB numbers. Genomic DNA of group 2 strains was digested with PstI, and A. oryzae RIB 40 was used as a positive control.
DISCUSSION
We have shown that a number of mutations exist in the aflR promoter region and in three ORFs (aflT, norA, and verA) within the aflatoxin biosynthesis gene homolog cluster in A. oryzae RIB 40 relative to the A. flavus sequence. These include deletions, frameshift mutations, and base pair substitutions (Fig. 1 and Table 2). In addition, a 1.5-kb deletion within the norB-cypA sequence was also detected, in agreement with observations made in other isolates of A. oryzae and in other A. flavus S isolates (15). In aflatoxin biosynthesis, it is thought that products of norA (7), nor-1 (44, 56), and norB (52) are involved in converting NA to averantin, which is an early step in aflatoxin biosynthesis (49, 54). Disruption of nor-1 has been reported to result in accumulation of NA and in a corresponding and substantial decrease in aflatoxin B1 (44). On the other hand, disruption of norA (7) or norB (52) did not severely influence aflatoxin production. It is thought that mutations of A. oryzae RIB 40 norA and norB sequences do not contribute to nonproductivity of aflatoxin in this strain.
The aflT gene, which encodes a major facilitator superfamily transporter, has been shown not to play a significant role in the production and secretion of aflatoxin (11). The partial deletion of the aflT sequence is thus presumed to have little share on the nonproductivity of aflatoxin by A. oryzae RIB 40. In addition to relatively low amino acid similarity found between A. oryzae and A. flavus (93%) or A. parasiticus (94%) homologs of verA (Table 2), which encodes a putative monooxygenase (52), a similar result (92%) was also found between A. flavus and A. parasiticus. The deduced amino acid similarity of cluster genes between A. flavus and A. parasiticus was relatively low compared to that between A. oryzae and A. flavus (data not shown).
As a result of the sequencing analysis of the norB-cypA region, three patterns of 1.5-kb (98 strains) and 0.8-kb (5 strains) deletions and no deletion (19 strains) were confirmed in group 1 strains. The aflR sequences of 19 no-deletion strains were consistent with that of A. sojae. Assuming that the 19 no-deletion strains are now classified as A. sojae, 95% of group 1 strains have the deletion structure of 1.5-kb in the norB-cypA region. It seems that 1.5-kb-deletion strains are the majority of A. oryzae group 1 strains. Strains having 1.5- and 0.8-kb deletions are also present in A. flavus (15). On the other hand, both A. parasiticus (15) and A. sojae have no deletion. The structures of the norB-cypA regions support the hypothesis that A. oryzae and A. sojae are differentiated from A. flavus and A. parasiticus, respectively.
The amino acid similarity of the deduced polypeptide encoded by aflR from A. oryzae RIB 40 and that from A. flavus was 99% (Table 2), and the AflR binding motifs present on the promoters of genes in the cluster (54) were completely conserved, except for one intergenic region between norB and cypA. From the results of RT-PCR (Fig. 3) and the real time Q-PCR, which are more sensitive than Northern analysis, the transcription level of aflR in group 1 strains is extremely low compared to that of A. parasiticus NFRI-95. Expression of aflR by real-time RT-PCR or RT-PCR is also detected in A. sojae strains, which have been proven nonaflatoxigenic (8), and A. flavus strains, which do not produce aflatoxin (38). It is thought that the reason for the lack of expression of avnA, vbs, verB, and omtA genes is a lower transcription level of the regulatory gene, aflR. However, it is possible that translation is not performed even if aflR is expressed slightly or AflR is degraded. Further work is needed to investigate these possibilities. In any case, it is obvious that group 1 strains cannot produce aflatoxin, because avnA, verB, vbs, and omtA genes, which are necessary for aflatoxin production, could not be confirmed by RT-PCR. In particular, avnA is considered essential to produce aflatoxin (53). The lack of expression of these genes in group 1 strains proves that A. oryzae does not produce aflatoxin. Furthermore, in the A. oryzae RIB 40 aflR promoter, base substitutions were found in putative AreA (17) and FacB (14) binding sites related to utilization of nitrogen (10, 34, 36) and carbon sources (43), respectively. However, it seems unlikely that base substitutions in those putative binding sites alone are the root cause for the nonproduction of aflatoxin by A. oryzae. At present, it is extremely difficult to determine a cause for nonproduction from the structural analysis. In addition, the laeA gene, which is involved in aflR expression, has recently been described (5), demonstrating the possibility that noncluster genes may cause nonproductivity of aflatoxin by A. oryzae.
Deletion of a large part of the aflatoxin biosynthesis gene homolog cluster, including aflR, was detected in 40% of the RIB strains (groups 2 and 3). The deletion of aflR in these strains (group 2) was confirmed by Southern analysis (Fig. 4). Furthermore, 60% of the RIB strains originating from tane-koji (the mold starter for making koji), used in sake, soy sauce, and miso production, belong to group 2 (data not shown). Group 3 strains amplify vbs at least, and few amplified patterns were confirmed. “Others” were strains which could not be classified into groups 1 to 3 and which may have misclassifications. Our examination focused on groups 1 and 2 in this study, because almost all RIB strains were classified in these two groups. However, group 3 and other strains need to be analyzed in detail and separately. This is the first report that has analyzed the aflatoxin biosynthesis gene homolog cluster in a large number of A. oryzae strains. We suggest that this deletion, found in such a large number of A. oryzae strains, may have been caused by a long history of use in the brewing industry or may be suitable for life under brewing conditions.
To our knowledge, this report is the first detailed characterization of the aflatoxin biosynthesis pathway gene homolog cluster in A. oryzae. While we have shown that the genes in the cluster in A. oryzae RIB 40 are not functional and that group 2 strains cannot produce aflatoxin, a determination of the cause cannot be made based on the structural analysis alone in the case of group 1 strains. Continued industrial use of group 2 strains appears to pose no risk of potential aflatoxin production. Attempts to develop easy methods to select group 2 strains are in progress.
Supplementary Material
Acknowledgments
We thank K. Yabe of the National Food Research Institute for kindly providing A. parasiticus NFRI-95.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Barbesgaard, P., H. P. Heldt-Hansen, and B. Diderichsen. 1992. On the safety of Aspergillus oryzae: a review. Appl. Microbiol. Biotechnol. 36:569-572. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, J. W. 1979. Aflatoxins and anthraquinones from diploids of Aspergillus parasiticus. J. Gen. Microbiol. 113:127-136. [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar, D., K. C. Ehrlich, and T. E. Cleveland. 2003. Molecular genetic analysis and regulation of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 61:83-93. [DOI] [PubMed] [Google Scholar]
- 4.Bhatnagar, D., A. H. Ullah, and T. E. Cleveland. 1988. Purification and characterization of a methyltransferase from Aspergillus parasiticus SRRC 163 involved in aflatoxin biosynthetic pathway. Prep. Biochem. 18:321-349. [DOI] [PubMed] [Google Scholar]
- 5.Bok, J. W., and N. P. Keller. 2004. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell 3:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cary, J. W., K. C. Ehrlich, M. Wright, P.-K. Chang, and D. Bhatnagar. 2000. Generation of aflR disruption mutants of Aspergillus parasiticus. Appl. Microbiol. Biotechnol. 53:680-684. [DOI] [PubMed] [Google Scholar]
- 7.Cary, J. W., M. Wright, D. Bhatnagar, R. Lee, and F. S. Chu. 1996. Molecular characterization of an Aspergillus parasiticus dehydrogenase gene, norA, located on the aflatoxin biosynthesis gene cluster. Appl. Environ. Microbiol. 62:360-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, P.-K. 2004. Lack of interaction between AFLR and AFLJ contributes to nonaflatoxigenicity of Aspergillus sojae. J. Biotechnol. 107:245-253. [DOI] [PubMed] [Google Scholar]
- 9.Chang, P.-K., K. C. Ehrlich, J. Yu, D. Bhatnagar, and T. E. Cleveland. 1995. Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl. Environ. Microbiol. 61:2372-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, P.-K., J. Yu, D. Bhatnagar, and T. E. Cleveland. 2000. Characterization of the Aspergillus parasiticus major nitrogen regulatory gene, areA. Biochim. Biophys. Acta 1491:263-266. [DOI] [PubMed] [Google Scholar]
- 11.Chang, P.-K., J. Yu, and J.-H. Yu. 2004. aflT, a MFS transporter-encoding gene located in the aflatoxin gene cluster, does not have a significant role in aflatoxin secretion. Fungal Genet. Biol. 41:911-920. [DOI] [PubMed] [Google Scholar]
- 12.Cotty, P. J. 1989. Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology 79:808-814. [Google Scholar]
- 13.Dutton, M. F. 1988. Enzymes and aflatoxin biosynthesis. Microbiol. Rev. 52:274-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrlich, K. C., J. W. Cary, and B. G. Montalbano. 1999. Characterization of the promoter for the gene encoding the aflatoxin biosynthetic pathway regulatory protein AFLR. Biochim. Biophys. Acta 1444:412-417. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlich, K. C., P.-K. Chang, J. Yu, and P. J. Cotty. 2004. Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Appl. Environ. Microbiol. 70:6518-6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrlich, K. C., B. G. Montalbano, and J. W. Cary. 1999. Binding of the C6-zinc cluster protein, AFLR, to the promoters of aflatoxin pathway biosynthesis genes in Aspergillus parasiticus. Gene 230:249-257. [DOI] [PubMed] [Google Scholar]
- 17.Ehrlich, K. C., B. G. Montalbano, and P. J. Cotty. 2003. Sequence comparison of aflR from different Aspergillus species provides evidence for variability in regulation of aflatoxin production. Fungal Genet. Biol. 38:63-74. [DOI] [PubMed] [Google Scholar]
- 18.Espeso, E. A., and H. N. Arst, Jr. 2000. On the mechanism by which alkaline pH prevents expression of an acid-expressed gene. Mol. Cell. Biol. 20:3355-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flaherty, J. E., and G. A. Payne. 1997. Overexpression of aflR leads to upregulation of pathway gene transcription and increased aflatoxin production in Aspergillus flavus. Appl. Environ. Microbiol. 63:3995-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiser, D. M., J. I. Pitt, and J. W. Taylor. 1998. Cryptic speciation and recombination in the aflatoxin-producing fungus Aspergillus flavus. Proc. Natl. Acad. Sci. USA 95:388-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomi, K., A. Tanaka, Y. Iimura, and K. Takahashi. 1989. Rapid differentiation of four related species of koji molds by agarose gel electrophoresis of genomic DNA digested with SmaI restriction enzyme. J. Gen. Appl. Microbiol. 35:225-232. [Google Scholar]
- 22.Hicks, J. K., K. Shimizu, and N. P. Keller. 2002. Genetics and biosynthesis of aflatoxins and sterigmatocystin, p. 55-69. In F. Kempken (ed.), The Mycota XI agricultural applications. Springer-Verlag, Berlin, Germany.
- 23.Kurtzman, C. P., M. J. Smiley, C. J. Robnett, and D. T. Wicklow. 1986. DNA relatedness among wild and domesticated species in the Aspergillus flavus group. Mycologia 78:955-959. [Google Scholar]
- 24.Kusumoto, K., T. Goto, and M. Manabe. 1990. Evaluation for conversion of sterigmatocystin to aflatoxin in koji-molds. Rep. Natl. Food Res. Inst. 54:14-17. [Google Scholar]
- 25.Kusumoto, K.-I., Y. Nogata, and H. Ohta. 2000. Directed deletions in the aflatoxin biosynthesis gene homolog cluster of Aspergillus oryzae. Curr. Genet. 37:104-111. [DOI] [PubMed] [Google Scholar]
- 26.Kusumoto, K.-I., K. Yabe, Y. Nogata, and H. Ohta. 1998. Transcript of a homolog of aflR, a regulatory gene for aflatoxin synthesis in Aspergillus parasiticus, was not detected in Aspergillus oryzae strains. FEMS Microbiol. Lett. 169:303-307. [DOI] [PubMed] [Google Scholar]
- 27.Lee, B. R., O. Yamada, K. Kitamoto, and K. Takahashi. 1996. Cloning, characterization and overexpression of a gene (pdiA) encoding protein disulfide isomerase of Aspergillus oryzae. J. Ferment. Bioeng. 82:538-543. [Google Scholar]
- 28.Liu, B.-H., and F. S. Chu. 1998. Regulation of aflR and its product, AflR, associated with aflatoxin biosynthesis. Appl. Environ. Microbiol. 64:3718-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manabe, M., S. Matsuura, and M. Nakano. 1968. Studies on the fluorescent compounds in fermented foods. Part I. Chloroform soluble fluorescent compounds produced by Koji-molds. Nippon Shokuhin Kogyogakkaishi 15:341-346. [Google Scholar]
- 30.Matsushima, K., P.-K. Chang, J. Yu, K. Abe, D. Bhatnagar, and T. E. Cleveland. 2001. Pre-termination in aflR of Aspergillus sojae inhibits aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 55:585-589. [DOI] [PubMed] [Google Scholar]
- 31.Matsushima, K., K. Yashiro, Y. Hanya, K. Abe, K. Yabe, and T. Hamasaki. 2001. Absence of aflatoxin biosynthesis in koji mold (Aspergillus sojae). Appl. Microbiol. Biotechnol. 55:771-776. [DOI] [PubMed] [Google Scholar]
- 32.Murakami, H. 1971. Classification of the koji mold. J. Gen. Appl. Microbiol. 17:281-309. [Google Scholar]
- 33.Murakami, H., S. Takase, and T. Ishii. 1967. Non-productivity of aflatoxin by Japanese industrial strains of Aspergillus. J. Gen. Appl. Microbiol. 13:323-334. [Google Scholar]
- 34.Muro-Pastor, M. I., R. Gonzalez, J. Strauss, F. Narendja, and C. Scazzocchio. 1999. The GATA factor AreA is essential for chromatin remodeling in a eukaryotic bidirectional promoter. EMBO J. 18:1584-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikkuni, S., H. Nakajima, S.-I. Hoshina, M. Ohno, C. Suzuki, Y. Kashiwagi, and K. Mori. 1998. Evolutionary relationships among Aspergillus oryzae and related species based on the sequences of 18S rRNA genes and internal transcribed spacers. J. Gen. Appl. Microbiol. 44:225-230. [DOI] [PubMed] [Google Scholar]
- 36.Ravagnani, A., L. Gorfinkiel, T. Langdon, G. Diallinas, E. Adjadj, S. Demais, D. Gorton, H. N. Arst, Jr., and C. Scazzocchio. 1997. Subtle hydrophobic interactions between the seventh residue of the zinc finger loop and the first base of an HGATAR sequence determine promoter-specific recognition by the Aspergillus nidulans GATA factor AreA. EMBO J. 16:3974-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigó, K., J. Varga, B. Tóth, J. Téren, A. Mesterházy, and Z. Kozakiewicz. 2002. Evolutionary relationships within Aspergillus section Flavi based on sequences of the intergenic transcribed spacer regions and the 5.8S rRNA gene. J. Gen. Appl. Microbiol. 48:9-16. [DOI] [PubMed] [Google Scholar]
- 38.Scherm, B., M. Palomba, D. Serra, A. Marcello, and Q. Migheli. 2005. Detection of transcripts of the aflatoxin genes aflD, aflO, and aflP by reverse transcription-polymerase chain reaction allows differentiation of aflatoxin-producing and non-producing isolates of Aspergillus flavus and Aspergillus parasiticus. Int. J. Food Microbiol. 98:201-210. [DOI] [PubMed] [Google Scholar]
- 39.Silva, J. C., B. E. Minto, C. E. Barry III, K. A. Holland, and C. A. Townsend. 1996. Isolation and characterization of the versicolorin B synthase gene from Aspergillus parasiticus. Expansion of the aflatoxin B1 biosynthetic gene cluster. J. Biol. Chem. 271:13600-13608. [DOI] [PubMed] [Google Scholar]
- 40.Sweeney, M. J., and A. D. W. Dobson. 1999. Molecular biology of mycotoxin biosynthesis. FEMS Microbiol. Lett. 175:149-163. [DOI] [PubMed] [Google Scholar]
- 41.Tailor, M. J., and T. Richardson. 1979. Applications of microbial enzymes in food systems and in biotechnology. Adv. Appl. Microbiol. 25:7-35. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi, T., P.-K. Chang, K. Matsushima, J. Yu, K. Abe, D. Bhatnagar, T. E. Cleveland, and Y. Koyama. 2002. Nonfunctionality of Aspergillus sojae aflR in a strain of Aspergillus parasiticus with a disrupted aflR gene. Appl. Environ. Microbiol. 68:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Todd, R. B., A. Andrianopoulos, M. A. Davis, and M. J. Hynes. 1998. FacB, the Aspergillus nidulans activator of acetate utilization genes, binds dissimilar DNA sequences. EMBO J. 17:2042-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trail, F., P.-K. Chang, J. Cary, and J. E. Linz. 1994. Structural and functional analysis of the nor-1 gene involved in the biosynthesis of aflatoxins by Aspergillus parasiticus. Appl. Environ. Microbiol. 60:4078-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trail, F., N. Mahanti, and J. Linz. 1995. Molecular biology of aflatoxin biosynthesis. Microbiology 141:755-765. [DOI] [PubMed] [Google Scholar]
- 46.Watson, A. J., L. J. Fuller, D. J. Jeenes, and D. B. Archer. 1999. Homologs of aflatoxin biosynthesis genes and sequence of aflR in Aspergillus oryzae and Aspergillus sojae. Appl. Environ. Microbiol. 65:307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woloshuk, C. P., K. R. Foutz, J. F. Brewer, D. Bhatnagar, T. E. Cleveland, and G. A. Payne. 1994. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl. Environ. Microbiol. 60:2408-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yabe, K., Y. Ando, and T. Hamasaki. 1991. A metabolic grid among versiconal hemiacetal acetate, versiconol acetate, versiconol and versiconal during aflatoxin biosynthesis. J. Gen. Microbiol. 137:2469-2475. [DOI] [PubMed] [Google Scholar]
- 49.Yabe, K., and H. Nakajima. 2004. Enzyme reactions and genes in aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 64:745-755. [DOI] [PubMed] [Google Scholar]
- 50.Yan, P.-S., Y. Song, E. Sakuno, H. Nakajima, H. Nakagawa, and K. Yabe. 2004. Cyclo(l-leucyl-l-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl. Environ. Microbiol. 70:7466-7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yokotsuka, T., M. Sasaki, T. Kikuchi, Y. Asao, and A. Nobuhara. 1967. Studies on the compounds produced by moulds. Part I. Fluorescent compounds produced by Japanese industrial moulds. Nippon Nougeikagaku Kaishi 41:32-38. [Google Scholar]
- 52.Yu, J., D. Bhatnagar, and T. E. Cleveland. 2004. Completed sequence of aflatoxin pathway gene cluster in Aspergillus parasiticus. FEBS Lett. 564:126-130. [DOI] [PubMed] [Google Scholar]
- 53.Yu, J., P.-K. Chang, J. W. Cary, D. Bhatnagar, and T. E. Cleveland. 1997. avnA, a gene encoding a cytochrome P-450 monooxygenase, is involved in the conversion of averantin or averfine in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 63:1349-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu, J., P.-K. Chang, K. C. Ehrlich, J. W. Cary, D. Bhatnagar, T. E. Cleveland, G. A. Payne, J. E. Linz, C. P. Woloshuk, and J. W. Bennett. 2004. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 70:1253-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu, J.-H., R. A. E. Butchko, M. Fernandes, N. P. Keller, T. J. Leonard, and T. H. Adams. 1996. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr. Genet. 29:549-555. [DOI] [PubMed] [Google Scholar]
- 56.Zhou, R., and J. E. Linz. 1999. Enzymatic function of the Nor-1 protein in aflatoxin biosynthesis in Aspergillus parasiticus. Appl. Environ. Microbiol. 65:5639-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.