Abstract
Specific amino acids, purine ribonucleosides, or a combination of the two is required for efficient germination of endospores of Bacillus cereus ATCC 14579. A survey including 20 different amino acids showed that l-alanine, l-cysteine, l-threonine, and l-glutamine are capable of initiating the germination of endospores of B. cereus ATCC 14579. In addition, the purine ribonucleosides inosine and adenosine can trigger germination of the spores. Advanced annotation of the B. cereus ATCC 14579 genome revealed the presence of seven putative germination (ger) operons, termed gerG, gerI, gerK, gerL, gerQ, gerR, and gerS. To determine the role of the encoded putative receptors in nutrient-induced germination, disruption mutants were constructed by the insertion of pMUTIN4 into each of the seven operons. Four of the seven mutants were affected in the germination response to amino acids or purine ribonucleosides, whereas no phenotype could be attributed to the mutants with disrupted gerK, gerL, and gerS loci. The strain with a disrupted gerR operon was severely hampered in the ability to germinate: germination occurred in response to l-glutamine but not in the presence of any of the other amino acids tested. The gerG mutant showed significantly reduced l-glutamine-induced germination, which points to a role of this receptor in the l-glutamine germination signaling pathway. gerR, gerI, and gerQ mutants showed reduced germination rates in the presence of inosine, suggesting a role for these operons in ribonucleoside signaling. Efficient germination by the combination of l-glutamine and inosine was shown to involve the gerG and gerI operons, since the germination of mutants lacking either one of these receptors was significantly reduced. Germination triggered by the combination of l-phenylalanine and inosine was lost in the gerI mutant, indicating that both molecules are effective at the GerI receptor.
Bacillus cereus is a spore-forming gram-positive pathogen that is widely distributed in the environment. Its frequent occurrence in the soil and on plant surfaces enables easy dissemination into the food chain, and the spores especially are generally capable of surviving the preservation conditions currently applied by the food industry (22). Once present in food in sufficient numbers, B. cereus can produce a heat-stable emetic toxin, leading to vomiting, while a second type of food poisoning is caused by enterotoxins that are produced during vegetative growth in the small intestine and result in diarrheal syndromes (7). Genetically, B. cereus is very closely related to Bacillus thuringiensis and Bacillus anthracis (15), and these are often regarded as one species (9). However, their phenotypic characteristics diverge substantially, as B. thuringiensis can be beneficial as an insecticide while B. anthracis is the causative agent of the often lethal disease anthrax.
Bacillus species have the ability to respond to nutrient depletion by sporulation, a process that results in metabolically dormant, highly resistant endospores. Under favorable conditions, endospores are able to escape their dormant state and return to the vegetative state by an irreversible process called germination. To do so, spores retain an alert sensory mechanism that allows them to monitor the presence of nutrients in their direct environment, an essential requirement for the resumption of vegetative growth. Besides exposure to specific nutrient molecules such as amino acids, ribosides, and sugars, germination can be initiated by enzymes, chemical compounds, or physical treatments such as high hydrostatic pressure (6, 26). Nutrient-induced germination can be initiated by small molecules called germinants, which are somehow able to activate putative germination receptors located in the inner membrane (11, 24), probably by an allosteric interaction with the receptor (38). After this initial interaction, the spore becomes committed to germination (16). Subsequent processes in germination are the release of metal ions from the spore core and the excretion of spore-specific molecules such as dipicolinic acid (DPA) (19, 26, 33). Swelling of the core through water uptake will start, and in the meantime the peptidoglycan cortex layer will be degraded by cortex lytic enzymes, giving room to the expanding core. Low-energy precursors present in the dormant spore are converted to utilizable energy sources such as ATP shortly after the initiation of germination (32). Afterwards, metabolic processes will resume and DNA transcription and protein synthesis will be initiated.
Amino acids and purine ribosides as single germinants or in concert are powerful initiators of the germination of spores of bacilli (5). Although germination responses upon the addition of germinant molecules have been studied extensively, little is known about the interaction of these molecules with the putative germination receptors. The germination receptors described so far are encoded by tricistronic ger operons and transcribed during sporulation under the control of a σG promoter (26). The three gene products are all required for the formation of a functional receptor, but the interplay between these proteins is not exactly understood (20). Transmembrane regions can be found in the A and B proteins, while the hydrophilic C protein, containing a prelipoprotein signal sequence, is expected to be transported across the membrane and anchored to the outer surface (12, 39). It has been suggested that germination receptors can act together to form a complex (2, 13, 16, 18), but there is no experimental evidence for their action in concert.
The genome of Bacillus subtilis 168 harbors five ger operons. Three of the putative receptors, encoded by gerA (39), gerB (4), and gerK (14), have been characterized. The putative receptor encoded by gerA can be activated by l-alanine, while the putative receptors encoded by gerB and gerK can be activated by a mixture of asparagine, glucose, fructose, and potassium (18, 21, 36). The functions of the two remaining ger operons remain to be elucidated (25, 26). For B. cereus 569, an l-alanine-activated receptor was described which is encoded by the gerL operon (1). Two more germination operons have been described for this strain, namely, the gerI operon, encoding a receptor involved in l-alanine- and inosine-induced germination (3), and the gerQ operon, encoding a receptor involved in inosine-mediated germination (1). The genome of B. cereus ATCC 14579 contains seven ger operons (15). We previously characterized the receptor encoded by the gerR operon, and this receptor also played a role in l-alanine- and inosine-initiated germination, although it was not a direct ortholog of the B. cereus 569 gerI operon (10). Finally, for B. anthracis, three germinant receptors have been described so far. The B. anthracis Sterne 7702 strain harbors the gerX operon on plasmid pXO1, and disruption of this operon resulted in diminished germination within phagocytic cells (8). Furthermore, two more chromosomal operons have been described. The proteins encoded by gerS were shown to be involved in aromatic amino acid responses, while the gerH-encoded proteins were involved in inosine-histidine and purine-alanine responses (13, 37).
For this work, we assessed the role of amino acids and purine ribonucleosides in the initiation of germination of B. cereus ATCC 14579 endospores. To examine the putative germination receptors of this strain in more detail, we constructed mutants for all seven ger operons present in this strain by disruption of each of the operons. Subsequently, the responses of spores of the wild-type and mutant strains to amino acids, purine ribonucleosides, and a combination of these germinants were investigated. Furthermore, we compared the ger operons of several B. cereus strains and B. anthracis Sterne to make a preliminary classification based on amino acid homology of the germination receptors and their nutrient specificities.
MATERIALS AND METHODS
Strains, plasmids, and endospore preparation.
The bacterial strains and plasmids used for this study are listed in Table 1. B. cereus ATCC 14579 was obtained from BGSC (Bacillus Genetic Stock Center) and cultured routinely on Luria broth (LB) (31) at 30°C and 225 rpm. Escherichia coli JM109 was cultured on LB at 37°C. Antibiotics were used at the following concentrations: for B. cereus, erythromycin was used in combination with lincomycin at concentrations of 1 and 25 μg/ml, respectively, and for E. coli, 100 μg/ml ampicillin was used. Spores of the B. cereus wild-type and mutant strains were prepared in chemically defined medium, harvested, and repeatedly washed as described previously (10). The sporulation properties of the mutants were identical to those of the wild-type strain. Spores were stored at 4°C in 1 mM phosphate buffer, pH 7.4, with 0.01% Tween 20 to prevent aggregation of the hydrophobic spores.
TABLE 1.
Strains and plasmids used for this study
| Strain or plasmid | Relevant genotype and/or phenotype | Source or reference |
|---|---|---|
| B. cereus strains | ||
| ATCC 14579 | Wild-type strain for this study | BGSCa |
| LH129 | gerRC1::pMUTIN4 Eryr | Hornstra (10) |
| LH130 | gerQA1::pMUTIN4 Eryr | This study |
| LH132 | gerGA1::pMUTIN4 Eryr | This study |
| LH140 | gerKA1::pMUTIN4 Eryr | This study |
| LH142 | gerLB1::pMUTIN4 Eryr | This study |
| LH144 | gerSA1::pMUTIN4 Eryr | This study |
| LH148 | gerIA1::pMUTIN4 Eryr | This study |
| Plasmids | ||
| pMUTIN4 | Ampr Eryr | Vagner (34) |
| pMUTIN4/gerRC1 | Ampr Eryr | Hornstra (10) |
| pMUTIN4/gerQA1 | Ampr Eryr | This study |
| pMUTIN4/gerGA1 | Ampr Eryr | This study |
| pMUTIN4/gerKA1 | Ampr Eryr | This study |
| pMUTIN4/gerLB1 | Ampr Eryr | This study |
| pMUTIN4/gerSA1 | Ampr Eryr | This study |
| pMUTIN4/gerIA1 | Ampr Eryr | This study |
BGSC, Bacillus Genetic Stock Center.
Construction of B. cereus ATCC 14579 ger mutant strains.
Gene inactivation was achieved by insertion of the pMUTIN4 vector (34) into each of the seven ger operons of B. cereus ATCC 14579. A fragment of the target gene was amplified from genomic DNA isolated from B. cereus ATCC 14579 and then ligated into pMUTIN4. The primers used and the fragments obtained are listed in Table 2. pMUTIN4 containing the target sequence inactivates the corresponding operon by integration via a single crossover event and has been described previously (10). Typically, 5 to 50 transformants were obtained per individual experiment. Five transformants from one gene inactivation event were selected for further analysis. Primers were designed for positions flanking the expected position of insertion, and in combination with pMUTIN4 primers, were used to verify the insertion position of the plasmid. Southern hybridization confirmed single-copy integration of the plasmid. For all gene inactivation events, the five selected transformants showed identical results in control PCRs and Southern hybridization assays, while spores of the transformants showed identical phenotypes in germination assays (data not shown).
TABLE 2.
Primers for construction of mutants
| Gene | ORFa | Target size (bp) | Primer name | Primer sequenceb (5′→3′) |
|---|---|---|---|---|
| gerQA | RZC04499 | 716 | Target forw. | CATGAAAAAGTACCTGTTCACTCc |
| Target rev. | CGGGATCCGGTGCTGTAAATGAGCTAAGAGC | |||
| gerGA | RZC10850 | 1,312 | Target forw. | GCCAAGCTTCGGAAGTTTAATATTGGACGCAC |
| Target rev. | CGGGATCCTGAATCGATATGGAACAGCAGG | |||
| gerKA | RZC05046 | 1,030 | Target forw. | GCCGAATTCCATCACTCCCTGAGAAC |
| Target rev. | CGGGATCCAACTCGGCTTGGTAAGCGAATAC | |||
| gerLB | RZC03031 | 1,035 | Target forw. | GCCAAGCTTCCCGTTTGCTCTTATTGCACC |
| Target rev. | CGGGATCCTCGCGATTCATATGCTGTCAAAG | |||
| gerSA | RZC02468 | 911 | Target forw. | GCCAAGCTTCCAAACTGTTTAGCGGTATTCCAG |
| Target rev. | CGGGATCCTGGCTCGAGATTCTCCAATC | |||
| gerIA | RZC04556 | 1,486 | Target forw. | GCCAAGCTTACGAAACAGATAATCAGGAGCAGC |
| Target rev. | CGGGATCCAATGTAGCGTACATCCAGGAGACG |
Corresponding to the Integrated Genomics B. cereus ATCC 14579 genome database (15).
Restriction sites are underlined. All PCR fragments were cloned by insertion into the pMUTIN4 BamHI/HindIII sites, except for the fragment disrupting the gerKA gene, which was cloned into EcoRI/HindIII sites.
Cloned by an internal HindIII site.
Germination assays.
Prior to germination, spores were washed with 1 mM phosphate buffer, pH 7.4, 0.01% Tween 20. Spores were resuspended and heat activated for 15 min at 75°C in sterile water. After heat activation, the spores were resuspended in ice-cold germination buffer (10 mM Tris-HCl, pH 7.4, 10 mM NaCl) to an optical density at 600 nm (OD600) of 1.0 in a Tecan Safire (Tecan Group, Switzerland) plate reader. The germination process was followed by monitoring the reduction in the OD600, which reflects the number of germination events in the whole spore population by a change in refractility of the spores from phase-bright to phase-dark. After the addition of germinants, the OD600 of the spore suspension was measured at 2-min intervals for 60 min at 30°C. Before each time point, the plate was shaken for 30 s to prevent settling of the spores. The percentage of germination was determined as follows. Maximum germination was observed after 60 min as a result of the addition of 100 mM alanine to spores of the wild-type strain. This resulted in a 62% drop in the initial OD. This OD reduction reflected 100% germination, as confirmed by phase-contrast microscopy, and other germination responses were related to this maximum response. Spores were also routinely checked for their germination behavior by phase-contrast microscopy after the addition of germinants. All amino acids used for the germination assays were l-isomers dissolved in sterile water at stock concentrations of 10 to 100 mM, depending on their solubility properties, and were sterilized by filtration using 0.45-μm-pore-size filters. The amino acids were used in the germination assays at a final concentration of 1 mM unless noted otherwise. Microscopic observation showed that a small percentage (<5%) of spores were triggered to germinate as a result of the heat activation step (Fig. 1).
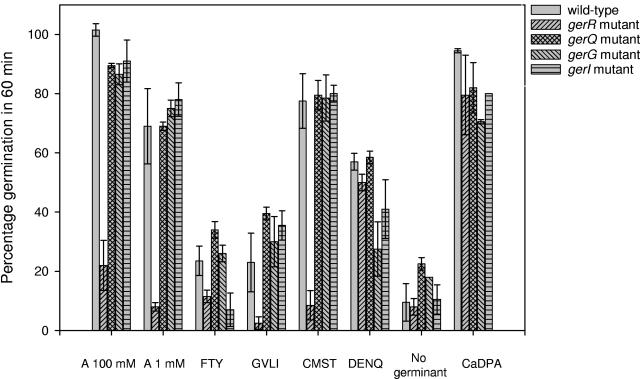
FIG. 1.
Germination survey of B. cereus ATCC 14579 wild-type spores and spores of the seven ger mutant strains with 20 amino acids. The percentage of germination was monitored after 60 min of incubation with the germinant molecules. The bars represent the responses of spores of the wild-type, gerR mutant, gerQ mutant, gerG mutant, and gerI mutant strains. The responses of the mutants with disrupted gerS, gerK, and gerL operons were similar to those of wild-type spores and were omitted for clarity. The amino acids alanine (A) and proline (not shown) were surveyed as single germinants, and the remaining amino acids were surveyed in groups in the following combinations: FTY (phenylalanine, tyrosine, and tryptophan), GVLI (glycine, valine, leucine, and isoleucine), CMST (cysteine, methionine, serine, and threonine), a group with lysine, arginine, and histidine (not shown), and DENQ (aspartate, glutamate, asparagine, and glutamine). The final concentration of each amino acid in these groups was 1 mM, except for tyrosine (0.5 mM). The addition of proline and the mixture of lysine, arginine, and histidine did not result in germination in any of the strains, and these results were omitted from the figure for clarity. Calcium DPA (CaDPA)-induced germination proceeds indirectly by activation of the cortex lytic enzymes, bypassing the germination receptors. Spores of all strains should have responded equally, and this was evaluated by assessing the germination response upon the addition of 50 mM calcium DPA. The results shown are the averages of duplicate experiments completed with two independent spore batches.
RESULTS
Germination survey with 20 amino acids and spores of B. cereus wild-type and ger mutant strains.
A number of amino acids have been shown to be of particular relevance to B. cereus germination (1, 3, 6, 27, 35), although predominantly alanine has been examined. To determine the specificities of germinant receptors for alanine and other amino acids, we exposed spores of the wild-type and mutant strains to 20 naturally utilized amino acids.
Spores of the wild-type strain reacted promptly upon addition of a strong germinant molecule such as cysteine, resulting in >50% germination within 20 min (Fig. 2). Subtle germination as a result of a suboptimal germinant concentration or a less powerful germinant molecule was not observed within this 20-min period but was assessed by determining the percentage of germinated spores after a 60-min incubation period (Fig. 1). As expected, alanine was capable of inducing the germination of spores of the wild type and six of the mutant strains, while the gerR mutant did not germinate, as reported previously (10). Proline, which is capable of inducing germination in Bacillus megaterium (30), did not induce germination in any of the strains tested.
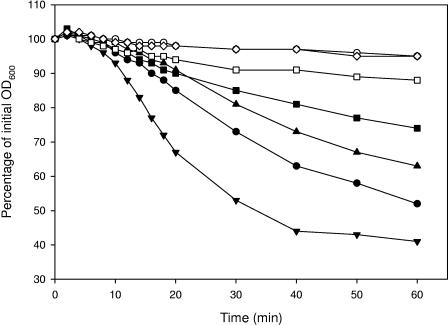
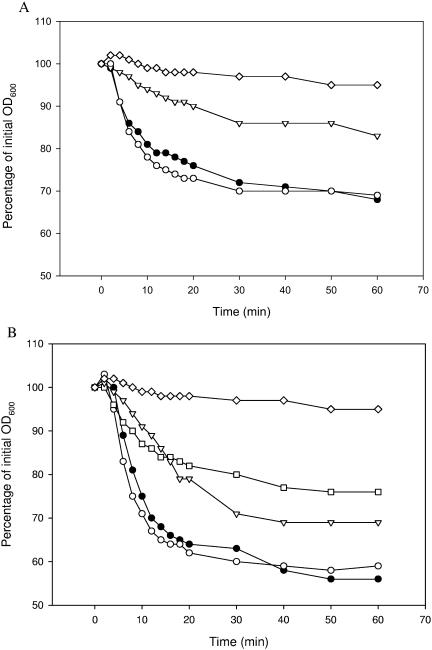
FIG. 2.
Effects of the amino acids alanine (1 mM), cysteine (1 mM), threonine (1 mM), and glutamine (1 mM) on the germination of spores of the wild-type strain and the gerR and gerG mutant strains. Germination was monitored as the fall in OD600 over 60 min. ▾, wild-type strain with 1 mM cysteine; •, wild-type strain with 1 mM alanine; ▴, wild-type strain with 1 mM threonine; ▪, wild-type strain with 1 mM glutamine; ○, gerR strain with 1 mM alanine; □, gerG strain with 1 mM glutamine; ⋄, wild-type strain with no germinant. Germination data for gerR spore responses to cysteine and threonine were identical to those for the response to alanine and were omitted for clarity. Germination data for the gerR, gerQ, gerK, gerL, gerS, and gerI mutant strains upon the addition of l-glutamine were similar to those for the wild type and were omitted for clarity.
The remaining 18 amino acids were tested in groups of three or four compounds, and if a group was able to initiate a clear germination response, single amino acids from this group were assessed for germination-inducing capacity. The mixture of lysine, arginine, and histidine did not initiate a germination response in any of the strains. The group consisting of the aromatic amino acids phenylalanine, tyrosine, and tryptophan and the group consisting of glycine, valine, leucine, and isoleucine both initiated slow germination responses within 60 min (Fig. 1) in all strains except the gerR mutant. gerI mutant spores did not respond to aromatic amino acids. The combination of cysteine, methionine, serine, and threonine resulted in quick and strong germination responses by spores of all strains, again except for spores of the gerR mutant. A separate examination of these four amino acids revealed that cysteine and threonine are able to initiate strong germination responses (Fig. 2) on their own. The combination of aspartate, glutamate, asparagine, and glutamine induced moderate responses in all strains, including the gerR mutant, and germination with single amino acids revealed that only glutamine was responsible for these responses. The mutant disrupted in the gerG operon, however, showed a significant reduction in the germination rate upon the addition of glutamine (Fig. 2).
Of the 20 amino acids studied, alanine, cysteine, threonine, and glutamine were able to deliver clear germination responses as single germinants at a 1 mM concentration. The receptor encoded by the gerR operon plays a prominent role in single amino acid-induced germination, as the gerR mutant failed to respond properly to all amino acids except glutamine. The reduced germination rate of spores of the gerG mutant suggests a unique role for the GerG receptor in glutamine-induced germination.
Germination assays with the ribosides inosine and adenosine.
Purine ribonucleosides are commonly used as germinants of B. cereus. Inosine is a known independent germinant of B. cereus spores and is important as a cogerminant in B. cereus and B. anthracis germination (3, 13).
To determine the germination behavior of spores of the wild-type and mutant strains upon exposure to ribosides, germination in response to inosine and adenosine was assayed. As single germinants, both inosine and adenosine are strong inducers of germination. Spores of the wild-type strain responded to 10 and 1 mM inosine with similar germination efficiencies. At 0.1 mM, the germination efficiency decreased, while at 0.01 mM, no clear germination response was measured (Fig. 3). Spores of all mutants germinated in response to inosine; however, spores of the gerR, gerQ, and gerI mutants showed delayed responses at concentrations of 1 mM or lower compared to those of wild-type spores and spores of the other mutants (Fig. 4). The response of gerR spores was affected most, as these spores did not germinate in 0.1 mM inosine, while gerQ and gerI spores were delayed in this response but were able to germinate almost to completion within 60 min (Fig. 3). The differences in the responses of spores of the gerR, gerQ, and gerI mutants were most distinct shortly after the addition of inosine. Spores of these mutants consistently showed significantly delayed responses compared to spores of the wild type and the other mutants (Fig. 4). A 0.01 mM inosine concentration did not induce germination in any of the strains. The involvement of the GerI and GerQ receptors in inosine-induced germination has previously been described for B. cereus 569 (1, 3).
FIG. 3.
Effects of the purine ribonucleosides inosine and adenosine on the germination of spores of the wild-type strain and ger mutant strains. The bars represent the responses of spores of the wild-type, gerR mutant, gerQ mutant, and gerI mutant strains. The responses of the mutants with disrupted gerG, gerK, gerL, and gerS operons were similar to those of wild-type spores and were omitted for clarity.
FIG. 4.
Effect of 1 mM inosine on the germination of spores of the wild-type strain and the gerR, gerI, and gerQ mutants. •, wild-type strain; ▵, gerQ spores; ▿, gerI spores; ○, gerR spores; ⋄, wild-type strain with no germinant. Spores of these mutants showed reduced germination rates compared to that of the wild type, although the spores of these strains were able to complete germination within 60 min. The responses of gerG, gerK, gerL, and gerS spores were coincident with those of wild-type spores.
Adenosine initiated germination at concentrations of 1 (Fig. 3) and 0.1 (data not shown) mM for spores of all strains except the gerR mutant.
Alanine as a cogerminant for amino acid-induced germination.
For B. anthracis, it has been reported that the addition of alanine can elicit amino acid-mediated germination (13). We examined this possibility, but we could not observe any stimulation of the germination rate of B. cereus ATCC 14579 spores when 0.1 mM alanine was combined with any 1 of the 20 amino acids tested (data not shown).
Inosine as a cogerminant for amino acid-induced germination.
The most powerful germination response is initiated by the combination of an amino acid and a riboside, and the combination of alanine and inosine is considered one of the most powerful germinant combinations for Bacillus spp. (5). For B. cereus as well as B. anthracis, germination responses to many of these synergistic combinations have been described (3, 13, 37). We studied the combination of amino acids and inosine by adding a subgerminal concentration of inosine (0.01 mM) to a single amino acid or a group of amino acids. A concentration as low as 0.1 mM alanine or 0.01 mM inosine as a single germinant did not induce germination, but combining the two resulted in strong responses from spores of all strains (Fig. 5). This synergistic effect is particularly remarkable for spores of the gerR mutant, as these spores showed no response to an alanine concentration of ≤100 mM or an inosine concentration of ≤0.1 mM but germinated normally in response to the surveyed alanine-inosine combination.
FIG. 5.
Germination survey of B. cereus wild-type spores and spores of the seven mutant strains with 20 amino acids, with inosine as a cogerminant. The concentrations of the amino acids used during this experiment were the same as those described in the legend to Fig. 1. The bars represent the responses of spores of the wild-type, gerR mutant, gerQ mutant, gerG mutant, and gerI mutant strains. The responses of mutant strains with disrupted gerK, gerL, and gerS operons were similar to those of wild-type spores and were omitted for clarity.
No stimulatory effect was detected for proline or the mixture of lysine, arginine, and histidine in conjunction with inosine. Combining 0.01 mM inosine with the aromatic amino acids phenylalanine, tyrosine, and tryptophan showed significant increases in germination (Fig. 5). A separate examination revealed a synergistic effect of the combination of phenylalanine with inosine for all mutants except the gerI mutant (Fig. 6A). The observation that spores of all mutants other than gerI spores germinate normally suggests that not only inosine but also phenylalanine plays a role in triggering this receptor. This is in line with the function of the gerI ortholog in B. anthracis, termed gerH, which encodes a receptor that is involved in aromatic amino acid responses (37). The addition of inosine slightly further enhanced germination with the mixture of glycine, valine, leucine, and isoleucine (Fig. 5), and the same was observed for the mixture of cysteine, methionine, serine, and threonine and the mixture of aspartate, glutamate, asparagine, and glutamine. With the latter mixture, spores of the gerG mutant strain were clearly slower to germinate. Germination induced by glutamine and inosine showed an involvement of the receptors encoded by gerG and gerI. Both receptors were required to initiate a germination response (Fig. 6B).
FIG. 6.
(A) Effect of 1 mM phenylalanine with 0.01 mM inosine as a cogerminant on the germination of spores of the wild-type strain (•), the gerR mutant (○), and the gerI mutant (▿). ⋄, wild-type strain without a germinant. Spores of the gerQ, gerG, gerK, gerL, and gerS mutant strains germinated like spores of the wild type (data not shown). (B) Effect of 1 mM glutamine with 0.01 mM inosine as a cogerminant on the germination of spores of the wild-type strain (•), gerR mutant spores (○), gerG mutant spores (□), and gerI mutant spores (▿). ⋄, wild-type strain without a germinant. Spores of strains with disrupted gerQ, gerK, gerL, and gerS loci responded similarly to spores of the wild-type strain (data not shown).
Comparison of the ger operons of B. cereus and B. anthracis.
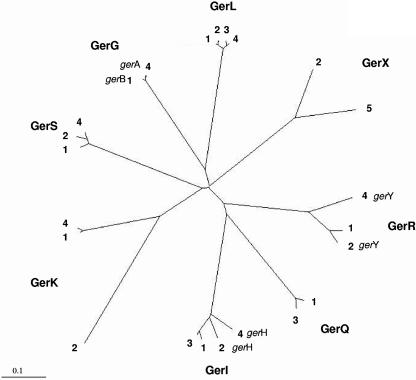
In the annotated B. cereus ATCC 14579 genome, seven ger operons could be identified (15), while the genome of B. cereus ATCC 10987 contains six ger operons (28), which are all located on the chromosome. B. anthracis strain Sterne possesses six ger operons on the chromosome. The sequence of the seventh operon, the pXO1 plasmid-located gerX operon, was derived from B. anthracis strain A2012 (29). Furthermore, three ger operons have been characterized for the nonsequenced strain B. cereus 569 (gerQ, gerI, and gerL) (1, 3). To identify congruence within the ger operon family, ClustalW analysis was performed with the amino acid sequences of the A cistrons of the ger operons, and mutual similarities are depicted in an unrooted phylogenetic tree (Fig. 7).
FIG. 7.
Unrooted phylogenetic tree based on the ger operons of B. cereus ATCC 14579 (GenBank accession number AE016877) (1), B. cereus ATCC 10987 (AE017194) (2), B. cereus 569 (AAD03541 to AAD03543 [gerI], AAK70461 to AAK70463 [gerL], and AAK63174 to AAK63176 [gerQ]) (3), and B. anthracis Sterne (AE017225) (4) and the plasmid-located gerX sequence derived from the genome sequence of B. anthracis A2012 (AE016879) (5). The tree is based on the amino acid sequence of the A cistron of each operon. The A cistrons of these strains clustered in eight distinct groups. If the operon annotation for the strain is distinct from the group name, the strain annotation is noted.
The surveyed ger operons of B. cereus and B. anthracis clustered into eight distinct groups (Fig. 7). Members of the gerL and gerI groups showed high levels of similarity and could be found in the three B. cereus strains, while members of the gerS, gerR, and gerK groups could be found in the two completely sequenced B. cereus strains. Members of the gerQ group were found in B. cereus 569 and B. cereus ATCC 14579 but not in B. cereus ATCC 10987. B. cereus ATCC 14579 and B. cereus ATCC 10987 each harbored an operon without an ortholog in any of the B. cereus strains, i.e., the gerG operon in strain ATCC 14579 and the gerX operon in strain ATCC 10987; however, these operons have an ortholog in B. anthracis. B. anthracis Sterne operons showed obvious clustering with the B. cereus operons. Common ger operons identified in the sequenced strains were gerS, gerI, and gerL. Members of the gerR and gerK groups were identified in these strains as well, although they showed less congruence. An ortholog of the gerX operon, located on the pXO1 plasmid of B. anthracis, was located on the chromosome of B. cereus ATCC 10987. The gerQ operon was not present in B. anthracis Sterne, as described before (1, 13).
DISCUSSION
For this study, we investigated the germination responses of spores of B. cereus ATCC 14579 mutant strains, each of which was disrupted in one of the seven ger operons. In addition to alanine, a frequently used germinant for B. cereus, we found that cysteine and threonine are strong inducers as well. A cysteine-induced response has been described before for B. cereus (17, 35) and for B. anthracis spores at high concentrations and after prolonged incubation (13). Threonine-initiated germination has not been described before for B. cereus. All of these responses seem to be mediated by the GerR receptor, which is apparently involved in all responses to single amino acids. The only exception that we observed was glutamine. Surprisingly, this amino acid was capable of inducing germination in gerR mutant spores, and this could indicate a different germination pathway for glutamine-induced germination. In this case, the GerG receptor was shown to play a role, as gerG-defective spores consistently showed a reduced germination rate, and we therefore propose the designation gerG for the operon encoding this receptor.
As reported previously for B. cereus and B. anthracis (3, 5, 13, 37), the addition of inosine as a cogerminant with amino acids enhanced the germination response of B. cereus ATCC 14579. This effect was particularly apparent at low concentrations of inosine in combination with glutamine or phenylalanine. The combination of glutamine and inosine required gerG and gerI, and the phenylalanine-inosine combination required only the gerI-encoded receptor. This indicates that the GerI receptor interacts with phenylalanine and inosine and that both molecules contribute to the initiation of germination. Spores lacking the GerR receptor, and therefore considerably hindered in the alanine and ribonucleoside response, germinated efficiently with a combination of the two at very low concentrations. It is unclear how a combination of alanine and inosine was able to initiate germination in the gerR mutant. The GerL receptor, involved in alanine germination in B. cereus 569, might take over the role of the nonfunctional GerR receptor, although the B. cereus ATCC 14579 gerL-disrupted mutant did not show an alanine germination-hindered phenotype in our experiments. Double mutants would be needed to unravel this response in more detail.
The nature of the stimulatory effect of inosine on amino acid-induced germination remains unclear. As the sole germinant, it initiates strong germination in B. cereus ATCC 14579, and even mutant strains disrupted in the inosine-involved operons gerR, gerQ, and gerI were able to complete their germination, although at reduced rates. B. anthracis contains at least three operons, gerS, gerR, and gerH, encoding putative receptors that could play a role in purine ribonucleoside-induced germination, but B. anthracis Sterne spores do not germinate with inosine as a single germinant. The GerS receptor has been shown to play a role in the enhancement of amino acid-induced germination by the addition of inosine as a cogerminant (13). It can be questioned if inosine initiates germination in a similar way to that by amino acids, as there are clear differences in the responses. First, inosine mutants were all able to finish germination relatively quickly compared to amino acid mutants. Second, no difference could be observed in the germination rate upon the addition of 1 or 10 mM of inosine (Fig. 3), indicating that inosine-induced germination is only partially concentration dependent, while the amino acid-induced germination rate is strongly affected by concentration (10). Instead of direct interaction with a receptor, inosine might stimulate germination by enhancing the signal resulting from an amino acid-activated receptor. In B. cereus, the signal delivered by inosine alone or in combination with endogenous spore amino acids such as alanine (35) is sufficient to initiate germination, but for B. anthracis this is apparently not the case (13).
The availability of an increasing number of Bacillus genome sequences allows ger operon comparisons among species and strains, permitting homology-based classification. In this study, we showed that substantial variation exists in the number and set of ger operons, even among strains. It is unknown if mutual comparisons of ger operon homologues of strains of the same or related species result in valid and reliable predictions of their function. Until now, the germinant specificities of some germination receptors have been proven experimentally, but for most of these receptors the ligands and precise activation conditions are not known. The comparison of B. cereus and B. anthracis genomes in this study revealed close congruence, but a comparison of related germination mutants showed diverging phenotypes. The cistrons in the gerL operon group showed high similarity, and this operon was studied in detail with B. cereus 569, where a gerL mutant showed a delayed response upon alanine addition. In contrast, a disrupted gerL locus in B. cereus ATCC 14579 did not result in a hindered response to alanine. The gerQ operon was shown to be involved in inosine-induced germination in B. cereus 569 (it is not present in B. cereus 10987 and B. anthracis Sterne), and although an inosine-related response could be detected for B. cereus ATCC 14579 gerQ mutant spores, it was only detectable at specific concentrations. Parallels were found for the gerI operon, termed gerH in B. anthracis. The gerH locus is involved in inosine-amino acid-induced germination, partly analogous to the role of the gerI locus in B. cereus 569 (3). Furthermore, this locus was shown to be involved in phenylalanine-inosine-initiated germination, a phenotype previously described for the gerH-encoded receptor, similar to the result seen in this study (37).
Even in cases of high similarity within a group, receptors may differ specifically at the binding sites. In fact, the alteration of only a few amino acids within the germination proteins GerBA and GerBB changed the nutrient specificity from l-alanine to d-alanine in B. subtilis (23). This can explain phenotypical discrepancies even when the similarity is high. It is conceivable that a further increase in known ger operon phenotypes will allow for further refinement and robustness of future homology-based phenotype predictions.
Specific roles could not be demonstrated for the putative GerK, GerL, and GerS receptors, as mutants with disruption in these operons showed germination phenotypes identical to those of spores of the wild-type strain. It cannot be excluded that these operons do not result in functional receptors, although all ger operons of B. cereus ATCC 14579 are transcribed during sporulation (L. Hornstra, unpublished). It is also possible that the presence of a dominant receptor with an overlap in specificity, e.g., the GerR receptor, conceals the alanine response of, e.g., the GerL receptor. Furthermore, these receptors might require different ligands or might be activated under different circumstances from those tested here.
In this study, we have investigated the role of the seven putative germination receptors of B. cereus ATCC 14579 in amino acid- and purine ribonucleoside-induced germination. We identified matching germinant molecules for four of these putative receptors and new germinant molecules for B. cereus ATCC 14579. For three of the putative receptors, the germinant molecules remain to be elucidated.
Acknowledgments
We thank Marcel Zwietering for valuable discussions and critical reading of the manuscript.
REFERENCES
- 1.Barlass, P. J., C. W. Houston, M. O. Clements, and A. Moir. 2002. Germination of Bacillus cereus spores in response to l-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148:2089-2095. [DOI] [PubMed] [Google Scholar]
- 2.Cabrera-Martinez, R. M., F. Tovar-Rojo, V. R. Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clements, M. O., and A. Moir. 1998. Role of gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 180:6729-6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corfe, B. M., R. L. Sammons, D. A. Smith, and C. Mauel. 1994. The gerB region of the Bacillus subtilis 168 chromosome encodes a homologue of the gerA spore germination operon. Microbiology 140:471-478. [DOI] [PubMed] [Google Scholar]
- 5.Foerster, H. F., and J. W. Foster. 1966. Response of Bacillus spores to combinations of germinative compounds. J. Bacteriol. 91:1168-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould, G. W. 1969. Germination, p. 397-444. In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, New York, N.Y.
- 7.Granum, P. E. 2001. Bacillus cereus, p. 327-336. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. American Society for Microbiology, Washington, D.C.
- 8.Guidi-Rontani, C., Y. Pereira, S. Ruffie, J. C. Sirard, M. Weber-Levy, and M. Mock. 1999. Identification and characterization of a germination operon on the virulence plasmid pXO1 of Bacillus anthracis. Mol. Microbiol. 33:407-414. [DOI] [PubMed] [Google Scholar]
- 9.Helgason, E., O. A. Okstad, D. A. Caugant, H. A. Johansen, A. Fouet, M. Mock, I. Hegna, and Kolsto. 2000. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl. Environ. Microbiol. 66:2627-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornstra, L. M., Y. P. de Vries, W. M. de Vos, T. Abee, and M. H. J. Wells-Bennik. 2005. gerR, a novel ger operon involved in l-alanine and inosine germination of Bacillus cereus ATCC 14579. Appl. Environ. Microbiol. 71:774-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudson, K. D., B. M. Corfe, E. H. Kemp, I. M. Feavers, P. J. Coote, and A. Moir. 2001. Localization of GerAA and GerAC germination proteins in the Bacillus subtilis spore. J. Bacteriol. 183:4317-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Igarashi, T., B. Setlow, M. Paidhungat, and P. Setlow. 2004. Effects of a gerF (lgt) mutation on the germination of spores of Bacillus subtilis. J. Bacteriol. 186:2984-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ireland, J. A., and P. C. Hanna. 2002. Amino acid- and purine ribonucleoside-induced germination of Bacillus anthracis Delta Sterne endospores: gerS mediates responses to aromatic ring structures. J. Bacteriol. 184:1296-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irie, R., Y. Fujita, and M. Kobayashi. 1996. Nucleotide sequence and gene organisation of the gerK spore germination locus of Bacillus subtilis 168. J. Gen. Appl. Microbiol. 42:141-153. [Google Scholar]
- 15.Ivanova, N., A. Sorokin, I. Anderson, N. Galleron, B. Candelon, V. Kapatral, A. Bhattacharyya, G. Reznik, N. Mikhailova, A. Lapidus, L. Chu, M. Mazur, E. Goltsman, N. Larsen, M. D'Souza, T. Walunas, Y. Grechkin, G. Pusch, R. Haselkorn, M. Fonstein, S. D. Ehrlich, R. Overbeek, and N. Kyrpides. 2003. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature 423:87-91. [DOI] [PubMed] [Google Scholar]
- 16.Johnstone, K. 1994. The trigger mechanism of spore germination: current concepts. Soc. Appl. Bacteriol. Symp. Ser. 23:17S-24S. [DOI] [PubMed] [Google Scholar]
- 17.Krask, B. J. 1961. Discussion, p. 89-100. In H. O. Halvorson (ed.), Spores II. Burgess Publishing Co., Minneapolis, Minn.
- 18.McCann, K. P., C. Robinson, R. L. Sammons, D. A. Smith, and B. M. Corfe. 1996. Alanine germination receptors of Bacillus subtilis. Lett. Appl. Microbiol. 23:290-294. [DOI] [PubMed] [Google Scholar]
- 19.Moir, A., B. M. Corfe, and J. Behravan. 2002. Spore germination. Cell. Mol. Life Sci. 59:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moir, A., E. H. Kemp, C. Robinson, and B. M. Corfe. 1994. The genetic analysis of bacterial spore germination. J. Appl. Bacteriol. 77:9S-16S. [PubMed] [Google Scholar]
- 21.Moir, A., and D. A. Smith. 1990. The genetics of bacterial spore germination. Annu. Rev. Microbiol. 44:531-553. [DOI] [PubMed] [Google Scholar]
- 22.Newsome, R. 2003. Dormant microbes: research needs. Food Technol. 57:38-42. [Google Scholar]
- 23.Paidhungat, M., and P. Setlow. 1999. Isolation and characterization of mutations in Bacillus subtilis that allow spore germination in the novel germinant d-alanine. J. Bacteriol. 181:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paidhungat, M., and P. Setlow. 2001. Localization of a germinant receptor protein (GerBA) to the inner membrane of Bacillus subtilis spores. J. Bacteriol. 183:3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paidhungat, M., and P. Setlow. 2000. Role of Ger proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 182:2513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In J. A. Hoch, R. Losick, and A. L. Sonenshein (ed.), Bacillus subtilis and its relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 27.Preston, R. A., and H. A. Douthit. 1988. Functional relationships between l- and d-alanine, inosine and NH4Cl during germination of spores of Bacillus cereus T. J. Gen. Microbiol. 134:3001-3010. [DOI] [PubMed] [Google Scholar]
- 28.Rasko, D. A., J. Ravel, O. A. Okstad, E. Helgason, R. Z. Cer, L. Jiang, K. A. Shores, D. E. Fouts, N. J. Tourasse, S. V. Angiuoli, J. Kolonay, W. C. Nelson, A. B. Kolsto, C. M. Fraser, and T. D. Read. 2004. The genome sequence of Bacillus cereus ATCC 10987 reveals metabolic adaptations and a large plasmid related to Bacillus anthracis pXO1. Nucleic Acids Res. 32:977-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read, T. D., S. L. Salzberg, M. Pop, M. Shumway, L. Umayam, L. Jiang, E. Holtzapple, J. D. Busch, K. L. Smith, J. M. Schupp, D. Solomon, P. Keim, and C. M. Fraser. 2002. Comparative genome sequencing for discovery of novel polymorphisms in Bacillus anthracis. Science 296:2028-2033. [DOI] [PubMed] [Google Scholar]
- 30.Rossignol, D. P., and J. C. Vary. 1979. Biochemistry of l-proline-triggered germination of Bacillus megaterium spores. J. Bacteriol. 138:431-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Setlow, P. 1984. Germination and outgrowth, p. 211-254. In A. Hurst and G. W. Gould (ed.), The bacterial spore, vol. 2. Academic Press, London, United Kingdom. [Google Scholar]
- 33.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 34.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 35.Warren, S. C., and G. W. Gould. 1968. Bacillus cereus spore germination: absolute requirement for an amino acid. Biochim. Biophys. Acta 170:341-350. [DOI] [PubMed] [Google Scholar]
- 36.Wax, R., and E. Freese. 1968. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of l-alanine. J. Bacteriol. 95:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiner, M. A., T. D. Read, and P. C. Hanna. 2003. Identification and characterization of the gerH operon of Bacillus anthracis endospores: a differential role for purine nucleosides in germination. J. Bacteriol. 185:1462-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolgamott, G. D., and N. N. Durham. 1971. Initiation of spore germination in Bacillus cereus: a proposed allosteric receptor. Can. J. Microbiol. 17:1043-1048. [DOI] [PubMed] [Google Scholar]
- 39.Zuberi, A. R., A. Moir, and I. M. Feavers. 1987. The nucleotide sequence and gene organization of the gerA spore germination operon of Bacillus subtilis 168. Gene 51:1-11. [DOI] [PubMed] [Google Scholar]