Abstract
Ochratoxin A is a potent nephrotoxin and a possible human carcinogen that can contaminate various agricultural products, including grapes and wine. The capabilities of species other than Aspergillus carbonarius within Aspergillus section Nigri to produce ochratoxin A from grapes are uncertain, since strain identification is based primarily on morphological traits. We used amplified fragment length polymorphisms (AFLPs) and genomic DNA sequences (rRNA, calmodulin, and β-tubulin genes) to identify 77 black aspergilli isolated from grape berries collected in a 2-year survey in 16 vineyards throughout Italy. Four main clusters were distinguished, and they shared an AFLP similarity of <25%. Twenty-two of 23 strains of A. carbonarius produced ochratoxin A (6 to 7,500 μg/liter), 5 of 20 strains of A. tubingensis produced ochratoxin A (4 to 130 μg/liter), 3 of 15 strains of A. niger produced ochratoxin A (250 to 360 μg/liter), and none of the 19 strains of Aspergillus “uniseriate” produced ochratoxin A above the level of detection (4 μg/liter). These findings indicate that A. tubingensis is able to produce ochratoxin and that, together with A. carbonarius and A. niger, it may be responsible for the ochratoxin contamination of wine in Italy.
Ochratoxin A (OTA) is an important mycotoxin; is considered to be nephrotoxic, immunotoxic, genotoxic, and teratogenic; and has been classified by the International Agency for Research on Cancer as a possible human carcinogen (group 2B) (10). Ochratoxin A is produced by a small number of species in the genera Aspergillus, Petromyces, Neopetromyces, and Penicillium (14) and can contaminate various agricultural products, including grapes and wine (13, 34, 36). Accurate identification of ochratoxigenic fungi is of great importance because the toxin profiles of individual species vary and because the fungi that are present limit and define the potential toxicological risks (40). Unfortunately, the taxonomy of Aspergillus section Nigri is not completely resolved, especially within the Aspergillus niger aggregate (5, 20, 35, 38). The A. niger aggregate of Al Musallam (5) is currently described as two species, A. foetidus and A. niger, that are subdivided further into seven varieties, based on morphological and cultural criteria (5, 18, 38). Molecular studies support the division of the A. niger aggregate into two morphologically indistinguishable species, A. niger and A. tubingensis (20, 32, 48).
The presence of ochratoxin A in wine is a relatively recent mycotoxicological problem (31, 33, 44, 49) that is due to contamination by black aspergilli, primarily strains of A. carbonarius and others belonging to the A. niger species aggregate (8, 21, 46). These reports are all based on morphological identifications, which have limited ability to distinguish species in the A. niger aggregate. Amplified fragment length polymorphism (AFLP) analysis, described by Vos et al. (50), can be used for strain identification, especially at low taxonomic ranks (41, 42). Recent studies suggest that these markers can be used to evaluate genetic relatedness among fungal species (23, 51) and to clarify relationships within or between closely related groups or species (24, 47). The advantages of this technique are its high discriminatory power, reproducibility, and robustness. It also can be easily automated (4, 45), and numerous independent polymorphisms can be identified with relatively little change in the protocol.
Our objective in this study was to determine which strain types of the black aspergilli isolated from grapes in Italy, characterized by different molecular approaches (AFLP analysis and DNA sequencing of 28S, internal transcribed spacer [ITS], calmodulin, and β-tubulin genes), can produce ochratoxin A. This report is the first to combine a toxicological characterization of black aspergilli isolated from grapes with different molecular techniques of genetic identification.
MATERIALS AND METHODS
Strains.
Sixteen standard strains from species in the Aspergillus section Nigri were used for AFLP and sequence comparison; they were A. carbonarius IMI 016136 (ex-type strain, ITEM 4503, CBS 111.26) and IMI 41875 (ITEM 4504; CBS 420.64); A. niger IMI 50566 (ex-type strain, ITEM 4501, CBS 554.65), IMI 091881 (ITEM 4502; CBS 555.65), and IMI 015954 (ITEM 4506; CBS 126.48; also reported as A. foetidus); A. tubingensis CBS 134.48 (ex-type strain, ITEM 7040), IMI 172296 (ITEM 4500; CBS 115.29), and IMI 211395 (ITEM 4498; CBS 136.52; also reported as A. phoenicis); A. awamori IMI 211394 (ex-type strain, ITEM 4509, CBS 557.65); A. foetidus var. pallidus IMI 175963 (ex-type strain, ITEM 4508, CBS 565.65); A. japonicus CBS 114.51 (ex-type strain, ITEM 7034) and IMI 211387 (ITEM 4497; CBS 568.65); A. aculeatus IMI 211388 (ex-type strain, ITEM 7046, CBS 172.66); A. pulverulentus IMI 211396 (ITEM 4510; CBS 558.65); A. helicothrix IMI 278383 (ex-type strain, ITEM 4499, CBS 677.79). A. ellipticus IMI 172283 (ex-type strain, ITEM 4505, CBS 482.65); and A. heteromorphus IMI 172288a (ex-type strain, ITEM 7045, CBS 117.55). Seventy-seven Aspergillus strains isolated from grapes in several vineyards throughout Italy were characterized by molecular analysis and tested for ochratoxin A production.
Field sampling and fungal isolation.
Aspergilli were selected during a survey carried out in 2000 and 2001 in 16 vineyards. Five grape berries were taken from a single bunch of grapes collected from each of 10 plants along a diagonal transect in each vineyard. The berries were placed in moist chambers, i.e., petri dishes (9 cm in diameter) containing disks of blotting paper (8 cm in diameter) wetted with 2 ml of sterile water, that were then sealed with Parafilm and incubated at 25 ± 2°C for 7 days. Fungal colonies were transferred to standard identification media, Czapek agar, Czapek yeast agar, and malt extract agar (39); incubated at 25 ± 2°C in the dark for 7 days; and identified according to standard morphological criteria (17). Seventy-seven strains derived from single-conidial-head subcultures were assigned ITEM numbers and deposited in the culture collection of the Institute of Sciences of Food Production (http://www.ispa.cnr.it/Collection).
AFLP analysis.
Fungal strains were grown in shake cultures (150 rpm; 25°C; 2 days) in Wickerham's medium (glucose, 40 g; peptone, 5 g; yeast extract, 3 g; malt extract, 3 g; and distilled water to 1 liter). Genomic DNA was extracted with the E.Z.N.A. Fungal DNA Miniprep Kit (Omega Bio-tek, Doraville, GA) according to the manufacturer's protocol. DNA was dissolved in sterile water, diluted to 20 ng/μl, and stored at −20°C.
We used the AFLP Microbial Fingerprinting kit (Applied Biosystems-Perkin-Elmer Corporation, Foster City, CA) according to the manufacturer's instructions. Approximately 10 ng of genomic DNA from each isolate was cut with EcoRI and MseI (New England Biolabs, Hitchin, Hertfordshire, United Kingdom), and the DNA fragments were ligated to double-stranded restriction site-specific adaptors from the kit. A preselective PCR (72°C for 2 min; 20 cycles of 94°C for 20 s, 56°C for 30 s, and 72°C for 2 min; and then holding at 4°C) was carried out in a 20-μl (final volume) mixture. For the selective PCR, 1.5 μl of a 1:20 dilution of the first PCR product was amplified in a 10-μl (final volume) mixture using selective primers. Four separate primer combinations were utilized for the selective amplification: EcoRI+AC and MseI+CC; EcoRI+AT and MseI+CG; EcoRI+AC and MseI+CA; and EcoRI+G and MseI+CT. The EcoRI primers were labeled with fluorescent dye (Applied Biosystems). The PCR program for selective AFLP amplification was one cycle of 94°C for 2 min and one cycle of 94°C for 20 s, 66°C for 30 s, and 72°C for 2 min; this cycle was followed by nine cycles in which the annealing temperature was lowered each cycle by 1°C from 65°C to 57°C. After that, 20 cycles of 94°C for 20 s, 56°C for 30 s, and 72°C for 2 min were performed, followed by a final extension step at 60°C for 30 min, and then holding at 4°C indefinitely by using a model 9700 GeneAmp PCR system.
After amplification, 1 μl of reaction product was mixed with 20 μl formamide and 0.5 μl GeneScan-500 (ROX) size standard (Applied Biosystems), ranging from 35 to 500 bp in length. The mixture was heated for 2 min at 95°C and snap cooled on ice. The product was separated by capillary electrophoresis on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems). After electrophoresis, the pattern was extracted with GeneScan collection software version 3.1.2 (Applied Biosystems), and the fingerprints were analyzed with Genotyper software (Applied Biosystems). DNA samples from five strains were tested in triplicate, and DNA samples from the other strains were tested in duplicate. DNAs from three replicate cultures of five strains each were also tested.
Peak height thresholds were set at 200. The Genotyper software (Applied Biosystems) was set to medium smoothing. Bands of the same size in different individuals were assumed to be homologous and to represent the same allele. Bands of different sizes were treated as independent loci with two alleles (present and absent). Data were analyzed with an AFLP manager database developed by ACGT BioInformatica S.r.l. (Bari, Italy) and were exported in a binary format with “1” for the presence of a band/peak and “0” for its absence. For clustering, two different analyses were performed. Fragments between 100 and 500 bp and between 200 and 500 bp were analyzed with NTSYS software by using the Dice similarity coefficient and clustered by the unweighted pair group method (27); the clusters obtained were also found by neighbor-joining analysis and maximum-parsimony networks using MEGA version 3.0, with a bootstrap analysis of 1,000 repetitions (19).
Fungal-DNA amplification and sequencing.
The identities of the ochratoxigenic species (A. carbonarius, A. niger, and A. tubingensis) also were confirmed by DNA sequencing. The nuclear rRNA gene containing the ITS region and domains D1 and D2 at the 5′ end of the 28S rRNA gene were amplified with primers F65/R635 and ITS5/ITS4, respectively (28). The calmodulin and β-tubulin genes were amplified by using the conditions and primers described by O'Donnell et al. (29).
After amplification, the PCR products were purified by agarose gel electrophoresis and excised from the agarose gel using spin columns (DNA Gel Extraction Kit; Millipore Corporation, Bedford, MA). Sequence analysis was performed with the Big Dye Terminator Cycle Sequencing Ready Reaction Kit for both strands, and the sequences were aligned with the MT Navigator software (Applied Biosystems). All the sequencing reaction mixtures were purified by gel filtration through Sephadex G-50 (Amersham Pharmacia Biotech, Piscataway, NJ) equilibrated in double-distilled water and analyzed on the ABI PRISM 310 Genetic Analyzer (Applied Biosystems). The resulting sequences of all the isolates were aligned by the Clustal method with the DNAMAN program (Lynnon Corporation, Quebec, Canada).
Ochratoxin A production.
Strains were grown in triplicate in 100-ml stationary cultures of enriched Czapek yeast broth (10) in 500-ml Erlenmeyer flasks for 14 days at 25 ± 2°C in the dark. The cultures were homogenized for 30 min (Ultra-Turrax; IKA, Staufen, Germany) and filtered through folded filter paper (S&S 595; Schleicher & Schuell, Dassel, Germany). One hundred microliters of the filtrate was diluted with 900 μl of the high-performance liquid chromatography (HPLC) mobile phase (acetonitrile-2% acetic acid, 41:59) and then passed through a 0.45-μm nylon syringe filter (Micron Separations Inc., Westborough, Mass.) prior to HPLC analysis.
HPLC analyses.
An ochratoxin A standard was purchased from Sigma (St. Louis, MO). A solution of ochratoxin A (40 mg/ml in benzene-acetic acid, 99:1 [vol/vol]) was calibrated spectrophotometrically (Lambda 2; Perkin-Elmer Corp., Norwalk, Conn.) at 333 nm with 5,550 for the extinction coefficient (6) and stored at −20°C. Working standards were prepared by evaporating an exact volume of the calibrated solution under a stream of nitrogen and redissolving the residue in the mobile phase.
The HPLC system consisted of a Perkin-Elmer 200 instrument equipped with an ISS 200 sampling system (loop volume, 150 μl) and a Jasco FP-920 fluorescence detector set at 333-nm excitation and 470-nm emission. The system was controlled by Perkin-Elmer Turbochrom PC software. A Select B RP-8 column (5-μm particle size; 150- by 4-mm inside diameter; Merck, Darmstadt, Germany) was employed at ambient temperature (20 to 25°C), with a mobile phase of acetonitrile-2% acetic acid (41:59 for ochratoxin A and 55:45 for ochratoxin A methyl ester) at 1.2 ml/min. The injection volume was 30 μl. Ochratoxin A standards of 2 to 60 pg were injected. Peak areas were quantified with the Turbochrom PC software. Ochratoxin A in the extracts was methylated, and the extracts were reanalyzed by HPLC for qualitative confirmation of positive samples (52). The detection limit was 4 μg/liter. All analyses were run in triplicate, and the mean values were reported; standard deviations were <5% of the reported values.
Nucleotide sequence accession numbers.
The calmodulin sequences were deposited in the EMBL nucleotide sequence database (Table 1).
TABLE 1.
Fungal type cultures and sequence accession numbers
| Taxon namec | Accession no.
|
|||
|---|---|---|---|---|
| ITSa | 28Sa | β-Tubulina | Calmodulinb | |
| A. carbonarius | ||||
| CBS 111.26T, ITEM 4503, ATCC 1025, IMI 016136, NRRL 369, WB 369, QM 331 | AJ280011 | AJ280011 | AY585532 | AJ964873 |
| A. niger | ||||
| CBS 554.65T, ITEM 4501, ATCC 16888, IMI 050566, NRRL 326, WB 326 | AJ223852AY656630AY373852 | AY656630AY373852 | AY585536 | AJ964872 |
| A. tubingensis | ||||
| CBS 134.48T, ITEM 7040 | AJ223853 | 1d | AY 820007 | AJ964876 |
| A. awamori | ||||
| CBS 557.65T, ITEM 4509, ATCC 16877, IMI 211394, IOC 230, WB 4948 | AM087614 | 2d | AY 820001 | AJ964874 |
| A. japonicus | ||||
| CBS 114.51T, ITEM 7034 | AJ279985 | 3d | AY585542 | AJ964875 |
| A. aculeatus | ||||
| CBS 172.66T, ITEM 7046, ATCC 16872, IMI 211388, WB 5094 | AJ279988 | U28813 | AY585540 | AJ964877 |
RESULTS
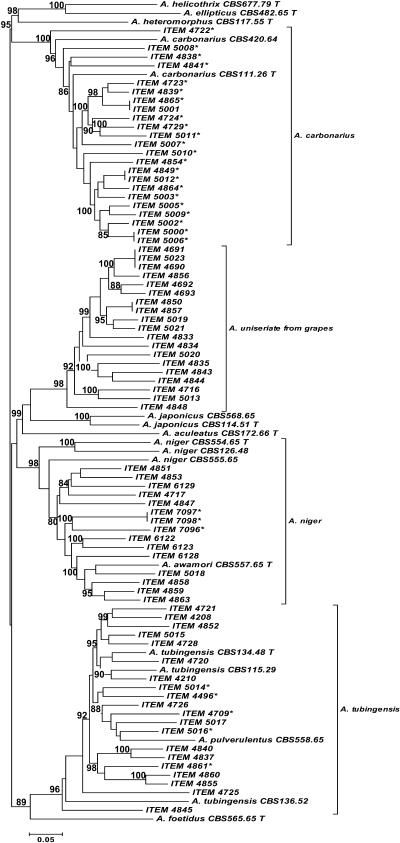
We examined 77 Aspergillus strains (Table 2) selected arbitrarily from 692 isolates of black aspergilli isolated from grapes in Italy during a 2-year survey. Clear polymorphisms both within and between species were obtained by AFLP analysis for each of the four primer pairs. Each primer combination consistently distinguished the taxa in section Nigri, as evidenced by the distribution of the ex-type strains in the neighbor-joining dendrogram (Fig. 1). Unweighted pair group method and maximum-parsimony analyses (data not shown) also divided the isolates into the same clusters, with a bootstrap support of 89 to 100% and 100%, respectively. The 77 strains analyzed clearly separated into four clusters: A. tubingensis, Aspergillus “uniseriate,” A. carbonarius, and A. niger (Fig. 1), with a similarity of <20% for strains in different clusters. Strains were assigned to a species if they shared more than 50% of the bands present in an ex-type strain. All strains of A. carbonarius and A. tubingensis had high similarity (more than 50%) with respect to their ex-type strains. The A. niger cluster strains were more similar to the ex-type strains of A. awamori (58%) than to the A. niger ex-type strain (40 to 45%). The Aspergillus “uniseriate” strains from grapes were at best distantly related to the ex-type strains of A. japonicus and A. aculeatus, with a similarity of >20%. We termed this cluster Aspergillus “uniseriate” because we could not assign the strains to any of the presently accepted uniseriate taxa in the Nigri section (Fig. 1). Both the AFLP analyses at fragment cutoffs of 100 and 200 bp gave the same grouping and similarity among the four clusters and their related ex-type strains.
TABLE 2.
OTA production by Aspergillus section Nigri strains isolated from grapes in different locations throughout Italy
| ITEM | Origina | OTAb (μg/liter) |
|---|---|---|
| A. carbonarius | ||
| 4722 | Brindisi | 110 ± 34 |
| 4723 | Lecce | 100 ± 21 |
| 4724 | Brindisi | 24 ± 9 |
| 4729 | Bari | 64 ± 8 |
| 4849 | Piacenza | 100 ± 30 |
| 4854 | Ravenna | 130 ± 11 |
| 4864 | Ragusa | 6 ± 4 |
| 4865 | Ragusa | 200 ± 53 |
| 5000 | Cuneo | 130 ± 11 |
| 5002 | Reggio Emilia | 120 ± 85 |
| 5003 | Reggio Emilia | 48 ± 7 |
| 5005 | Imola | 10 ± 5 |
| 5006 | Pesaro | 20 ± 5 |
| 5007 | Pesaro | 43 ± 15 |
| 5008 | Chieti | 1,100 ± 58 |
| 5009 | Chieti | 45 ± 16 |
| 5010 | Brindisi | 7,500 ± 390 |
| 5011 | Brindisi | 17 ± 9 |
| 5012 | Sassari | 2,100 ± 960 |
| 4838 | Ragusa | 82 ± 29 |
| 4839 | Ragusa | 60 ± 22 |
| 4841 | Trapani | 210 ± 260 |
| A. tubingensis | ||
| 4496 | Lecce | 130 ± 59 |
| 4709 | Bari | 2 ± 1.6 |
| 4861 | Brindisi | 7 ± 4 |
| 5014 | Brindisi | 7 ± 4 |
| 5016 | Ragusa | 21 ± 5 |
| A. niger | ||
| 7096 | Brindisi | 310 ± 14 |
| 7097 | Piacenza | 360 ± 29 |
| 7098 | Verona | 250 ± 34 |
Locality in Italy (province).
Mean of three repetitions ± standard error.
FIG. 1.
Dendrogram of 94 isolates of Aspergillus section Nigri based on cluster analysis with the neighbor-joining method using the Dice genetic distance coefficient on AFLP data obtained with four primer pairs generated by MEGA 3 software. The number at each node indicates the percentage bootstrap support (out of 1,000) for clusters with ≥80% support. Strains labeled with an asterisk are ochratoxin A producers.
Aspergillus carbonarius.
The A. carbonarius cluster contained all 23 strains identified morphologically as A. carbonarius. All the isolates clustered at a similarity of 68 to 78% with the A. carbonarius ex-type, CBS 111.26 (Fig. 1), while, among them, most of the members of the population shared ≥78% of the bands. Aspergillus carbonarius was the most toxigenic species, with 22/23 strains positive for ochratoxin A production and with ITEM 5001 the only strain in the species that did not produce OTA. The amount of ochratoxin A produced varied widely by strain, from 6 μg/liter by ITEM 4864 to 7,500 μg/liter by ITEM 5010 (Table 2). Sequences of rRNA, calmodulin, and β-tubulin genes from the ex-type strain, CBS 111.26, and the 23 Aspergillus strains characterized as A. carbonarius by AFLP analysis were identical.
Aspergillus tubingensis.
Twenty strains shared 62 to 85% of their bands with the ex-type strain of A. tubingensis, CBS 134.48. The A. phoenicis ex-type strain, CBS 136.52, molecularly reidentified as A. tubingensis, and the A. pulverulentus ex-type strain, CBS 558.65, also belonged to this cluster (Fig. 1). Strains in this cluster could not be clearly identified as A. tubingensis by morphological criteria. Five of the 20 strains produced detectable levels of ochratoxin A (4 to 130 μg/liter) (Table 2). The strains that did not produce OTA were ITEM 4208, 4210, 4720, 4721, 4725, 4726, 4728, 4837, 4840, 4845, 4852, 4855, 4860, 5015, and 5017. The sequences of the rRNA, calmodulin, and β-tubulin genes of ex-type strain CBS 134.48 and the 20 Aspergillus strains characterized as A. tubingensis by AFLP analysis were identical. The ex-type strains of A. phoenicis and A. pulverulentus had the same sequences for the rRNA, calmodulin, and β-tubulin genes as did the A. tubingensis type strain.
Aspergillus niger.
Fifteen strains were grouped in the A. niger cluster with an AFLP similarity of 40 to 46% to the ex-type of A. niger, CBS 554.65, and with a similarity of 58 to 75% to the ex-type strain of A. awamori, CBS 557.65, also identified as A. niger by β-tubulin analysis (39). However, the AFLP analysis at a cutoff of 200 bp revealed a greater distance between the ex-type of A. niger, CBS 554.65, and the 15 strains (35% similarity). The sequences of the rRNA genes for the ex-type strains and the 15 field strains in this group were identical. The calmodulin and β-tubulin genes of the 15 strains had identities of 98% and 100% with those from the A. niger and A. awamori type strains, respectively. Three of the 15 strains in this group produced detectable ochratoxin A (250 to 360 μg/liter) (Table 2). The OTA-nonproducing strains were ITEM 4717, 4847, 4851, 4853, 4858, 4859, 4863, 5018, 6122, 6123, 6128, and 6129. All 15 strains were morphologically indistinguishable from A. tubingensis.
Aspergillus “uniseriate.”
All 19 strains morphologically identified as A. japonicus clustered together and averaged 68% similarity to one another. This cluster was genetically different from the A. japonicus ex-type strain, CBS 114.51, and the A. aculeatus ex-type strain (similarity, <20%) (Fig. 1). None of the 19 strains tested produced detectable ochratoxin A, and they are available as ITEM 4690, 4691, 4692, 4693, 4716, 4833, 4834, 4835, 4843, 4844, 4848, 4850, 4856, 4857, 5013, 5019, 5020, 5021, and 5023.
DISCUSSION
Aspergillus carbonarius contained both the highest proportion of toxigenic strains (95%) and the highest producers of ochratoxin A (up to 7.5 μg/ml). Previous reports of toxin production by A. carbonarius found that 25 to 100% of the strains examined produced ochratoxin A in amounts ranging from 0.3 to 234 μg/g when cultured in an agar medium (12, 37). Further study is needed to determine the cause of this variation. A. carbonarius is a source of ochratoxin A in crops such as coffee and dried vine fruits (1, 16), but its incidence probably is underreported, since all of the black aspergilli are commonly reported only as “A. niger” (2). This is the first report of ochratoxin A production by molecularly characterized strains of A. carbonarius from grapes. Although A. carbonarius has distinctive morphological traits that are easily identified in a microscopic evaluation, molecular tools can speed the identification process and make it more objective. In this respect, AFLP analysis also can be used for identification purposes (42) and for the development of species-specific PCR primers based on unique AFLP fragments (43).
The other ochratoxin-A-producing Aspergillus species we found were A. tubingensis and A. niger. These species are difficult to differentiate on the basis of morphology, with reliable characteristics for distinguishing A. niger, A. foetidus, and A. tubingensis remaining to be identified (40). Aspergillus tubingensis was first described by Mosseray (25) and then by Raper and Fennel (35) as a species in the A. niger group. However, its morphological characteristics are very similar to those of other species in the A. niger species aggregate. Al Musallam (5) in her revision of the A. niger aggregate suggested that “A. niger” was composed of two species, namely, A. foetidus and A. niger sensu stricto, and that A. tubingensis was one of the six varieties in A. niger. Molecular studies (2, 20, 32, 40), however, strongly support our results, which are consistent with the hypothesis that A. tubingensis is clearly distinct from A. niger. Of special importance is our determination that A. tubingensis can produce ochratoxin A. It is not clear what proportion of the strains of “A. niger” previously reported to produce this mycotoxin and recovered from coffee beans (26), vine fruits (15, 21), and animal feed (11), or available from culture collections (3, 30, 46), are A. tubingensis. The identity of the strains from grapes as A. tubingensis is consistent with DNA sequence comparisons made with four previously analyzed diagnostic genes (3, 20, 32, 40). In contrast with our results, Accensi et al. (3) evaluated isolates of the A. niger aggregate (none from grapes) and found that “N-type” A. niger strains produced ochratoxin A but that A. tubingensis strains (termed the “T type”) could not.
The strains of A. tubingensis and A. niger differ from those of A. carbonarius both in the percentages of ochratoxin A-producing isolates recovered (25%, 20%, and 95%, respectively) and in the amounts of ochratoxin produced in liquid cultures (4 to 130, 255 to 357, and 6 to 7,500 μg/liter, respectively). These results confirm that A. carbonarius is an important ochratoxin-producing fungus in wine and that, together with A. tubingensis and A. niger, it may be responsible for the ochratoxin A contamination of wine in Italy.
Although two recent studies (9, 11) reported the production of ochratoxin A by A. japonicus strains isolated from grapes and animal feed, none of our Aspergillus “uniseriate” strains produced detectable ochratoxin A. The genetic distance, based on AFLPs, of our strains from the ex-type strains, however, leaves the taxonomic status of our cultures in need of further definition and resolution, as they could represent a new population or an as-yet-undescribed Aspergillus species.
AFLP analysis is a useful and powerful tool for studying the genetic diversity of black aspergilli. Our AFLP results, combined with DNA sequence analysis of diagnostic genes, confirm the validity and utility of the AFLP technique for evaluating genetic relatedness among fungal species (23, 51), and in particular, for resolving relationships within or between closely related groups or species (24, 47). Previous studies have shown that this technique is suitable for differentiating fungal strains at and below the species level (7, 22, 41, 42). Our study suggests that AFLPs have utility for this purpose in this group of Aspergillus species as well. A combination of biochemical and molecular methods is needed to correctly evaluate the potential toxicological risk in grapes caused by these fungi.
Acknowledgments
This research was supported in part by the European Union through project QLK1-CT-2001-01761.
We thank Filomena Epifani and Gaetano Stea for technical assistance and Zofia Kozakiewicz (CAB International Mycological Institute, Kew, Surrey, United Kingdom) for providing type strains and confirming the morphological identifications of some strains.
REFERENCES
- 1.Abarca, M. L., F. Accensi, M. R. Bragulat, G. Castella, and F. J. Cabanes. 2003. Aspergillus carbonarius as the main source of ochratoxin A contamination in dried vine fruits from the Spanish market. J. Food Prot. 66:504-506. [DOI] [PubMed] [Google Scholar]
- 2.Abarca, M. L., F. Accensi, J. Cano, and F. J. Cabanes. 2004. Taxonomy and significance of black aspergilli. Antonie Leeuwenhoek 86:33-49. [DOI] [PubMed] [Google Scholar]
- 3.Accensi, F., M. L. Abarca, J. Cano, L. Figuera, and F. J. Cabanes. 2001. Distribution of ochratoxin producing strains in the A. niger aggregate. Antonie Leeuwenhoek 79:365-370. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed, N., M. Alam, A. A. Majeed, S. A. Rahman, A. Cataldi, D. Cousins, and S. E. Hasnain. 2003. Genome sequence based, comparative analysis of the fluorescent amplified fragment length polymorphisms (FAFLP) of tubercle bacilli from seals provides molecular evidence for a new species within the Mycobacterium tuberculosis complex. Infect. Genet. Evol. 2:193-199. [DOI] [PubMed] [Google Scholar]
- 5.Al Musallam, A. 1980. Revision of the black Aspergillus species. Ph.D. thesis. University of Utrecht, Utrecht, The Netherlands.
- 6.Association of Official Analytical Chemists. 1995. Official methods of analysis, p. 38. AOAC International, Arlington, Va.
- 7.Bakkeren, G., J. W. Kronstad, and C. A. Lèvesque. 2000. Comparison of AFLP fingerprints and ITS sequences as phylogenetic markers in Ustilaginomycetes. Mycologia 92:510-521. [Google Scholar]
- 8.Battilani, P., and A. Pietri. 2002. Ochratoxin A in grapes and wine. Eur. J. Plant Pathol. 108:639-643. [Google Scholar]
- 9.Battilani, P., A. Pietri, T. Bertuzzi, L. Languasco, P. Giorni, and Z. Kozakiewicz. 2003. Occurrence of ochratoxin A-producing fungi in grapes grown in Italy. J. Food Prot. 66:633-636. [DOI] [PubMed] [Google Scholar]
- 10.Castegnaro, M., and C. P. Wild. 1995. IARC activities in mycotoxin research. Nat. Tox. 3:327-331. [DOI] [PubMed] [Google Scholar]
- 11.Dalcero, A., C. Magnoli, C. Hallak, S. M. Chiacchiera, G. Palacio, and C. A. Rosa. 2002. Detection of ochratoxin A in animal feeds and capacity to produce this mycotoxin by Aspergillus section Nigri in Argentina. Food Addit. Contam. 19:1065-1072. [DOI] [PubMed] [Google Scholar]
- 12.Da Rocha Rosa, C. A., V. Palacios, M. Combina, M. E. Fraga, A. De Oliveira Reckson, C. E. Magnoli, and A. M. Dalcero. 2002. Potential ochratoxin A producers from wine grapes in Argentina and Brazil. Food Addit. Contam. 19:408-414. [DOI] [PubMed] [Google Scholar]
- 13.Frisvad, J. C. 1995. Mycotoxins and mycotoxigenic fungi in storage, p. 251-288. In D. S. Jayas, N. D. White, and W. E. Muir. (ed.), Stored grain ecosystem. Marcell Dekker, New York, N.Y.
- 14.Frisvad, J. C., and R. A. Samson. 2000. Neopetromyces gen. nov. and an overview of teleomorphs of Aspergillus subgenus Circumdati. Stud. Mycol. 45:201-207. [Google Scholar]
- 15.Heenan, C. N., K. J. Shaw, and J. I. Pitt. 1998. Ochratoxin A production by Aspergillus carbonarius and A. niger isolates and detection using coconut cream agar. J. Food Mycol. 1:67-72. [Google Scholar]
- 16.Joosten, H. M. L. J., J. Goetz, A. Pittet, M. Schellenberg, and P. Bucheli. 2001. Production of ochratoxin A by Aspergillus carbonarius on coffee cherries. Int. J. Food Microbiol. 65:39-44. [DOI] [PubMed] [Google Scholar]
- 17.Klich, M. A. 2000. Identification of common Aspergillus species. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 18.Kozakiewicz, Z. 1989. Aspergillus species on stored products. Mycol. Pap. 161:1-188. [Google Scholar]
- 19.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 20.Kuster van Someren, M. A., R. A. Samson, and J. Visser. 1991. The use of RFLP analysis in classification of the black Aspergilli. Reinterpretation of Aspergillus niger aggregate. Curr. Genet. 19:21-26. [Google Scholar]
- 21.Magnoli, C., M. Violante, M. Combina, G. Palacio, and A. Dalcero. 2003. Mycoflora and ochratoxin-A producing strains of Aspergillus section Nigri in wine grapes in Argentina. Lett. Appl. Microbiol. 37:179-184. [DOI] [PubMed] [Google Scholar]
- 22.Majer, D., R. Mithen, B. G. Lewis, P. Vos, and R. P. Oliver. 1996. The use of AFLP fingerprinting for the detection of genetic variation in fungi. Mycol. Res. 9:1107-1111. [Google Scholar]
- 23.Marasas, W. F. O., J. P. Rheeder, S. C. Lamprecht, K. A. Zeller, and J. F. Leslie. 2001. Fusarium andiyazi sp. nov., a new species from sorghum. Mycologia 93:1203-1210. [Google Scholar]
- 24.Montiel, D., M. J. Dickinson, H. A. Lee, P. S. Dyer, D. J. Jeenes, I. N. Roberts, S. James, L. J. Fuller, K. Matsuchima, and D. B. Archer. 2003. Genetic differentiation of the Aspergillus section Flavi complex using AFLP fingerprints. Mycol. Res. 107:1427-1434. [DOI] [PubMed] [Google Scholar]
- 25.Mosseray, R. 1934. Les Aspergillus de la section “Niger” Thom et Church. Cellule 43:203-285. [Google Scholar]
- 26.Nakajima, M., H. Tsubuouchi, M. Miyabe, and Y. Ueno. 1997. Survey of aflatoxin B1 and ochratoxin A in commercial green coffee beans by high-performance liquid chromatography linked with immunoaffinity chromatography. Food Agr. Immunol. 9:77-83. [Google Scholar]
- 27.Nei, M., and W. H. Li. 1979. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 76:5269-5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Donnell, K., and E. Cigelnik. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7:103-116. [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell, K., H. I. Nirenberg, T. Aoki, and E. Cigelnik. 2000. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additional phylogenetically distinct species. Mycoscience 41:61-78. [Google Scholar]
- 30.Ono, H., A. Kataoka, M. Koakutsu, K. Tanaka, S. Kawasugi, M. Wakazawa, Y. Ueno, and M. Manabe. 1995. Ochratoxin A production by strains of the Aspergillus niger group stored in IFO culture collection. Mycotoxins 41:47-51. [Google Scholar]
- 31.Otteneder, H., and P. Majerus. 2000. Occurrence of ochratoxin A (OTA) in wines: influence of the type of wine and its geographical origin. Food Addit. Contam. 17:793-798. [DOI] [PubMed] [Google Scholar]
- 32.Parenicova, L., M. E. G. Suykerbuyk, R. A. Samson, and J. Visser. 1997. Evaluation of RFLP analysis for the classification of selected black Aspergilli. Mycol. Res. 101:810-814. [Google Scholar]
- 33.Pietri, A., T. Bertuzzi, L. Pallaroni, and G. Piva. 2001. Occurrence of ochratoxin A in Italian wines. Food Addit. Contam. 18:647-654. [DOI] [PubMed] [Google Scholar]
- 34.Pitt, J. I. 2000. Toxigenic fungi: which are important? Med. Mycol. 38S:17-22. [PubMed] [Google Scholar]
- 35.Raper, K. B., and D. I. Fennell. 1965. The genus Aspergillus. Williams & Wilkins, Baltimore, Md.
- 36.Romani, S., G. Saccheti, C. Chaves Lòpez, G. G. Pinnavia, and M. Dalla Rosa. 2000. Screening on the occurrence of ochratoxin A in green coffee beans of different origins and types. J. Agric. Food Chem. 48:3616-3619. [DOI] [PubMed] [Google Scholar]
- 37.Sage, L., S. Krivobok, E. Delbos, F. Seigle-Murandi, and E. E. Creppy. 2002. Fungal flora and ochratoxin A production in grapes and musts from France. J. Agric. Food Chem. 50:1306-1311. [DOI] [PubMed] [Google Scholar]
- 38.Samson, R. A. 1992. Current taxonomic schemes of the genus Aspergillus and teleomorphs, p. 355-390. In J. W. Bennett and M. A. Klich (ed.), Aspergillus: biology and industrial applications. Butterworth-Heinemann, Boston, Mass. [PubMed]
- 39.Samson, R. A., E. S. Hoekstra, J. C. Frisvad, and O. Filtenborg. 2000. Introduction to food- and airborne fungi, 6th ed. Centraalbureau Voor Schimmelcultures, Utrecht, The Netherlands.
- 40.Samson, R. A., J. A. M. P. Houbraken, A. F. A. Kuijpers, J. M. Frank, and J. C. Frisvad. 2004. New ochratoxin A or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 50:45-61. [Google Scholar]
- 41.Savelkoul, P. H. M., H. J. M. Aarts, J. De Haas, L. Dijkshoorn, B. Duim, M. Otsen, J. L. W. Rademaker, L. Schouls, and J. A. Lenstra. 1999. Amplified-fragment length polymorphism analysis: the state of an art. J. Clin. Microbiol. 37:3083-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt, H., M. Ehrmann, R. F. Vogel, M. H. Taniwaki, and L. Niessen. 2003. Molecular typing of Aspergillus ochraceus and construction of species specific SCAR-primers based on AFLP. Syst. Appl. Microbiol. 26:138-146. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt, H., M. H. Taniwaki, R. F. Vogel, and L. Niessen. 2004. Utilization of AFLP markers for PCR-based identification of Aspergillus carbonarius and indication of its presence in green coffee samples. J. Appl. Microbiol. 97:899-909. [DOI] [PubMed] [Google Scholar]
- 44.Serra, R., L. Abrunhosa, Z. Kozakiewicz, and A. Venancio. 2003. Black Aspergillus species as ochratoxin A producers in Portuguese wine grapes. Int. J. Food Microbiol. 88:63-68. [DOI] [PubMed] [Google Scholar]
- 45.Susca, A., G. Stea, and G. Mulè. 2002. Genome analysis of toxigenic fungi by automated fluorescent AFLP. J. Appl. Genet. 43A:115-124. [Google Scholar]
- 46.Téren, J., J. Varga, Z. Hamari, É. Rinyu, and F. Kevei. 1996. Immunochemical detection of ochratoxin A in black Aspergillus strains. Mycopathologia 134:171-176. [DOI] [PubMed] [Google Scholar]
- 47.Treadway, L. P., J. F. White, Jr., B. S. Gaut, P. V. Reddy, M. D. Richardson, and B. B. Clarke. 1999. Phylogenetic relationships within and between Epichloë and Neotyphodium endophytes as estimated by AFLP markers and rDNA sequences. Mycol. Res. 103:1593-1603. [Google Scholar]
- 48.Varga, J., F. Kevei, A. Vriesma, F. Debets, Z. Kozakiewicz, and J. H. Croft. 1994. Mitochondrial DNA restriction fragment length polymorphisms in field isolates of the Aspergillus niger aggregate. Can. J. Microbiol. 40:612-621. [DOI] [PubMed] [Google Scholar]
- 49.Visconti, A., M. Pascale, and G. Centonze. 1999. Determination of ochratoxin A in wine by means of immunoaffinity column clean-up and high performance liquid chromatography. J. Chromatogr. A 864:89-101. [DOI] [PubMed] [Google Scholar]
- 50.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. Van de Lee, M. Hornes, A. Fruters, J. Pot, J. Peleman, M. Kuiper, and M. Zabeau. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeller, K. A., B. A. Summerell, S. Bullock, and J. F. Leslie. 2003. Gibberella konza (Fusarium konzum) sp. nov. from prairie grasses, a new species in the Gibberella fujikuroi species complex. Mycologia 95:943-954. [PubMed] [Google Scholar]
- 52.Zimmerli, B., and R. Dick. 1995. Determination of ochratoxin A at the ppt level in human blood, serum, milk and some foodstuffs by HPLC with enhanced fluorescence detection and immunoaffinity column cleanup: methodology and Swiss data. J. Chromatogr. B 666:85-99. [DOI] [PubMed] [Google Scholar]