Abstract
The increasing incidence of harmful algal blooms around the world and their associated health and economic effects require the development of methods to rapidly and accurately detect and enumerate the target species. Here we describe use of a solid-phase cytometer to detect and enumerate the toxic alga Prymnesium parvum in natural samples, using a specific monoclonal antibody and indirect immunofluorescence. The immunoglobulin G antibody 16E4 exhibited narrow specificity in that it recognized several P. parvum strains and a Prymnesium nemamethecum strain but it did not cross-react with P. parvum strains from Scandinavia or any other algal strains, including species of the closely related genus Chrysochromulina. Prymnesium sp. cells labeled with 16E4 were readily detected by the solid-phase cytometer because of the large fluorescence signal and the signal/noise ratio. Immunofluorescence detection and enumeration of cultured P. parvum cells preserved with different fixatives showed that the highest cell counts were obtained when cells were fixed with either glutaraldehyde or formaldehyde plus the cell protectant Pluronic F-68, whereas the use of formaldehyde alone resulted in significantly lower counts. Immunofluorescence labeling and analysis with the solid-phase cytometer of fixed natural samples from a bloom of P. parvum occurring in Lake Colorado in Texas gave cell counts that were close to those obtained by the traditional method of counting using light microscopy. These results show that a solid-phase cytometer can be used to rapidly enumerate natural P. parvum cells and that it could be used to detect other toxic algae, with an appropriate antibody or DNA probe.
It is widely accepted that the occurrence of harmful algal blooms and their negative effects on aquatic resources and human health have increased worldwide over the last few decades (2, 10, 28). At present, the majority of monitoring programs rely on classical methods, light and electron microscopy, for identification of algae by observation of morphological features. This can be particularly difficult for small species (<10 μm), which may not have many discriminatory features and which may be difficult to discern in a background consisting of other species and debris, particularly if the algae are present at low concentrations. Therefore, correct identification and enumeration of the species of interest can be extremely time-consuming and require a researcher with considerable taxonomic experience. To understand further the mechanisms of bloom formation and to allow development of predictive models, sampling of harmful algal blooms needs to be increased both in time and in space. Over the last two decades many research programs have been initiated to develop molecular probes, primarily antibodies and DNA probes, to accurately detect the species of interest. Monoclonal antibodies (MAbs) and polyclonal antibodies have been used to detect cultured cells of a wide variety of harmful algae (1, 23, 32, 33). Because of their high specificity, MAbs have also been used very successfully for enumerating the brown tide alga Aureococcus anophagefferens in natural samples (5). Immunofluorescence techniques using MAbs targeted to the cell surface are also advantageous because no cell permeabilization is required and the fluorescence intensity is usually far greater than that of DNA probes and is less affected by the physiological state of the cell (3).
The haptophyte genus Prymnesium is composed predominantly of toxic species which can form harmful blooms that are usually restricted to brackish waters (7). The major bloom-forming species Prymnesium parvum and P. parvum f. patelliferum (formerly known as Prymnesium patelliferum) were initially thought to be two separate species of Prymnesium on the basis of body scale morphology. However, studies of DNA ploidy and phylogenetic analyses have suggested that the different morphologies represent two stages of the haploid-diploid life cycle of the same species, P. parvum (14, 15). Blooms of P. parvum and P. parvum f. patelliferum have been responsible for mass mortality of fish and significant economic losses in Europe and North America (7, 21), and recurrent blooms in numerous lakes, reservoirs, and rivers in Texas have been attributed to P. parvum (13, 31). Currently, Prymnesium spp. in natural samples are identified and counted by light or epifluorescence microscopy using a hemocytometer (30). This task is difficult because fresh samples must be examined since the morphology of Prymnesium cells is distorted by fixation. Furthermore, identification to the species level and differentiation of Prymnesium from the closely related genus Chrysochromulina require viewing of the body scales by transmission electron microscopy (TEM). Although specific oligonucleotide probes have been used successfully to discriminate members of a mixture of cultured cells of Prymnesium sp. and Chrysochromulina sp. using flow cytometry (FCM) (26, 27), these probes were not tested with natural samples.
An alternative instrument for semiautomated detection of fluorescently labeled microorganisms is the solid-phase cytometer (SPC), which scans the entire surface of a membrane with a laser, processes signals from the individual fluorescent events, and then applies a set of discriminants to distinguish true positive results from false-positive results (autofluorescent particles or cells). The advantages of the SPC are that the theoretical limit of detection is one cell per membrane, which allows detection of low cell concentrations (4, 17, 19, 25), and that the identity of the cells detected can be subsequently verified by microscopy.
Here we describe development of a rapid test for detection of P. parvum using, for the first time, a specific MAb and solid-phase cytometry, which was optimized initially with cultured Prymnesium sp. and then used to enumerate cells from natural samples.
MATERIALS AND METHODS
Natural samples.
Natural blooms of P. parvum were sampled on 1 July 2003 at two sites, one on the shore and the other located about 1 km offshore, at Lake Colorado in west central Texas. These sampling sites are referred to below as “Shore” and “Offshore,” respectively. At both sites samples (500 ml) were collected from the top 1 m of surface water with labeled plastic bottles (which were completely filled). Each bottle was wrapped with paper towels moistened with lake water and placed into plastic zip-lock bags to maintain the ambient lake temperature. The samples were then packed in a Styrofoam-lined box, sealed, and shipped via overnight courier to the Center for Marine Science, University of North Carolina, Wilmington, for microscopic observation.
Microscopic observation and counting of P. parvum in the natural samples.
Live subsamples of the Shore and Offshore samples were immediately examined using a Nikon Diaphot inverted microscope equipped with differential interference contrast and epifluorescence optics. Morphological features, such as chloroplast number, location of the nucleus, insertion and length of the flagella, and haptonema and cellular inclusions, were confirmed with a bright-field microscope. Subsamples were also fixed with 4% formaldehyde or 1% glutaraldehyde for SPC analysis or with 1% Lugol's iodine solution for bright-field microscopy and were stored in the dark at 4°C until counting. Bright-field microscopic counting was performed within 3 days of sample collection using the settling method as described previously (12). In brief, for each of the six replicate counts obtained for each sample, a 25-ml subsample of the well-mixed preserved sample was placed into a settling chamber and allowed to settle for 24 to 30 h. Counts were obtained for an appropriate number of fields (30) until more than 300 cells had been observed, using a Nikon inverted microscope. The algal counts for each site were calculated by averaging the counts for the six replicates.
Isolation of cultures from natural samples.
Clonal cultures of P. parvum from the Texas lake were established by single-cell isolation with a pipette and transfer of each cell to diluted (1/10) Guillard's F/2 medium (low salinity [∼5‰]). The cultures were incubated at 22°C with a cycle consisting of 12 h of light and 12 h of darkness by using an irradiance of 50 μmol · m−2 · s−1 (cool white light). Once established, the clonal cultures were maintained under the conditions described above except that the medium was full-strength F/2 medium. These isolates were used in the TEM and hemolytic studies described below.
TEM analysis.
To further confirm the identity of P. parvum, natural samples from Lake Colorado, as well as clones isolated from these samples (see above), were used to determine scale morphology by TEM. Whole-mount samples were prepared by using methods described previously (22). In brief, samples were dried at 60°C, shadow cast with a Denton DV502 vacuum evaporator, and observed with a Philips CM12 TEM. Distal and proximal scale morphology and other morphological features (presence and position of the haptonema, flagellar structure, and structure of the chloroplast) were used to confirm the identity of P. parvum.
Hemolytic activity assay.
Hemolytic activities of natural samples and cultures were determined with pelleted cells, supernatants from samples, and whole samples, using the microtiter assay described previously (8). A 50-ml aliquot of each natural sample or culture was centrifuged at 3,000 × g for 5 min at 4°C to obtain a cell pellet and a cell-free supernatant. The pellet was resuspended in 2 ml of assay buffer (150 mM NaCl, 3.2 mM KCl, 1.25 mM MgSO4, 3.75 mM CaCl2, 12.2 mM Trizma; pH 7.4) and sonicated at 50 W for 30 s. Portions (125 μl) of the pellet slurry concentrate or dilutions of the concentrate (0.5, 0.1, and 0.01) were pipetted into microtiter plate wells before addition of 125-μl aliquots of human erythrocytes that had been washed twice in assay buffer and resuspended in the same buffer to a concentration of about 290,000 cells/ml. Whole and supernatant samples were added to the microtiter plates as described above for the pellet concentrate. The negative control well contained 125 μl of erythrocytes in assay buffer alone, and the total-lysis control well contained an erythrocyte suspension that was totally lysed by addition of saponin (160 μg/ml; catalog no. S4521; Sigma). The plates were incubated at 4°C for 24 h, and then the optical density at 415 nm of each well was determined using a microplate scanning spectrophotometer (BIO-TEK PowerWavex). All values were corrected by subtracting the value for the negative control, and the hemolytic activity was then expressed as a percentage of the total-lysis control value.
Algal cultures.
The algal species and strains used in this study and their origins are shown in Table 1. All strains were grown in Larsen's TL medium (16) at a salinity of 30‰ and temperature of 15°C, with the following exceptions: Prymnesium strains K-0081 and K-0082 were grown in Larsen's TL medium at a salinity of 10‰ and temperature of 15°C, Prymnesium strain K-0635 was grown in Larsen's TL medium at a salinity of 30‰ and temperature of 20°C, Alexandrium minutum A5 was grown Larsen's TL medium at a salinity of 15‰ and temperature of 20°C, and A. minutum strains Morlaix and AL7V were grown in Guillard's F/2 medium at a salinity of 15‰ and temperature of 18°C. The irradiance was approximately 50 μmol · m−2 · s−1 with a cycle consisting of 12 h of light and 12 h of darkness. Cells were fixed with 3.8% (vol/vol) formaldehyde or 0.1% (vol/vol) glutaraldehyde at 4°C for at least 1 h (see Results).
TABLE 1.
Algal strains used in this study and their reactivities with MAb 16E4
| Species | Strain | Origin | Reactivity with MAb 16E4 |
|---|---|---|---|
| Prymnesiophyceae | |||
| Prymnesium parvum | K-0623 | Germany | + |
| P. parvum | K-0081 | Denmark | − |
| P. parvum f. patelliferum | K-0082 | England | + |
| P. parvum f. patelliferum | K-0252 | Australia | + |
| P. parvum f. patelliferum | K-0374 | Norway | − |
| P. parvum f. patelliferum | K-0594 | Spain | + |
| P. parvum f. patelliferum | K-0635 | Thailand | − |
| P. faveolatum | K-0636 | Spain | − |
| P. nemamethecum | K-0394 | Denmark | +/− |
| Chrysochromulina acantha | K-0643 | Norway | − |
| C. ahrengotii | K-0588 | Denmark | − |
| C. apheles | K-0290 | Denmark | − |
| C. brevifilum | K-0560 | Denmark | − |
| C. ericina | K-0562 | Denmark | − |
| C. kappa | K-0563 | Denmark | − |
| C. limonia | K-0567 | Denmark | − |
| C. polylepis | K-0259 | Sweden | − |
| C. polylepis | K-0618 | Sweden | − |
| C. polylepis | K-0617 | Sweden | − |
| C. rotunda | K-0644 | Norway | − |
| C. scutellum | K-0598 | Denmark | − |
| C. scutellum | K-0353 | Denmark | − |
| C. simplex | K-0272 | Australia | − |
| C. throndsenii | K-0589 | Norway | − |
| Isochrysis sp. | K-0633 | Denmark | − |
| Pavlova sp. | K-0465 | Japan | − |
| Pavlova sp. | K-0013 | New Zealand | − |
| Cryptophyceae | |||
| Rhodomonas marina | K-0435 | Denmark | − |
| Dinophyceae | |||
| Alexandrium minutum | A5 | Denmark | − |
| A. minutum | Morlaix | Northwest France | − |
| A. minutum | AL7V | Northwest Spain | − |
| A. tamarense | K-0056 | Norway | − |
| A. tamarense | AT02 | Southeast France | − |
| Karenia sp. | GM94GAB | Tunisia | − |
| Heterocapsa rotundata | K-0479 | Denmark | − |
| Prasinophyceae | |||
| Mantoniella squamata | K-0284a | Denmark | − |
| Nephroselmis pyriformis | K-0557 | Thailand | − |
| Pyramimonas gorlestonia | K-0261 | Australia | − |
| P. moestrupii | K-0265 | Australia | − |
| Tetraselmis sp. | K-0950 | England | − |
Preparation and screening of MAbs.
Cells in unialgal cultures of five P. parvum strains (strains K-0623, K-0081, K-0082, K-0252, and K-0374) (Table 1) were counted using a hemocytometer chamber, and equal numbers of cells from the five cultures were mixed before concentration by centrifugation to obtain approximately 105 cells/100 μl. Aliquots of the concentrated, unpreserved cells were stored at −20°C. MAbs were prepared with the assistance of CovalAb, Lyon, France, using standard methods described previously (11). The first immunization of four BALB/c mice (on day 0) was performed by intraperitoneal injection of thawed aliquots of concentrated P. parvum cells (100 μl containing 105 cells) mixed with an equal volume of Freund's complete adjuvant. The nine subsequent intraperitoneal injections were performed at intervals of about 20 days like the first injection, except that an equal volume of Freund's incomplete adjuvant was mixed with each 100-μl aliquot of thawed cells. The immune response of each mouse was monitored four times during the 10 immunizations by testing serum samples for antibody reactivity against P. parvum cells using an indirect immunofluorescence assay (IFA) on slides (see below) and by serotyping. On day 226, the mouse whose serum cross-reacted with P. parvum cells and showed the highest titer of antibodies was inoculated intraperitoneally with another aliquot of P. parvum cells (100 μl of cells without adjuvant) in order to boost the antibody titer before the mouse was sacrificed for spleen cell fusion 3 days later. Spleen cells were fused with Sp2/0 myeloma cells in the presence of 50% polyethylene glycol 4000, and the resulting hybridomas were resuspended in selective medium (RPMI-1640 [Sigma] supplemented with 1× hypoxanthine-aminopterin-thymine, 20% fetal calf serum, and 1× Hybridokine) to obtain a density of 5.5 × 105 cells/ml before 250-μl portions were distributed into 96-well plates. After 3 weeks, the hybridoma supernatants were first screened for antibody secretion by an enzyme-linked immunosorbent assay (11). Secreting hybridomas were screened further by IFA on slides, using a mixture of P. parvum strains in each well (see below). Supernatants that exhibited a positive reaction were then screened further by carrying out the same assay with individual Prymnesium sp. strains in order to assess antibody specificity. The hybridoma of interest was cloned by limiting dilution (11) by preparing serial dilutions in 96-well plates to obtain theoretical concentrations of 10, 5, 1, and 0.5 hybridomas per well. The medium used for limiting dilution was the same as the medium used for fusion except that it lacked aminopterin. The resulting clones were screened as described above by IFA to confirm antibody reactivity. For large-scale production of the MAb, the clone was introduced into BALB/c mice by intraperitoneal injection to produce ascites. The antibody-rich ascites liquid was collected by aspiration, and the antibody was purified by affinity chromatography.
Immunofluorescence assay on slides.
To immobilize cells on multiwell glass slides (Fisher), 5 μl of polylysine (10 mg ml−1) was spotted in each well and allowed to dry at 37°C. Some P. parvum strains (strains K-0623, K-0081, K-0082, K-0252, and K-0374) (Table 1) were fixed with 3.8% formaldehyde as described above, 5- to 10-μl portions of the cells were then added either as a mixture or individually to the wells before the slides were placed in a humid chamber, and the cells were allowed to settle for at least 2 h or overnight. The slides were rinsed by immersing them in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.2 mM Na2HPO4 · 7H2O, 1.4 mM KH2PO4; pH 7.3) for 1 min, and then excess buffer around the wells was removed by blotting before 5 μl of blocking solution (normal goat serum [Sigma] diluted to 1/10 in PBS) was added and incubation for 30 min at room temperature in a humid chamber. The slides were rinsed in PBS and blotted as described above before 5 μl of primary antibody was added to each well and incubation for 1 h at room temperature in a humid chamber. The primary antibody was either undiluted hybridoma supernatant or the pure MAb diluted 1/1,000 or 1/500 in PBS supplemented with 0.05% (final concentration) Tween 20 (PBS-T). The slides were washed by immersing them in PBS-T for 10 min and then blotted as described above before 5 μl of the secondary antibody (anti-mouse fluorescein isothiocyanate conjugate [Sigma] diluted 1/500 in PBS-T) was added to each well and incubation for 1 h at room temperature in a humid chamber. The slides were then washed as described above in PBS-T. Excess buffer was removed from the slides by blotting, after which the slides were mounted with the antifade agent Citifluor AF1 (Citifluor Ltd., London, United Kingdom). The slides were viewed by epifluorescence microscopy using an Olympus Provis AX70 microscope equipped with a filter (U-MWB) for blue excitation (450 to 480 nm), and images were captured with a Zeiss Axiocam HR digital camera.
Immunofluorescence assay on filters.
For the initial qualitative analyses with cultured Prymnesium sp. strains, 50 to 100 μl of fixed cells (3.8% formaldehyde) was deposited on 25-mm-diameter, 2.0-μm-pore-size black polyester membranes (Cyclopore track etched; Whatman) by placing the filter on a filter pad moistened with PBS and allowing 1 drop of cells to be absorbed by capillary action. For the quantitative analyses (stability of fixed cultured cells, spiking of seawater, and analysis of natural samples) 50 to 100 μl of fixed cultured cells or a fixed natural sample was diluted in 1 ml of PBS before filtering onto the same membranes using low vacuum pressure (<100 mm Hg). The IFA method used for membranes was the same as the method used for slides in terms of incubation times and temperatures; the main difference was that the immunolabeling and washing solutions were placed under the membrane and reached the cells by capillary action. The blocking and antibody incubation procedures were done in petri slides (Millipore) in order to maintain a humid atmosphere, and each membrane was placed on top of a 80-μl drop of either the blocking or antibody solution. After incubation with the primary and secondary antibodies, the membranes were washed twice by placing them on filter pads soaked in PBS-T for 5 min and then transferring them to fresh PBS-T-soaked filter pads for a further 5 min. Finally, the membranes were transferred onto pads soaked in PBS and stored at 4°C in the dark for a maximum of 24 h before analysis with the SPC.
Detection and enumeration of cells with the SPC.
After IFA labeling, each membrane in turn was placed onto the sample holder of the SPC (ChemScan RDI; Chemunex, Ivry-sur-Seine, France). The ChemScan RDI SPC is the successor of the ChemScan system that has been described elsewhere (20). A series of discriminating parameters were applied to the raw data (“Total Events”) to differentiate between immunolabeled organisms and autofluorescent particles. The discriminant parameters were as follows: peak intensity (PI), 500 to 6,000; secondary/primary ratio, 0.2 to 0.9; number of lines, 8 to 50; and number of samples, 15 to 60. When the discriminating parameters were being tested, raw data were validated and immunofluorescent organisms and autofluorescent particles were manually discriminated with the microscope using its motorized stage, under control of the SPC as described previously (20). This allowed comparison of the counts obtained by the SPC using the automatic validation procedure (application of discriminants) with the counts obtained using a manual discrimination step. The entire process was performed in less than 10 min.
Stability of cultured Prymnesium cells fixed in the presence or absence of Pluronic F-68.
Portions (10 ml) of a culture of P. parvum f. patelliferum K-0082 were fixed with either 0.1% glutaraldehyde or 3.8% formaldehyde for 1 h at 4°C, with or without addition of the cell surfactant Pluronic F-68 (Sigma) at a final concentration of 0.1%. After 1 h of fixation (designated time zero), 100-μl triplicate samples that received each of the four treatments were diluted 1/10 in PBS containing the same concentration of fixative (with or without Pluronic F-68) and were analyzed by flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson). In addition, duplicate 100-μl portions were filtered onto membranes and immunolabeled for analysis with the SPC (as described above). A further negative control membrane was prepared with 100 μl of sample and labeled as usual, except that primary antibody 16E4 was omitted. The fixed cultures were stored at 4°C, and samples were subsequently taken at weekly intervals in triplicate for flow cytometry and in duplicate for SPC analysis, except for the samples taken at 28 h, when sampling was also done in triplicate. For the statistical analyses, the normality and homogeneity of the variances were verified before nonparametric (Kruskal-Wallis) (29) or parametric (analysis of variance) (29) tests were performed.
Enumeration of cultured Prymnesium cells in spiked seawater samples.
Triplicate 1-ml samples of a glutaraldehyde-fixed (0.1%) culture of P. parvum f. patelliferum K-0082 were enumerated by flow cytometry (see above), and the mean number of cells/ml was calculated. Dilutions of the glutaraldehyde-fixed Prymnesium cells were prepared with seawater collected from the shore of Banyuls sur mer (France) to which 0.1% glutaraldehyde had been added in order to obtain concentrations of 5,000, 500, and 50 cells per ml. Portions (100 μl) of each dilution or seawater alone were filtered onto 2.0-μm membranes (as described above) in triplicate. An extra membrane was prepared using 100 μl of the 5,000-cell/ml dilution as the control membrane (no antibody). Immunolabeling and detection by the SPC were performed as described above.
SPC detection of Prymnesium in natural samples.
The Lake Colorado bloom samples, one from the Offshore site (fixed with 1% glutaraldehyde) and two from the Shore site (fixed with 4% formaldehyde or 1% glutaraldehyde), were analyzed by IFA, followed by detection with the SPC. For each of the three samples, quadruplicate aliquots (50 μl) were first diluted in 1 ml of PBS before they were filtered onto 2.0-μm membranes (as described above). For each sample, IFA was carried out as described above using MAb 16E4 for three of the membranes; for the fourth membrane, the primary antibody was omitted, and this was the negative control.
RESULTS
Production of MAbs against Prymnesium species and specificity testing.
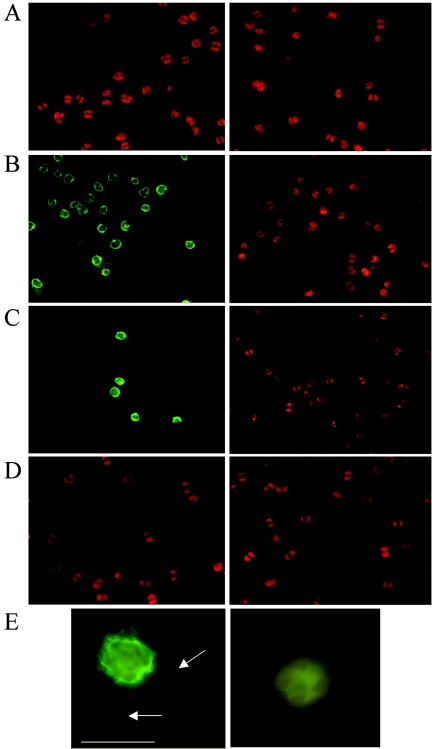
After fusion, 13.5% of the hybridomas exhibiting antibody activity (n = 310) showed positive immunoreactivity when they were tested against a mixture of Prymnesium strains (see Materials and Methods and Table 1). One hybridoma, designated 16E4, which produced immunoglobulin G antibodies, was selected for further cloning to obtain MAbs. The specificity of purified MAb 16E4 was confirmed by performing IFA on slides with a large number of different algal species (Table 1). The antibody proved to be highly specific, recognizing four of the seven strains of P. parvum (P. parvum or P. parvum f. patelliferum) tested, but it did not react with Prymnesium faveolatum and reacted only weakly with Prymnesium nemamethecum. The three P. parvum strains not recognized originated from Denmark (K-0081), Norway (K-0374), and Thailand (K-0635). Representative epifluorescence images of target and nontarget cells after immunolabeling with 16E4 are shown in Fig. 1A to D. The cross-reaction of the antibody with the surface of the target species (P. parvum f. patelliferum strain K-0082 or K-0252) was visible as a green halo around the cell, whereas the red fluorescence in the cell cytoplasm was due to the photosynthetic pigments. Specificity tests for 16E4 were carried out with other unicellular algal species and strains, including Chrysochromulina species that are morphologically similar to and can coexist with Prymnesium spp. in the environment. As Table 1 shows, we did not observe any cross-reactivity with any other prymnesiophyte or with any of the other algal species tested.
FIG. 1.
Epifluorescence images showing immunofluorescent labeling of cultured cells of Prymnesium strains K-0081 (A), K-0082 (B), and K-0252 (C) and Chrysochromulina strain K-0617 (D) and cells of P. parvum in natural samples (E) with MAb 16E4. (A to D) Left panels, IFA with MAb 16E4; right panels, control with only secondary antibody. (E) P. parvum cell from a natural sample from Lake Colorado (Offshore) in Texas labeled with MAb 16E4 (left panel) and unlabeled control (right panel). The arrows indicate labeling of the flagella. Bar, 10 μm. Green fluorescence is conferred by the secondary antibody-fluorescein isothiocyanate conjugate bound to the primary MAb, and red fluorescence is due to autofluorescence of the photosynthetic pigments.
Detection of cultured Prymnesium sp. with the SPC and establishment of the discriminants.
IFA labeling experiments were initially carried out with cultured cells of Prymnesium sp. and nontarget algal species to determine if the intensity of the immunofluorescence signal conferred by 16E4 was high enough to be detected by the SPC and if labeled target cells could be discriminated from nontarget cells and particles (Table 2). The species shown previously by IFA on slides to cross-react strongly with the antibody were also readily detected by the SPC, and the mean peak intensity values (average peak green fluorescence values for the cells) were greater than 1,000 (Table 2). Although IFA with P. nemamethecum on slides showed that there was a weak cross-reaction, this species could be readily detected by the SPC, although the mean PI was lower than that obtained for P. parvum strains (Table 2). The discriminant parameters (see Materials and Methods) were defined to allow detection of the labeled Prymnesium cells but rejection of the maximum number of autofluorescent particles and unlabeled cells. For example, the range used for the mean PI was 500 to 6,000. The parameters were very effective in distinguishing labeled cells from particles, since for cross-reacting species, 95 to 99% of the events validated were target cells and for nonreactive species fewer than five events had to be validated by microscopy (Table 2). The negative control cells (no primary antibody) always gave low mean PI values (less than 500), and no cells were detected after discrimination (data not shown).
TABLE 2.
Detection of IFA-labeled cultured algae by the SPC
| Species | Strain | SPC resulta | No. validated as algaeb | Mean PI (SD) |
|---|---|---|---|---|
| P. parvum | K-0623 | 171 | 162 | 1,827 (353) |
| P. parvum f. patelliferum | K-0082 | 286 | 277 | 1,432 (360) |
| P. parvum | K-0081 | 2 | 1 | 1,313 |
| P. nemamethecum | K-0394 | 281 | 278 | 924 (198) |
| P. parvum f. patelliferum | K-0374 | 1 | 0 | |
| C. kappa | K-0563 | 0 | 0 | |
| C. polylepsis | K-0259 | 0 | 0 | |
| A. tamarense | K-0056 | 4 | 0 | |
| A. minutum | AL7V | 3 | 0 | |
| A. tamarense | AT02 | 1 | 0 | |
| A. minutum | Morlaix | 3 | 0 |
Number of fluorescent events after discrimination.
Microscopic validation.
Stability of fixed cultured cells assessed by the SPC.
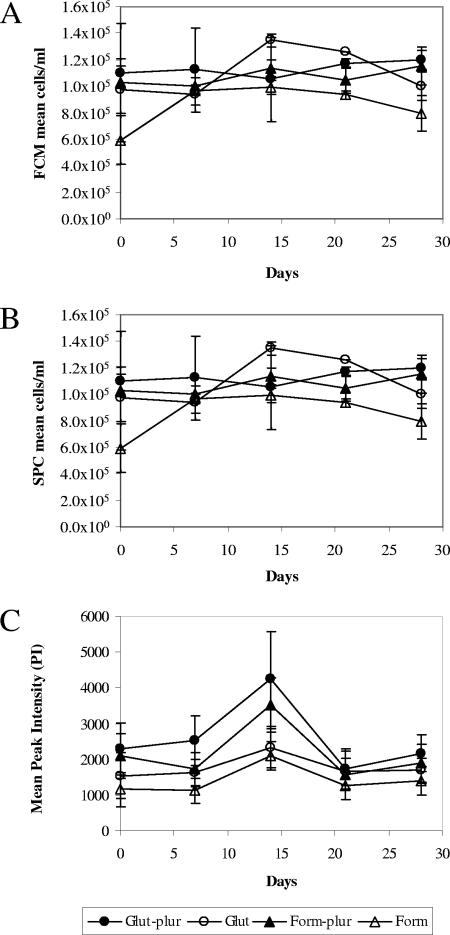
Since the method of preservation of a natural sample can have a significant impact on the number of cells detected, it was important to determine if the antigenicity of the cells (which influences the fluorescence signal) and their subsequent enumeration changed over time depending on the method of fixation. The FCM and SPC counts remained relatively stable (Fig. 2A and B) over the 4-week period, with no significant decrease for any of the fixation methods, and only the counts for the first time examined (after 1 h of fixation) were significantly different from the counts for the remaining times (P = 0.0104). When the different fixation methods were considered, fixation of cells with formaldehyde alone gave significantly lower counts than the other three treatments (P < 0.0001) in both the FCM and SPC analyses, as shown in Fig. 2A and B.
FIG. 2.
Graphs showing the stability of fixed P. parvum f. patelliferum K-0082 cells over time with respect to cell counts (A and B) and IFA labeling intensity (C) after preservation in formaldehyde (Form) or glutaraldehyde (Glut), with or without Pluronic F-68 (plur).
The mean PI did not vary significantly over the 4-week period except for the samples examined at day 14 (P < 0.0001), when it was higher for all treatments (Fig. 2C). There was also a significant difference in the mean PI values for the different fixation methods (P < 0.0001). However, even the lowest mean PI values observed for the formaldehyde-fixed cells were comfortably above the threshold (500) required to detect the cells. The counts obtained with the SPC after IFA labeling were not significantly different from those obtained by FCM for any of the times or fixation methods (P = 0.6194).
Enumeration of cultured P. parvum cells in spiked seawater samples.
Before environmental samples were tested, a quantitative experiment was carried out in order to determine the number of cells of a cultured P. parvum strain in the presence of a background of other organisms in a natural seawater sample by spiking the sample with known numbers of cells before labeling by IFA (Table 3). The mean number of algae detected by the SPC was in good agreement with the expected number of cells for all dilutions, and the mean PI values were also similar and well above the detection threshold (500). No labeled algae were detected on the membranes lacking spiked cells. Although the standard deviations were similar orders of magnitude for all dilutions of cells, the coefficient of variation increased with decreasing mean cell counts (1.8%, 12.2%, and 50.8% for 500, 50, and 5 cells per membrane, respectively), demonstrating that there was greater variability between the replicates.
TABLE 3.
Enumeration of P. parvum f. patelliferum K-0082 cells in spiked seawater samples
| No. of spiked cells per filter | Mean no. of algae (SD) | Mean PI (SD) |
|---|---|---|
| 500 | 551.3 (9.9) | 2,310.3 (816.0) |
| 50 | 50.0 (6.1) | 2,594.0 (783.0) |
| 5 | 6.3 (3.2) | 2,542.0 (723.3) |
| 0 | 0 |
Validation of the SPC with natural Prymnesium sp. samples.
Samples of a Prymnesium bloom were obtained from Lake Colorado in Texas at two sites, Shore and Offshore. The causative species was confirmed to be P. parvum by observation of the body scales by TEM (data not shown), and toxicity was demonstrated by performing an erythrocyte lysis assay (see Materials and Methods). Elevated hemolytic activity was observed in 10-ml concentrated Shore and Offshore samples, which showed 92% and 78% lysis, respectively, compared to the saponin control.
Shore samples fixed with glutaraldehyde or formaldehyde and an Offshore sample fixed with glutaraldehyde were subjected to IFA labeling with 16E4 on SPC filters as described above. Epifluorescence images of one representative labeled cell and an unlabeled cell are shown in Fig. 1E. As observed for cultured cells, the label was clearly located around the surface of the cell, and the flagella also appeared to be labeled. The results obtained by enumeration of the labeled cells from the two sampling sites on triplicate filters are shown in Table 4. High concentrations of P. parvum were detected by the SPC in both the Shore and Offshore samples. A comparison of the results obtained for the Shore sample fixed with glutaraldehyde and the Shore sample fixed with formaldehyde demonstrated that formaldehyde fixation gave lower counts and a lower mean PI than glutaraldehyde fixation for the same sample. However, for the Shore sample fixed with formaldehyde, the level of background fluorescent particles was lower as the total number of fluorescent events detected by the SPC (before discrimination) was approximately four times less than the number for the same sample fixed with glutaraldehyde. In addition, the proportion of fluorescent events discriminated that were validated as algae was higher for the formaldehyde-fixed Shore sample than for the glutaraldehyde-fixed Shore sample (96% and 93%, respectively) (Table 4). The traditional light microscopic counts for the Shore sample were in very good agreement with the counts obtained with the SPC, but the traditional light microscopic counts for the Offshore sample were slightly lower than the counts obtained with the SPC.
TABLE 4.
Enumeration of Prymnesium sp. in natural samples by IFA labeling and detection by the SPC or by direct microscopic counting
| Method | Sample | Fixative | No. of fluorescent eventsa | No. of algaeb | Mean PI (SD) | Concn of algae (103 cells ml−1) |
|---|---|---|---|---|---|---|
| SPC | Shore | Glutaraldehyde | 620.7 | 578.7 | 1,346.7 (347.0) | 11.3-11.9 |
| Formaldehyde | 406.0 | 390.0 | 883.3 (229.0) | 7.4-8.2 | ||
| Offshore | Glutaraldehyde | 579.0 | 559.3 | 1,096.7 (218.0) | 9.8-12.6 | |
| Microscopic counting | Shore | 10.6-12.2 | ||||
| Offshore | 7.8-10.2 |
Number of fluorescent events after discrimination (means for three membranes).
Number of events validated as algae by microscopy (means for three membranes).
DISCUSSION
Improving the monitoring of toxic algae by increasing sampling frequency and the rapidity of the analyses is very important for obtaining a better understanding of bloom dynamics and for mitigating the impact of blooms on human health and the economy. Monitoring the fish-killing alga Prymnesium sp. is difficult because blooms are inconspicuous, the cells are small (<12 μm) and fragile due to the absence of a cell wall, and, in order to avoid morphological distortion by fixatives, fresh samples must be observed. At present, Prymnesium sp. is enumerated in counting chambers, a method which is time-consuming and which requires considerable expertise to accurately identify the cells. In this paper we describe for the first time an IFA labeling method performed with membranes to rapidly enumerate P. parvum in a natural bloom, using a specific MAb in combination with solid-phase cytometry. The scope of MAb 16E4, the development of the IFA method, and the applicability of this method to natural samples are discussed below.
Although polyclonal antibodies are less costly and can give a brighter fluorescent signal than MAbs, the latter generally exhibit greater specificity and the hybridoma cell lines are immortal, which allows consistent production of antibodies. In this study an MAb was raised against a mixture of P. parvum strains having different origins in order to increase the chances of selecting an antibody clone that recognized all strains of P. parvum, the species that has been implicated in the majority of harmful blooms (7, 21). However, specificity tests with cultured Prymnesium strains showed that MAb 16E4 did not recognize three of the P. parvum strains tested, two of which originated from Scandinavia. These observations may have a genetic basis since phylogenetic analyses of the sequence and length variation of an internal transcribed spacer (ITS1) of the calmodulin gene showed that all the P. parvum strains originating from Scandinavia were very closely related and formed a cluster distinct from the strains originating from England and Australia (15). Therefore, the lack of binding or reduced binding of MAb 16E4 to the Scandinavian strains may be due to the absence or significant modification of the epitope recognized by the antibody. The specificity of MAb 16E4 was also demonstrated by the lack of cross-reactions with other algal species, including Chrysochromulina kappa and Chrysochromulina polylepis, which are more closely related to P. parvum and P. nemamethecum than to other Chrysochromulina species (6).
For detection of labeled cells by the SPC, the IFA method had to be adapted for membranes. Initially, the antibody or washing solutions were pipetted onto the filters, but this was found to cause considerable cell loss (data not shown). Subsequently, the labeling and washing steps of the IFA method were performed from beneath the membrane, which allowed the solutions to reach the surface of the membrane by capillary action. The high mean PI obtained and the good agreement of the cell numbers measured by the FCM and SPC methods after IFA labeling demonstrated that the cells were well labeled and that there was no significant loss of cells from the membrane (Table 3). Furthermore, the washing of the membranes was adequate since none of the nontarget species were detected by the SPC (Table 2). To eliminate the possibility of nonspecific binding by the secondary antibody, negative control membranes (no primary antibody) were always prepared. Cells on these membranes were never detected by the SPC since the mean PI was always well below the threshold (500), and subsequent manual visualization of the membranes by epifluorescence microscopy confirmed the absence of binding of the secondary antibody.
The method of fixation had a significant impact on the enumeration of cultured P. parvum cells by FCM (total cell counts) or by the SPC (cell counts after IFA labeling). Formaldehyde is commonly used for preservation of phytoplankton and other biological samples for immunofluorescence studies. However, in this study, significantly lower counts were obtained by FCM and by SPC when cells were fixed in formaldehyde than when cells were fixed in glutaraldehyde. As lower cell counts were obtained with both cytometers, this suggests that some of the formaldehyde-fixed cells were lost rather than that there was reduced antibody binding to the cell surface epitopes, which would have led to lower cell counts only for the SPC analysis. Formaldehyde is known to be less efficient at cross-linking than glutaraldehyde, and cell structure is not as well preserved. Prymnesium cells can be damaged by filtration (18); therefore, some cells from the formaldehyde-fixed culture may have been ruptured during the different steps of sample processing (for example, pipetting, vortexing, or filtration). This explanation is supported by the apparent protective effect of Pluronic F-68 since fixation of cultured cells with formaldehyde in the presence of Pluronic F-68, a surfactant used in cell cultures to reduce shearing effects, resulted in cell counts that were comparable to those obtained after glutaraldehyde fixation. For all of the preservation methods there was no significant decrease in the SPC cell counts or the mean PI over the 4-week period, demonstrating that the antigenicity of the cells was well preserved and that cells could be stored fixed for at least 1 month before IFA was performed. A study in which an MAb was used for enumeration of the alga Aureococcus anophagefferens showed that cells fixed with glutaraldehyde and stored at 4°C remained morphologically stable and retained their antigenicity for more than 1 year (5). Although certain epitopes may be more stable than others, it is possible that Prymnesium cells could also be preserved for longer times before IFA labeling, although this should be verified with cultured cells and natural samples. The cell counts at time zero (after 1 h of fixation) were found to be different from the cell counts at other times for both cytometers, and the SPC counts of formaldehyde-fixed cells appeared to be significantly lower. This was probably due to the lower rate of cross-linking of formaldehyde than of cross-linking of glutaraldehyde, and hence the cells may not have been adequately fixed before analysis. Therefore, Prymnesium cultures or natural samples should be fixed either with glutaraldehyde or with formaldehyde plus Pluronic F-68 for several hours or overnight before an IFA is performed.
Spiking of natural (unfiltered) seawater with known concentrations of cultured P. parvum cells revealed that even very low numbers of cells could be enumerated by the SPC after IFA labeling against a background of other phytoplankton. The absence of labeled cells on the control membranes was further confirmation of the lack of nonspecific binding of MAb 16E4 to other species present in the seawater. The good agreement of the SPC-determined mean cell counts with the expected counts showed that there was good quantitative recovery of the cells, although the variability between the triplicate membranes was greater when the lowest concentration of cells was studied. As the whole membrane is scanned by the SPC, the observed variability is not affected by uneven distribution of cells on the membrane, but it probably is affected by the different numbers of cells sampled when cells are pipetted onto the replicate membranes. Therefore, for enumeration of samples containing low concentrations of algae, the reproducibility of the counts could be improved by increasing the number of replicate membranes.
The application of antibody probes to natural samples can be challenging for several reasons. The probes may react with nontarget species in nature that do not have cultured representatives, or they may show poor recognition of the target species due to differences in antigen expression. Furthermore, properties of the natural sample (for example, the level of particulate matter) may interfere with the assay. A P. parvum bloom in Lake Colorado in Texas allowed us to test the applicability of MAb 16E4 for detecting this species in natural samples and to compare the data obtained with the SPC with the data obtained by traditional microscopy, which is currently used for monitoring the development of P. parvum blooms. MAb 16E4 exhibited an excellent cross-reaction with the Lake Colorado P. parvum bloom samples, as shown by the bright green fluorescence around the cell surface and the flagella (Fig. 1E). Although the fluorescence intensity (mean PI) of the cells was lower than that observed for cultured P. parvum strains, the mean PI was well above the threshold used to discriminate positively labeled cells, and this suggests that all or the great majority of P. parvum cells were detected by the SPC. This was also corroborated by the good agreement between the SPC counts and the traditional counts. The lower cell counts obtained for the formaldehyde-fixed Shore sample than for the same sample fixed with glutaraldehyde was in agreement with the culture studies and further confirmed the importance of the method used for preservation for accurate quantification of P. parvum in natural samples. However, a significant advantage of formaldehyde fixation was that the level of background generated was lower than the level generated with glutaraldehyde fixation. As there is an upper limit to the number of total fluorescent events that can be processed by the SPC, the level of the background is an important consideration since this influences the volume of the sample that can be filtered and hence the detection limit for the target species. Therefore, further tests should be carried out with natural samples fixed with formaldehyde and Pluronic F-68 to determine if this method of preservation gives low background values while maintaining the integrity of P. parvum cells. For the Lake Colorado natural samples, the theoretical maximum volume that could be filtered before the processing threshold of the SPC was reached was about 1.4 ml for a formaldehyde-fixed sample. As the spiking experiment demonstrated, just a few cells per membrane could be enumerated, but the variability between replicate membranes resulted in less accurate quantification. Therefore, even if the more conservative minimum number of cells per membrane was 50, P. parvum concentrations as low as 3.6 × 104 cells liter−1 may be quantified. This concentration is much lower than the minimum concentration of P. parvum observed to have a negative impact on the ecosystem (1 × 106 cells liter−1) (7). Detection of subbloom levels of P. parvum could allow early implementation of mitigation strategies, such as treatment of the affected water with ammonium sulfate, which is known to cause lysis of P. parvum cells (9).
The specificity tests with cultured Prymnesium sp. showed that MAb 16E4 did not cross-react with the P. parvum strains isolated from Scandinavia, suggesting that it may not be useful for detection of P. parvum in this region. However, when a natural sample from Denmark containing low numbers of P. parvum cells was analyzed by IFA with MAb 16E4, labeled Prymnesium cells were observed (Gert Hansen, unpublished results). Therefore, additional natural samples from Scandinavia and other geographical regions need to be analyzed by this IFA technique, and the results should be compared with the results of traditional methods to determine if the antibody response to the cultured P. parvum strains reflects the response of natural populations. Another cultured Prymnesium species, P. nemamethecum, was also recognized by the MAb. Although we cannot rule out the possibility that cells of these species cannot be detected by MAb 16E4 in water being monitored for P. parvum, this is unlikely since P. nemamethecum is a marine species (24) and the majority of Prymnesium blooms have been recorded in brackish waters (7). Furthermore, to our knowledge, there have not been any reports of blooms caused by P. nemamethecum.
In conclusion, an IFA method using an MAb was used successfully in combination with solid-phase cytometry to quantify P. parvum in natural samples. The assay can be performed in less than 3 h, which permits higher-frequency sampling and improved monitoring of this toxic species. The great strengths of the SPC include rapid scanning of entire membranes and use of discriminant parameters that allow labeled cells to be validated and enumerated in a fraction of the time that it takes to count the cells on a whole membrane manually by epifluorescence microscopy. Although the IFA-SPC technique has a lower throughput than an enzyme-linked immunosorbent assay-based method and the SPC instrument is costly, this technique could be a useful component of an algal monitoring program, especially as it is one of the only ways to detect and monitor subbloom levels.
Acknowledgments
This work was supported by the European project Detal (grant QLRT-1999-30778) of the Fifth Framework Programme (R&D Project) and by the national French program PNEC ART3-. The electron microscope and hemolytic assay component was supported by a CDC NCDOHH grant to C.T.
We thank Covalab for its assistance in the production of antibodies and Louis Peperzak for kindly donating natural samples. We are also grateful to Philippe Catala for technical aid, to Marie-Jo Dinet for helpful discussions, and to Yves Desdevises for help with the statistical analyses. We thank Joan Glass from Texas Parks and Wildlife for collecting and transmitting natural bloom samples.
REFERENCES
- 1.Adachi, M., Y. Sako, and Y. Ishida. 1993. The identification of conspecific dinoflagellates Alexandrium tamarense from Japan and Thailand by monoclonal antibodies. Nippon Suisan Gakkaishi 59:327-332. [Google Scholar]
- 2.Anderson, D. M. 1987. Toxic algal blooms and red tides: a global perspective, p. 11-16. In T. Okaichi, D. M. Anderson, and T. Nemoto (ed.), Red tides: biology, environmental science, and toxicology. Elsevier, New York, N.Y.
- 3.Anderson, D. M., D. M. Kulis, B. A. Keafer, and E. Berdalet. 1999. Detection of the toxic dinoflagellate Alexandrium fundyense (Dinophyceae) with oligonucleotide and antibody probes: variability in labeling intensity with physiological condition. J. Phycol. 35:870-883. [Google Scholar]
- 4.Baudart, J., J. Coallier, P. Laurent, and M. Prévost. 2002. Rapid and sensitive enumeration of viable diluted cells of members of the family Enterobacteriaceae in freshwater and drinking water. Appl. Environ. Microbiol. 68:5057-5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caron, D. A., M. R. Dennett, D. M. Moran, R. A. Schaffner, D. J. Lonsdale, C. J. Gobler, R. Nuzzi, and T. I. McLean. 2003. Development and application of a monoclonal-antibody technique for counting Aureococcus anophagefferens, an alga causing recurrent brown tides in the Mid-Atlantic United States. Appl. Environ. Microbiol. 69:5492-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edvardsen, B., W. Eikrem, J. C. Green, R. A. Andersen, S. Y. Moon-van der Staay, and L. K. Medlin. 2000. Phylogenetic reconstructions of the Haptophyta inferred from 18S ribosomal DNA sequences and available morphological data. Phycologia 39:19-35. [Google Scholar]
- 7.Edvardsen, B., and E. Paasche. 1998. Bloom dynamics and physiology of Prymnesium and Chrysochromulina, p. 193-208. In D. M. Anderson, A. D. Cemballa, and G. M. Hallegraeff (ed.), Physiological ecology of harmful algal blooms, vol. G 41. Springer, New York, N.Y. [Google Scholar]
- 8.Eschbach, E., J. P. Scharsack, U. John, and L. K. Medlin. 2001. Improved erythrocyte lysis assay in microtitre plates for sensitive detection and efficient measurement of haemolytic compounds from ichthyotoxic alga. J. Appl. Toxicol. 21:513-519. [DOI] [PubMed] [Google Scholar]
- 9.Guo, M. X., P. J. Harrison, and F. J. R. Taylor. 1996. Fish kills related to Prymnesium parvum N Carter (Haptophyta) in the People's Republic of China. J. Appl. Phycol. 8:111-117. [Google Scholar]
- 10.Hallegraeff, G. M. 1993. A review of harmful algal blooms and their apparent global increase. Phycologia 32:79-99. [Google Scholar]
- 11.Harlow, H., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 12.Hasle, G. R. 1978. The inverted-microscope method, p. 88-96. In A. Sournia (ed.), Phytoplankton manual, vol. 6. UNESCO, Paris, France. [Google Scholar]
- 13.James, T. L., and A. de la Cruz. 1989. Prymnesium parvum Carter (Chrysophyceae) as a suspect of mass mortalities of fish and shellfish communities in western Texas. Tex. J. Sci. 41:429-430. [Google Scholar]
- 14.Larsen, A., and B. Edvardsen. 1998. Relative ploidy levels in Prymnesium parvum and P. patelliferum (Haptophyta) analyzed by flow cytometry. Phycologia 37:412-424. [Google Scholar]
- 15.Larsen, A., and L. K. Medlin. 1997. Inter- and intraspecific genetic variation in twelve Prymnesium (Haptophyceae) clones. J. Phycol. 33:1007-1015. [Google Scholar]
- 16.Larsen, N. H., Ø. Moestrup, and P. M. Pedersen. 1994. Scandinavian Culture Centre for Algae and Protozoa catalogue 1994. Botanical Institute, University of Copenhagen, Copenhagen, Denmark.
- 17.Lemarchand, K., N. Parthuisot, P. Catala, and P. Lebaron. 2001. Comparative assessment of epifluorescence microscopy, flow cytometry and solid-phase cytometry used in the enumeration of specific bacteria in water. Aquat. Microb. Ecol. 25:301-309. [Google Scholar]
- 18.Lindholm, T., P. Ohman, K. Kurki-Helasmo, B. Kincaid, and J. Meriluoto. 1999. Toxic algae and fish mortality in a brackish-water lake in Angstrom Land, SW Finland. Hydrobiologia 397:109-120. [Google Scholar]
- 19.Lisle, J. T., M. A. Hamilton, A. R. Willse, and G. A. McFeters. 2004. Comparison of fluorescence microscopy and solid-phase cytometry methods for counting bacteria in water. Appl. Environ. Microbiol. 70:5343-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mignon-Godefroy, K., J. G. Guillet, and C. Butor. 1997. Solid phase cytometry for detection of rare events. Cytometry 27:336-344. [PubMed] [Google Scholar]
- 21.Moestrup, O. 1994. Economic aspects: ‘blooms,’ nuisance species and toxins, p. 265-285. In J. C. Green and B. S. C. Leadbeater (ed.), The haptophyte algae, vol. 51. Clarendon Press, Oxford, United Kingdom. [Google Scholar]
- 22.Moestrup, O., and H. A. Thompsen. 1980. Preparation of shadow-cast whole mounts, p. 385-390. In E. Gannt (ed.), Handbook of phycological methods. Developmental and cytological methods. Cambridge University Press, Cambridge, United Kingdom.
- 23.Nakanishi, K., I. Karube, S. Hiroshi, A. Uchida, and Y. Ishida. 1996. Detection of the red tide-causing plankton Chattonella marina using a piezoelectric immunosensor. Anal. Chim. Acta 325:73-80. [Google Scholar]
- 24.Pienaar, R. N., and M. Birkhead. 1994. Ultrastructure of Prymnesium nemamethecum sp. nov (Prymnesiophyceae). J. Phycol. 30:291-300. [Google Scholar]
- 25.Reynolds, D. T., and C. R. Fricker. 1999. Application of laser scanning for the rapid and automated detection of bacteria in water samples. J. Appl. Microbiol. 86:785-795. [DOI] [PubMed] [Google Scholar]
- 26.Simon, N., J. Brenner, B. Edvardsen, and L. K. Medlin. 1997. The identification of Chrysochromulina and Prymnesium species (Haptophyta, Prymnesiophyceae) using fluorescent or chemiluminescent oligonucleotide probes: a means for improving studies on toxic algae. Eur. J. Phycol. 32:393-401. [Google Scholar]
- 27.Simon, N., L. Campbell, E. Örnolfsdottir, R. Groben, L. Guillou, M. Lange, and L. K. Medlin. 2000. Oligonucleotide probes for the identification of three algal groups by dot blot and fluorescent whole-cell hybridization. J. Eukaryot. Microbiol. 47:76-84. [DOI] [PubMed] [Google Scholar]
- 28.Smayda, T. J. 1990. Novel and nuisance phytoplankton blooms in the sea: evidence for a global epidemic, p. 29-40. In B. S. E. Granéli, L. Edler, and D. M. Anderson (ed.), Toxic marine phytoplankton. Elsevier, New York, N.Y.
- 29.Sokal, R. R., and F. J. Rohlf. 1995. Biometry: the principles and practice of statistics in biological research. W. H. Freeman, New York, N.Y.
- 30.Sournia, A. (ed.). 1978. Phytoplankton manual, vol. 6. UNESCO, Paris, France.
- 31.Tomas, C. R., J. Glass, J. Ralph, and A. Lewitus. Blooms of the ichthyotoxic flagellate Prymnesium parvum in U.S. waters: an emerging or a perennial problem? In K. Steidinger, J. Landsberg, C. Tomas, and G. Vargo (ed.), Harmful algae 2002. Proceedings of the Xth International Conference on Harmful Algae, in press. Florida Fish and Wildlife Conservation Commission and Intergovernmental Oceanographic Commission of UNESCO, St. Petersburg, Fla.
- 32.Vrieling, E. G., R. P. T. Koeman, C. A. Scholin, P. Scheerman, L. Peperzak, M. Veenhuis, and W. W. C. Gieskes. 1996. Identification of a domoic acid-producing Pseudonitzschia species (Bacillariophyceae) in the Dutch Wadden Sea with electron microscopy and molecular probes. Eur. J. Phycol. 31:333-340. [Google Scholar]
- 33.Vrieling, E. G., L. Peperzak, W. W. C. Gieskes, and M. Veenhuis. 1994. Detection of the ichthyotoxic dinoflagellate Gyrodinium (cf.) aureolum and morphologically related Gymnodinium species using monoclonal antibodies: a specific immunological tool. Mar. Ecol. Prog. Ser. 103:165-174. [Google Scholar]