Abstract
The acid waters (pH 2.7 to 3.4) originating from the Carnoulès mine tailings contain high concentrations of dissolved arsenic (80 to 350 mg · liter−1), iron (750 to 2,700 mg · liter−1), and sulfate (2,000 to 7,500 mg · liter−1). During the first 30 m of downflow in Reigous creek issuing from the mine tailings, 20 to 60% of the dissolved arsenic is removed by coprecipitation with Fe(III). The microbial communities along the creek have been characterized using terminal-restriction fragment length polymorphism (T-RFLP) and 16S rRNA gene library analyses. The results indicate a low bacterial diversity in comparison with unpolluted water. Eighty percent of the sequences obtained are related to sequences from uncultured, newly described organisms or recently associated with acid mine drainage. As expected owing to the water chemistry, the sequences recovered are mainly related to bacteria involved in the geochemical Fe and S cycles. Among them, sequences related to uncultured TrefC4 affiliated with Gallionella ferruginea, a neutrophilic Fe-oxidizing bacterium, are dominant. The description of the bacterial community structure and its dynamics lead to a better understanding of the natural remediation processes occurring at this site.
The processing of sulfide-rich ores in the recovery of base metals, such as copper, lead, zinc, and gold, has produced large quantities of pyrite wastes (20). When exposed to rain, this material generates acid mine drainage (AMD) which contains large amounts of sulfate, iron, arsenic, and heavy metals. Despite their toxicity, such waters host organisms, both prokaryotes and eukaryotes, which are able to cope with the pollution (2, 33). Some of them have the capacity to modify the physicochemical conditions of the water either by detoxification or by metabolic exploitation. For example, efficient oxidation of As by bacteria has been reported in AMD or in chemically somewhat similar waters like those from hot springs (3, 7, 21, 25, 30). Because of their elevated Fe concentration, the development of iron-oxidizing bacteria is favored in AMD (16) where Acidithiobacillus ferrooxidans and Leptospirillum ferrooxidans are often observed (2).
Owing to its ability to oxidize Fe, the bacterial consortium in AMD plays a major role in the immobilization of the elements that exhibit a strong affinity for solid Fe oxide phases such as Sr, Cs, Pb, U (14), and As (8, 24). In addition, the ability of several bacterial strains in AMD to oxidize As further contributes to reduction of its toxicity in water, because As(III) is considered to be more toxic than As(V) (28) and because arsenate adsorbs more strongly than arsenite to Fe(III) oxides and hydroxides at acidic pH (5, 26).
Owing to their tolerance of heavy metals and the ability of some to promote transformations that make some metals less toxic, bacteria in acid mine waters may be useful in AMD bioremediation or that of some other industrial effluents. In order to develop remediation processes or optimize them, further knowledge of the bacteria living in the extreme environment of AMD is required.
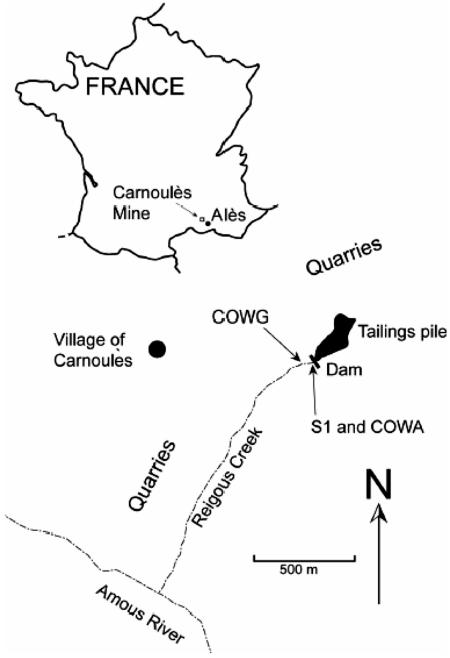
This study aims to investigate the microbial community of a small creek (the Reigous, present at Carnoulès, France). The Carnoulès mine (Fig. 1) has been inactive since 1962. Its exploitation has left about 1.5 megatons of tailings containing 0.7% Pb, 10% FeS2, and 0.2% As. The tailings are contained behind a dam. Water percolating through the tailings emerges at the base of the dam, forming the head of the Reigous creek. The head waters of the creek are characterized by low pH (2.7 to 3.4) and high concentrations of As (100 to 350 mg · liter−1), Fe (750 to 2,700 mg · liter−1), and SO42− (2,000 to 7,500 mg · liter−1).
FIG. 1.
Map of the Carnoulès mining site and location of sampling stations. Sampling stations were Reigous spring (S1), 3 m downstream of the spring (COWA), and 30 m downstream of the spring (COWG).
The As and Fe behavior in creek water has been intensively studied (8, 22, 23, 24). As(III) is the dominant As type, whereas Fe occurs as Fe(II). Along the first 30 m of the creek (about 1 h of residence time), the bacterially mediated oxidation of Fe(II) leads to the coprecipitation of 20 to 60% of the dissolved As. The precipitate which contains up to 22% of As is mainly composed of As(III)-Fe(III) oxy-hydroxide in the wet season while As(V)-Fe(III) oxy-hydroxide compounds predominate in the dry season. Several phenotypes of Acidithiobacillus ferrooxidans have been isolated, and their role in the oxidation of Fe(II) and the coprecipitation of As has been demonstrated in laboratory experiments (8, 11). Additionally, Bruneel et al. (7) isolated at this site three different strains of Thiomonas spp. closely related to Thiomonas sp. strain Ynys1 able to promote As oxidation in laboratory conditions.
The present study combines terminal-restriction fragment length polymorphism (T-RFLP) analysis in order to investigate the dynamics of the bacterial communities and 16S rRNA gene library analysis to identify the dominant bacterial group.
MATERIALS AND METHODS
Sampling procedure and physicochemical determinations in situ.
Water samples for molecular analysis of microbial populations were collected in October 2002 and January 2003 in the spring and at two other locations in the creek over a distance of 30 m (Fig. 1). A volume of 200 ml of water was filtered through a sterile 0.22-μm nucleopore filter. These filters were then transferred to a tube, frozen in liquid nitrogen, and stored at −20°C until further analysis.
DNA isolation.
Genomic DNA was extracted from filtered water using the UltraClean Soil DNA Isolation kit according to the recommendation of the manufacturer (MoBio Laboratories, Inc.). All extracted genomic DNA samples were stored at −20°C until further processing.
T-RFLP analysis.
Primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1489R (5′-TACCTTGTTACGACTTCA-3′) (19, 31) were used for T-RFLP analysis to assess the bacterial community structures. Forward (8F) and reverse (1489R) primers were fluorescently labeled with tetrachlorofluorescein phosphoramidite and hexachlorofluorescein phosphoramidite (E.S.G.S. Cybergene Group), respectively. The PCR amplification mixture contained 12.5 μl Hot Start Taq polymerase master mix (QIAGEN), 0.5 μl of each primer (20 μM), and 10 ng of DNA template. A final volume of 50 μl was adjusted with distilled water. 16S rRNA gene amplification reactions were cycled in a PTC200 thermocycler (MJ Research) with a hot start step at 94°C for 15 min followed by 35 cycles of 94°C for 1 min, 52°C for 1.5 min, and 72°C for 1 min, with a final extension step at 72°C for 10 min. The amount of PCR product was determined by comparison to known concentrations by the “dots method” (Smartlader; Eurogentec) after migration on agarose gel. PCR products were purified with the GFX PCR DNA purification kit (Amersham-Pharmacia).
Purified PCR products (600 to 700 ng) were digested with 12 U of enzyme HaeIII or HinfI (New England Biolabs). The lengths of terminal-restriction fragments (T-RFs) from the digested PCR products were determined by capillary electrophoresis on an ABI prism 310 (Applied Biosystems). About 50 ng of the digested DNA from each sample was mixed with 10 μl of deionized formamide and 0.25 μl of 6-carboxytetramethylrhodamine size standard, denatured at 94°C for 2 min, and immediately chilled on ice prior to electrophoresis. After an injection step of 10 s, electrophoresis was carried out for up to 30 min, applying a voltage of 15 kV. T-RFLP profiles were performed using GeneScan software (ABI).
Dominant operational taxonomic units represent T-RFs whose fluorescence was higher than 100 fluorescence units for at least one sample. Predictive digestions were made on the RDP web site (http://rdp.cme.msu.edu/html/index.html) using the T-RFLP Analysis Program.
Cloning and restriction analysis.
To further characterize the bacterial populations inhabiting the creek in each sampling period and sampling point, the bacterial diversity was analyzed by cloning PCR amplified 16S rRNA genes. For S1 and COWG, libraries were constructed for each sampling period. For COWA, a library was constructed only for October, since the comparison between October and January T-RFLP profiles showed mainly a disappearance of T-RFs in January. Bacterial 16S rRNA genes were amplified with unlabeled 8F and 1489R primers. These PCR products were cloned in Escherichia coli TOP10 using the pCR2.1 Topo TA cloning kit (Invitrogen, Inc.). Cloned 16S rRNA gene fragments were amplified using the primers TOP1 (5′-GTGTGCTGGAATTCGCCCTT-3′) and TOP2 (5′-TATCTGCAGAATTCGCCCTT-3′), located on the vector and surrounding the inserted PCR fragment, and then were digested with the enzyme HaeIII or HinfI. Restriction profiles were analyzed using 2.5% agarose gel electrophoresis (small-fragment resolution agarose; QA agarose; QBiogène, Inc.). Sixty clones from each library were analyzed and grouped according to their RFLP patterns (HaeIII and HinfI digestion). Only sequences from dominant groups were determined
16S rRNA gene sequencing.
Partial sequences of the 16S rRNA gene (from 8 to 336 according to E. coli numbering) were determined by the dideoxy nucleotide chain termination method using a BigDye cycle sequencing kit (Applied Biosystems) on an ABI PRISM 310 Genetic analyzer (Applied Biosystems). DNA sequence analyses were performed via the infobiogen server (http://www.infobiogen.fr) by using the FASTA, BLAST, ALIGNN, and CLUSTALW programs (1, 13, 29). Phylogenetic trees were constructed by using the PHYLIP computer package (13). The confidence level of the phylogenetic tree topology was evaluated by performing 100 bootstrap replications with the SEQBOOK program.
RESULTS
Bacterial community structures.
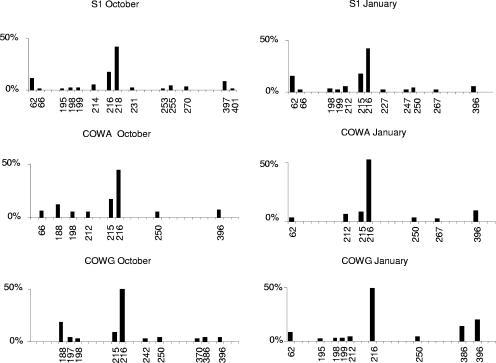
The results of the T-RFLP analysis of bacterial community structure are presented in Fig. 2. The average T-RF number was relatively small (about 10) both in October and January, reflecting low bacterial diversity. The bacterial population characterized by a 216-bp (± 2 bp) T-RF was generally the most abundant, except at station S1 in October. Abundance of this 216-bp T-RF generally increased between October and January, except at station COWG, where the variations were minor.
FIG. 2.
Seasonal comparison of bacterial community T-RFLP fingerprints from the AMD of Carnoulès, France, in October and January samples.
Composition of bacterial communities.
The most representative sequences of the dominant clones are summarized in Table 1, and the phylogenetic analysis of all the obtained sequences are presented in Fig. 3 to 5.
TABLE 1.
Most representative sequences of bacterial clones found in October in the three stations of the Reigous Creek (S1, COWA, and COWG) and in two stations in January (S1 and COWG)c
| Sampling period | Station | Clone | Accession no. | Phylogenetic group | Closest relative (postulated metabolism) | % Similarity | Relative abundance of clones (%)a | T-RFs (bp)b |
|---|---|---|---|---|---|---|---|---|
| October | S1 | S1Oct9 | AJ877944 | δ-Proteobacteria | Desulfobacterium indolicum (sulfate reduction) | 96 | 10 | 202 |
| S1Oct7 | AJ877952 | γ-Proteobacteria | A. ferrooxidans (sulfide/iron oxidation and iron reduction) | 99 | 8 | 251-197 | ||
| S1Oct43 | AJ877956 | Actinobacteria | Actinomycetales spp. (sulfide/iron oxidation) | 94 | 8 | 160 | ||
| S1Oct11 | AJ877924 | β-Proteobacteria | Gallionella ferruginea (iron oxidation) | 94 | 5 | 217 | ||
| COWA | COWAOct18 | AJ877926 | β-Proteobacteria | Gallionella ferruginea (iron oxidation) | 95 | 28 | 217 | |
| COWAOct77 | AJ877946 | δ-Proteobacteria | Desulfobacterium indolicum (sulfate reduction) | 94 | 8 | 202 | ||
| COWG | COWGOct61 | AJ877929 | β-Proteobacteria | Gallionella ferruginea (iron oxidation) | 94 | 22 | 217 | |
| COWGOct52 | AJ877947 | δ-Proteobacteria | Desulfobacterium indolicum (sulfate reduction) | 91 | 5 | 202 | ||
| January | S1 | S1Jan1 | AJ877934 | β-Proteobacteria | Gallionella ferruginea (iron oxidation) | 97 | 66 | 217 |
| S1Jan50 | AJ877949 | δ-Proteobacteria | Desulfobacterium indolicum (sulfate reduction) | 93 | 5 | 202 | ||
| COWG | COWGJan9 | AJ877935 | β-Proteobacteria | Gallionella ferruginea (iron oxidation) | 95 | 70 | 217 | |
| COWGJan29 | AJ877957 | β-Proteobacteria | Thiobacillus plumbophilus (sulfide oxidation) | 91 | 5 | 219 | ||
| COWGJan20 | AJ877953 | γ-Proteobacteria | A. ferrooxidans (sulfide/iron oxidation and iron reduction) | 95 | 4 | 250-197 | ||
| COWGJan58 | AJ877951 | δ-Proteobacteria | Desulfomonile tiedjei (sulfate reduction) | 91 | 1 | 202 |
Corresponds to the relative abundance of clones for each library.
T-RFs correspond to expected terminal fragments with HaeIII predictive digestion.
The phylogenetic group, closest relatives, and postulated metabolism and relative abundance of clones are indicated.
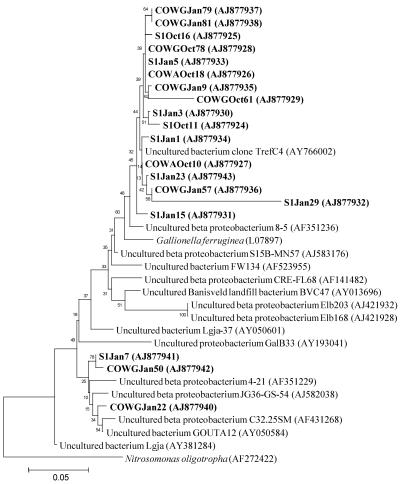
FIG. 3.
Phylogenetic analysis of 16S rRNA gene sequences affiliated with the Gallionella division from the AMD of Carnoulès, France. Clone names in boldface correspond to sequences found in October (Oct) and January (Jan) within the three stations along the Reigous Creek, S1, COWA, and COWG.
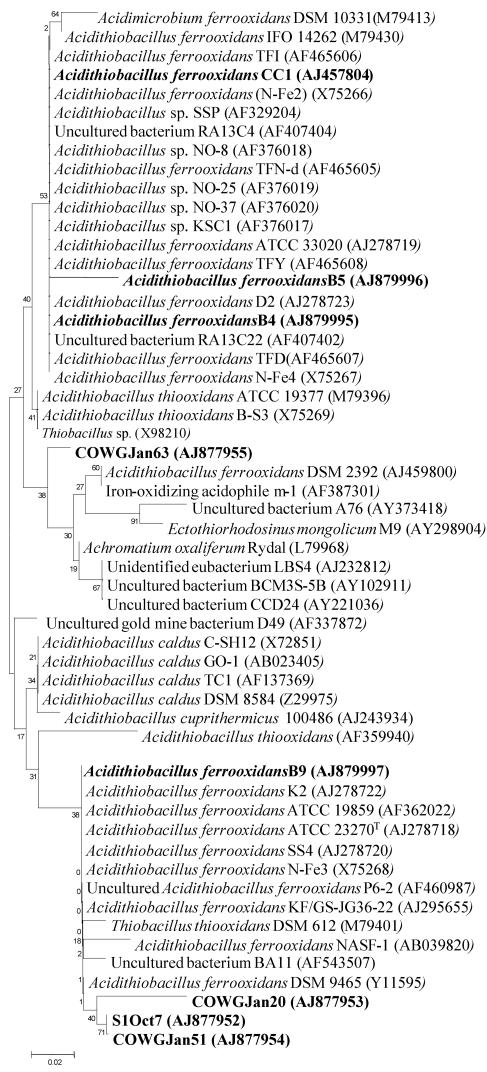
FIG. 5.
Phylogenetic analysis of 16S rRNA gene sequences affiliated with the Acidithiobacillus division from the AMD of Carnoulès, France. Clone names in boldface correspond to sequences found in October (Oct) and January (Jan) within the three stations along the Reigous Creek, S1, COWA, and COWG. Strains in boldface (CC1, B5, B4, and B9) represent the bacteria isolated in the Carnoulès Creek.
The most abundant sequence types are positioned within the beta subdivision of the Proteobacteria (Table 1). They were recovered at all stations during both sampling periods, accounting for 5 to 28% of the clones in October and more than 65% in January. Numerous clone sequences of this group displayed around 95% homology with a sequence isolated from an acid- and iron-rich stream in the United Kingdom (GenBank accession no. AY766002) (unpublished data). The phylogenetic analyses (Fig. 3) did not allow affiliation of the clone sequences with any representative of the subdivision. The closest relative (91%) is Gallionella ferruginea, a neutrophilic iron-oxidizing bacterium.
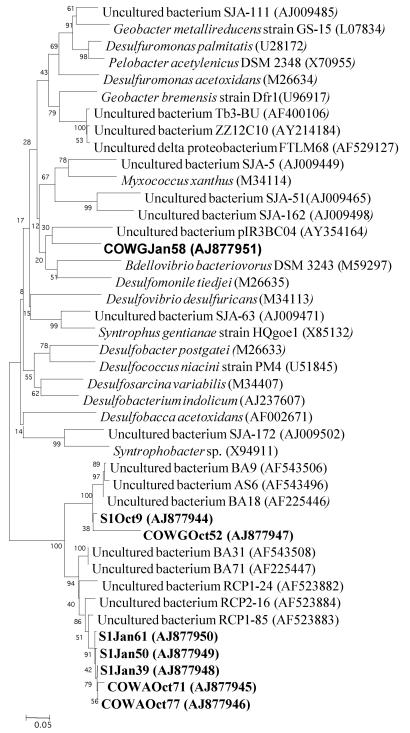
The sequences representing the second-most abundant type are positioned within the delta subdivision of the Proteobacteria (Table 1, Fig. 4). These sequences were more abundant in October, representing 10, 8, and 5% of the clones at S1, COWA, and COWG, respectively, than in January, with 5 and 1% at S1 and COWG. In October, all the clones were similar (more than 90% similarity) to clones found in an AMD at Iron Mountain (4). In contrast, the clones of January were similar (94% similarity) to those found in a forested wetland impacted by sulfate-rich waters from coal piles (6). As for the main sequence, the phylogenetic analyses did not allow the affiliation of the clone sequences with any representative of the subdivision. The closest relative was Desulfobacterium indolicum, a sulfate-reducing bacterium (18). For the clone from COWG in January that was phylogenetically distant from the others (Fig. 4), the closest relative is Desulfomonile tiedjei, a sulfate-reducing bacterium (12).
FIG. 4.
Phylogenetic analysis of 16S rRNA gene sequences affiliated with the Desulfobacterium division from the AMD of Carnoulès, France. Clone names in boldface correspond to sequences found in October (Oct) and January (Jan) within the three stations along the Reigous Creek, S1, COWA, and COWG.
The next most abundant sequence types, representing 8% of the clones at S1 in October and 4% at COWG in January (Table 1), were firmly positioned in the Acidithiobacillus ferrooxidans group (Fig. 5). Three sequences are related (99% similarity) to uncultured A. ferrooxidans KF/GS-JG36-22 (27) isolated in waste piles of a uranium mine, and one sequence was related to A. ferrooxidans DSM 2392 (Fig. 5).
The next group, representing 8% of the clones at S1 in October, was associated with the Actinobacteria group, with 94% similarity with sequences recovered in forested wetland exposed to coal effluent (6). The phylogenetic analysis could not affiliate the sequence with any isolated bacterium (data not shown).
The last sequence found, detected only in January at COWG, representing 5% of the clones, was firmly positioned in the Thiobacillus group with 91% similarity with T. plumbophilus DSM 6690. These strains were isolated from a uranium mine, and they grew by oxidation of H2S, galena (PbS), and H2 (10).
Finally, sequences closely related to 16S rRNA genes from a chloroplast of Euglena spp. were also detected (data not shown). This was not surprising, since the 16S rRNA gene of chloroplasts is closely related to the bacterial 16S rRNA gene and therefore can be amplified by primers 8F and 1489R. Moreover, this is consistent with previous work indicating that these organisms are able to accumulate and oxidize As in the cell (9).
DISCUSSION
In the Reigous creek, the low bacterial diversity as revealed by molecular-based methods is consistent with the results of Baker and Banfield (2) in a similar environment. This may reflect the limited number of different electron donors and acceptors available in AMD and the toxicity of heavy metals and low pH.
Numerous sequences in the libraries are related to sequences previously found in AMD, indicating that the clone libraries were not contaminated. Nevertheless, 80% of the sequences could not be closely related to cultured organisms, suggesting that they may constitute new taxa. As long as the bacterial strains were not isolated, their physiological role in the creek ecology will remain uncertain.
Both molecular methods revealed that the dominant population (216-bp [±2 bp] T-RF) can be related to Gallionella ferruginea sequences, as indicated by predictive digestion (217 bp) and 16S rRNA gene library analyses. Gallionella ferruginea is a neutrophilic bacterium that oxidizes Fe. It has been shown to efficiently remove Fe, As(III), and As(V) in water (17). It is possible that an acid-tolerant relative of this bacterium has the ability to oxidize iron under acid pH conditions. In the creek, the abundance of this population was much more significant in January (more than 65%) than in October (less than 30%). Such variations are consistent with the occurrence of higher Fe and As precipitation rates in the rainy seasons than in other seasons, as reported by Casiot et al. (8). In addition to the Gallionella ferruginea sequences, the library analyses show the presence of other uncultured bacterial groups related to the Fe cycle, such as the Actinobacteria group. Members of this group, previously reported in AMD, are iron-oxidizing, heterotrophic, acidophilic bacteria capable of autotrophic growth. Some of them may play a synergistic role, removing organic carbon (4). Finally, A. ferrooxidans constituted a minor group in the Fe-oxidizing bacterial population contrary to expectations from previous findings based on isolation and culturing techniques (8).
With respect to bacteria involved in S cycling, the sequences recovered, in addition to A. ferrooxidans, are related to members of the Desulfobacterium genera, which contains sulfate-reducing bacteria (18). As the water of the Carnoulès creek is fully oxygenated, the presence of bacteria from this group, which is characterized by anaerobic respiration, may be surprising. Nevertheless, this is in agreement with several studies that have recently reported sulfate- and iron-reducing bacteria under acidic conditions (15, 32).
Considering the small population, sequence analyses indicate the presence of bacteria from the β subdivision of the Proteobacteria affiliated with the Thiobacillus group. A member of this group was recently described as a galena and hydrogen oxidizer (11). A Thiomonas sp., which has been isolated and shown to be very active in the oxidation of As (7), was not detected by molecular techniques, probably reflecting its low abundance.
Analyses of the most abundant clones strongly indicate that further efforts have to be exerted to fully understand AMD systems. They include isolation and identification of the organisms represented by these clones in order to define their ecophysiological roles.
Acknowledgments
This study was financed by the project GEOMEX from the CNRS, the project Environnement Vie et Sociétés (CNRS-INSU), the ACI-Ecologie Quantitative, and the ACI-ECCODYN (French Ministry of Research).
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Baker, B. J., and J. F. Banfield. 2003. Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 44:139-152. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia-Brunet, F., M.-C. Dictor, F. Garrido, C. Crouzet, D. Morin, K. Dekeyser, M. Clarens, and P. Baranger. 2002. An arsenic(III)-oxidizing bacterial population: selection, characterization, and performance in reactors. J. Appl. Microbiol. 93:656-667. [DOI] [PubMed] [Google Scholar]
- 4.Bond, P. L., S. P. Smriga, and J. F. Banfield. 2000. Phylogeny of microoorgansims populating a thick, subaerial, predominantly lithotrophic biofilm at an extreme acid mine drainage site. Appl. Environ. Microbiol. 66:3842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowell, R. J. 1994. Sorption of arsenic by iron oxides and oxyhydroxides in soils. Appl. Geochem. 9:279-286. [Google Scholar]
- 6.Brofft, J. E., J. V. McArthur, and L. J. Shimkets. 2002. Recovery of novel bacterial diversity from a forested wetland impacted by reject coal. Environ. Microbiol. 4:764-769. [DOI] [PubMed] [Google Scholar]
- 7.Bruneel, O., J.-C. Personné, C. Casiot, M. Leblanc, F. Elbaz-Poulichet, B. J. Mahler, A. Le Flèche, and P. A. D. Grimont. 2003. Mediation of arsenic oxidation by Thiomonas sp. in acid mine drainage (Carnoulès, France). J. Appl. Microbiol. 95:492-499. [DOI] [PubMed] [Google Scholar]
- 8.Casiot, C., G. Morin, F. Juillot, O. Bruneel, J. C. Personné, M. Leblanc, K. Duquesne, V. Bonnefoy, and F. Elbaz-Poulichet. 2003. Bacterial immobilization of arsenic in acid mine drainage (Carnoulès creek, France). Water Res. 37:2929-2936. [DOI] [PubMed] [Google Scholar]
- 9.Casiot, C., O. Bruneel, J.-C. Personné, M. Leblanc, and F. Elbaz-Poulichet. 2003. Arsenic oxidation and bioaccumulation by the acidophilic protozoan, Euglena mutabilis, in acid mine drainage (Carnoulès, France). Sci. Total Environ. 320:259-267. [DOI] [PubMed] [Google Scholar]
- 10.Drobner, E., H. Huber, R. Rachel, and K. O. Stetter. 1992. Thiobacillus plumbophilus spec. nov., a novel galena and hydrogen oxidizer. Arch. Microbiol. 157:213-217. [DOI] [PubMed] [Google Scholar]
- 11.Duquesne, K., S. Lebrun, C. Casiot, O. Bruneel, J.-C. Personné, M. Leblanc, F. Elbaz-Poulichet, G. Morin, and V. Bonnefoy. 2003. Immobilization of arsenite and ferric iron by Acidithiobacillus ferrooxidans in acid mine drainage. Appl. Environ. Microbiol. 69:6165-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Fantroussi, S., J. Mahillon, H. Naveau, and S. N. Agathos. 1997. Introduction of anaerobic dechlorinating bacteria into soil slurry microcosms and nested-PCR monitoring. Appl. Environ. Microbiol. 63:806-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felsenstein, J. 1993. PHYLIP-phylogeny inference package (version 3.5c), Department of Genetics, University of Washington, Seattle, WA.
- 14.Ferris, F. G., R. O. Hallberg, B. Lyvén, and K. Pedersen. 2000. Retention of strontium, cesium, lead and uranium by bacterial iron oxides from a subterranean environment. Appl. Geochem. 15:1035-1042. [Google Scholar]
- 15.Friese, K., K. Wendt-Potthoff, D. W. Zachmann, A. Fauville, B. Mayer, and J. Veizer. 1998. Biogeochemistry of iron and sulfur in sediments of an acidic mining lake in Lusatia, Germany. Water Air Soil Poll. 108:231-247. [Google Scholar]
- 16.Hallberg, K. B., and D. B. Johnson. 2003. Novel acidophiles isolated from moderately acidic mine drainage waters. Hydrometallurgy 71:139-148. [Google Scholar]
- 17.Katsoyiannis, I. A., and A. I. Zouboulis. 2004. Application of biological processes for the removal of arsenic from groundwater. Water Res. 38:17-26. [DOI] [PubMed] [Google Scholar]
- 18.Kuever, J., M. Konneke, A. Galushko, and O. Drzyzga. 2001. Reclassification of Desulfobacterium phenolicum as Desulfobacula phenolica comb. nov. and description of strain SaxT as Desulfotignum balticum gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 51:171-177. [DOI] [PubMed] [Google Scholar]
- 19.Lane, D. J. 1991. rRNA sequencing, p. 115-175. In G. M. E. Stachenbradt (ed.), Nucleic acid techniques in bacterial systematics. Wiley, Chichester, United Kingdom.
- 20.Langmuir, D. 1997. Acid mine waters, p. 457-478. In R. McConnin (ed.), Aqueous environmental geochemistry. Prentice-Hall, Englewood Cliffs, N.J.
- 21.Langner, H. W., C. R. Jackson, T. R. Mcdermott, and W. P. Inskeep. 2001. Rapid oxidation of arsenite in a hot spring ecosystem, Yellowstone National Park. Environ. Sci. Technol. 35:3302-3309. [DOI] [PubMed] [Google Scholar]
- 22.Leblanc, M., B. Achard, D. Benothman, J. Bertrand-Sarfati, J. M. Luck, and J. C. Personné. 1996. Accumulation of arsenic from acidic mine waters by ferruginous bacterial accretions (stromatolites). Appl. Geochem. 11:541-554. [Google Scholar]
- 23.Michard, G., and J. Faucherre. 1970. Etude géochimique de l’altération des minerais sulfurés de St. Sébastien d’Aigrefeuille. Chem. Geol. 6:63-84. [Google Scholar]
- 24.Morin, G., F. Juillot, C. Casiot, O. Bruneel, J.-C. Personné, F. Elbaz-Poulichet, M. Leblanc, P. Ildefonse, and G. Calas. 2003. Bacterial formation of tooeleite and mixed arsenic(III) or arsenic(V)-Iron(III) gels in the Carnoulès acid mine drainage, France. A XANES, XRD, and SEM study. Environ. Sci. Technol. 37:1705-1712. [DOI] [PubMed] [Google Scholar]
- 25.Oremland, R. S., and J. F. Stolz. 2003. The ecology of arsenic. Science 300:939-944. [DOI] [PubMed] [Google Scholar]
- 26.Sadiq, M. 1997. Arsenic chemistry in soils: an overview of thermodynamic predictions and field observations. Water Air Soil Poll. 93:117-136. [Google Scholar]
- 27.Selenska-Pobell, S., G. Kampf, K. Flemming, G. Radeva, and G. Satchanska. 2001. Bacterial diversity in soil samples from two uranium waste piles as determined by rep-APD, RISA and 16S rDNA retrieval. Antonie Leeuwenhoek 79:149-161. [DOI] [PubMed] [Google Scholar]
- 28.Squibb, K. S., and B. A. Fowler. 1983. The toxicity of arsenic and its compounds, p. 233-269. In B. A. Fowler (ed.), Biological and environmental effects of arsenic. Elsevier, New York, N.Y.
- 29.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakao, N., H. Koyatsu, Y. Komai, H. Shimokawara, Y. Sakurai, and H. Shiota. 1988. Microbial oxidation of arsenite and occurrence of arsenite-oxidizing bacteria in acid mine water from a sulfur-pyrite mine. Geomicrobiol. J. 6:11-24. [Google Scholar]
- 31.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wielinga, B., J. K. Lucy, J. N. Moore, O. F. Seastone, and J. E. Gannon. 1999. Microbiological and geochemical characterization of fluvially deposited sulfidic mine tailings. Appl. Environ. Microbiol. 65:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zettler, L. A. A., F. Gómez, E. Zettler, B. G. Keenan, R. Amils, and M. L. Sogin. 2002. Eukaryotic diversity in Spain’s river of fire. Nature 417:137. [DOI] [PubMed] [Google Scholar]