Abstract
Clostridium acetobutylicum is not able to grow on glycerol as the sole carbon source since it cannot reoxidize the excess of NADH generated by glycerol catabolism. Nevertheless, when the pSPD5 plasmid, carrying the NADH-consuming 1,3-propanediol pathway from C. butyricum VPI 3266, was introduced into C. acetobutylicum DG1, growth on glycerol was achieved, and 1,3-propanediol was produced. In order to compare the physiological behavior of the recombinant C. acetobutylicum DG1(pSPD5) strain with that of the natural 1,3-propanediol producer C. butyricum VPI 3266, both strains were grown in chemostat cultures with glycerol as the sole carbon source. The same “global behavior” was observed for both strains: 1,3-propanediol was the main fermentation product, and the qH2 flux was very low. However, when looking at key intracellular enzyme levels, significant differences were observed. Firstly, the pathway for glycerol oxidation was different: C. butyricum uses a glycerol dehydrogenase and a dihydroxyacetone kinase, while C. acetobutylicum uses a glycerol kinase and a glycerol-3-phosphate dehydrogenase. Secondly, the electron flow is differentially regulated: (i) in C. butyricum VPI 3266, the in vitro hydrogenase activity is 10-fold lower than that in C. acetobutylicum DG1(pSPD5), and (ii) while the ferredoxin-NAD+ reductase activity is high and the NADH-ferredoxin reductase activity is low in C. acetobutylicum DG1(pSPD5), the reverse is observed for C. butyricum VPI 3266. Thirdly, lactate dehydrogenase activity is only detected in the C. acetobutylicum DG1(pSPD5) culture, explaining why this microorganism produces lactate.
For a long time, 1,3-propanediol has been considered a specialty chemical. However, the recent development of a new polyester called poly(propylene terephthalate), with unique properties for the fiber industry (23, 29), necessitates a drastic increase in the production of this chemical. There are currently two processes for the chemical synthesis of 1,3-propanediol. Both of these processes produce toxic intermediates and require a reduction step under high hydrogen pressure (35). The biological production of 1,3-propanediol from glycerol was demonstrated for several bacterial species, e.g., Lactobacillus brevis, Lactobacillus buchnerii (32, 33), Bacillus welchii (15), Citrobacter freundii, Klebsiella pneumoniae (14, 34), Clostridium pasteurianum (7), and Clostridium butyricum (2, 13, 31). Among these microorganisms, C. butyricum is, to our knowledge, the best “natural producer” in terms of both the yield and titer of 1,3-propanediol produced (30). Moreover, unlike the case with other bacteria, the production of 1,3-propanediol by this microorganism is not a vitamin B12-dependent process, which is clearly an economical advantage for an industrial application. The B12-independent pathway converting glycerol to 1,3-propanediol in C. butyricum has been recently characterized from a biochemical (31) and a molecular point of view (27). To develop an economical process of 1,3-propanediol production, it is necessary to further improve the process by a metabolic engineering approach with the strain. No genetic tools are currently available for C. butyricum, and all our efforts to develop them so far have been unsuccessful. This work opens the possibility of converting other clostridia to 1,3-propanediol producers by heterologous expression of the genes encoding the B12-independent 1,3-propanediol pathway. Among the clostridia, Clostridium acetobutylicum is a microorganism of choice, as (i) it has already been used for the industrial production of solvent (5) and (ii) the genetic tools for gene knockout or gene overexpression are currently available (12, 21).
In this article, we describe a novel genetically engineered strain of Clostridium acetobutylicum containing the genes for 1,3-propanediol production from C. butyricum VPI 3266 on the pSPD5 plasmid. In addition, the novel strain of C. acetobutylicum and the natural 1,3-propanediol producer C. butyricum were studied and compared from a physiological point of view by determining the types and levels of enzymes in the key metabolic pathways in association with the nucleotide pools.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used for or derived from this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used for this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| C. acetobutylicum DG1 | Cured of pSOL1 | 24 |
| E. coli ER 2275 | RecA− McrBC− | New England Biolabs |
| C. butyricum VPI 3266 | Wild type | Virginia Polytechnic Institute |
| pSPD5 plasmid | MLSr Apr; dhaB1 dhaB2 dhaT | 27 |
| pIMP1 plasmid | MLSr Apr; control plasmid | 21 |
| pAN1 plasmid | Cmr; Φ3TI | 21 |
Abbreviations: RecA−, homologous recombination abolished; McrBC−, lacking methylcytosine-specific restriction system; MLSr, macrolide, lincosamide, and streptogramin B resistance; dhaB1 and dhaB2. glycerol dehydratase genes; dhaT, 1,3-propanediol dehydrogenase gene; Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Φ3TI, Φ3T methylase.
DNA isolation and manipulation.
Plasmid DNA was extracted from Escherichia coli with a Qiaprep kit (QIAGEN, Courtaboeuf, France). DNA restriction enzymes, calf intestinal alkaline phosphatase, and T4 DNA ligase were obtained from New England Biolabs (Beverly, Mass.) or GIBCO/BRL (Life Technologies, Cergy Pontoise, France) and used according to the manufacturer's instructions.
Plasmids and genetic construction.
Prior to the transformation of C. acetobutylicum DG1, the pPSD5 plasmid was methylated in vivo in E. coli ER2275(pAN1) (21) and then concentrated and desalted in a Microcon 100 microconcentrator (Amicon, Inc., Beverly, Mass.). Methylated plasmid DNA was used to transform C. acetobutylicum by electroporation as previously described (22).
Culture media.
E. coli strains were grown aerobically at 37°C in Luria-Bertani medium supplemented with chloramphenicol (30 μg/ml) and ampicillin (100 μg/ml). The synthetic medium used for clostridium cultivations contained the following reagents per liter of deionized water: glycerol, 60 g; KH2PO4, 0.5 g; K2HPO4, 0.5 g; MgSO4 · 7H2O, 0.2 g; CoCl2 · 6H2O, 0.01 g; FeSO4 · 7H2O, 0.01 g; biotin, 0.04 mg; p-aminobenzoic acid, 8 mg; and acetic acid, 2 g. The medium pH was adjusted to 6.5 with 6 N NH4OH. The feed medium for continuous cultures was the synthetic medium described above without acetic acid and with 0.028 g liter−1 of FeSO4 · 7H2O (instead of 0.01 g liter−1), 1.5 g liter−1 of NH4Cl, and 1 ml of 17.4 M H2SO4; the medium pH was not adjusted in this case.
Continuous culture conditions.
Continuous cultures were performed in a 2-liter Biostat MD bioreactor (Braun, Melsungen, Germany), with a working volume of 1,250 ml, and in a 600-ml glass bioreactor, with a working volume of 500 ml. The cultures were stirred at 200 rpm, the temperature was set to 35°C, and a constant pH was maintained by the automatic addition of 6 N NH4OH. To create anaerobic conditions, the sterilized medium in the vessels was flushed with sterile O2-free nitrogen until room temperature was attained. A growing culture taken at the early exponential growth phase was used as the inoculum (10% [vol/vol]). The cultures were first grown in batches, and continuous feeding was started once the exponential growth phase was reached. After sterilization, the feed medium was sparged with sterile O2-free nitrogen until it reached room temperature. During the experiments, the feed medium was maintained under nitrogen at 3 kPa to avoid O2 entry. All tubing was made of butyl rubber, and the bioreactor gas outlet was protected with a pyrogallol arrangement (37).
Analytical procedures.
The cell concentration was measured turbidimetrically at 620 nm and correlated with the cell dry weight, which was determined directly. Glycerol, 1,3-propanediol, ethanol, and acetic, butyric, and lactic acid concentrations were determined by high-performance liquid chromatography analysis. Separation was performed on a Bio-Rad Aminex HPX-87H column (300 mm × 7.8 mm), and detection was achieved by using a refractive index. Operating conditions were as follows: mobile phase, 0.5 mM sulfuric acid; flow rate, 0.5 ml/min; and temperature, 30°C. The protein concentration was determined by use of Coomassie brilliant blue G250 (4).
Preparation of cell extracts.
The extraction method used was carried out following the method described by Vasconcelos et al. (37). Anaerobic conditions were maintained throughout the entire procedure. Sixty-milliliter samples were removed with a sterilized syringe from the bioreactor and centrifuged (8,000 × g for 10 min at 4°C) in 25-ml Beckman centrifugation tubes, which were sealed with a butyl rubber stopper and capped with an aluminum top. These tubes were previously filled with the gaseous mixture (80% N2, 10% CO2, and 10% H2) used within an anaerobic chamber (model 855-AC; PLAS LABS). The cell pellets were resuspended and washed in a buffer containing 100 mM O2-free Tris-HCl, pH 7.6, with 2 mM of dithiothreitol (DTT) and centrifuged anaerobically (8,000 × g for 10 min at 4°C). Harvested cells were resuspended in 3 ml of the previously mentioned buffer and sonicated in the anaerobic chamber with an ultrasonic disintegrator (Labsonic U; B. Braun Diessel Biotech) at 0°C, with four cycles of 30 s each at 2-min intervals. Cell debris was removed by centrifugation at 14,000 rpm for 15 min. The cell extract was kept on ice during the enzyme assays.
Enzyme assays.
All enzyme assays were performed in duplicate or triplicate with two different culture samples (between each sampling, 2.5 to 5 residence time periods were allowed) under the strictly anaerobic conditions provided by the above-mentioned anaerobic chamber. The results given are the average values of four to six measurements. One unit of enzyme activity is defined as the amount of enzyme that catalyzes the conversion of 1 μmol of substrate per minute. Assays for the following products were adopted from the work of Vasconcelos et al. (37): hydrogenase in the hydrogen evolution direction, ferredoxin-NAD+ reductase, NADH-ferredoxin reductase, phosphotransacetylase and phosphotransbutyrylase, acetate and butyrate kinases (in the nonphysiological direction), and pyruvate-ferredoxin oxidoreductase.
Glycerol dehydrogenase activity was measured spectrophotometrically by following the glycerol-dependent formation of NADH at 340 nm by a method adapted from the work of Ruch et al. (28). The assay mixture contained 2 mM NAD+, 30 mM ammonium sulfate, 100 mM potassium bicarbonate (pH 9.0), and 200 mM glycerol.
Glycerol kinase activity was measured spectrophotometrically by following the glycerol-dependent formation of NAD+ at 340 nm by a method adapted from the work of Kremer and Hensen (18). The assay mixture contained 100 mM potassium bicarbonate (pH 9.0), 5 mM MgCl2, 0.5 mM NAD+, 2.5 mM ATP, 5.5 U of glycerophosphate dehydrogenase from rabbit muscle, and 10 mM glycerol.
Glycerol-3-phosphate dehydrogenase activity was measured spectrophotometrically as described by Blomberg and Adler (3). The assay mixture contained 20 mM imidazole-HCl (pH 7.0), 1 mM DTT, 1 mM MgCl2, 0.67 mM dihydroxyacetone phosphate (DHA phosphate), and 0.09 mM NADH.
Dihydroxyacetone kinase activity was followed in a coupled system, where the NADH-dependent reduction of the reaction product (DHA phosphate) to glycerol-3-phosphate was measured in a modified assay based on the method described by Johnson et al. (16). The assay mixture was prepared with 50 mM potassium bicarbonate (pH 9.0) containing 2 mM DTT, 2.5 mM ATP, 0.4 mM NADH, 15 mM MgCl2, 18 U glycerophosphate dehydrogenase from rabbit muscle, and 10 mM DHA.
1,3-Propanediol dehydrogenase activity was measured in the oxidative direction as described by Heyndrickx et al. (13). The assay mixture contained 2 mM NAD+, 30 mM ammonium sulfate, 100 mM potassium bicarbonate (pH 9.0), and 100 mM 1,3-propanediol.
Glycerol dehydratase activity was measured in a modified assay derived from the method described by Toraya et al. (36), based on NADH consumption when the aldehydes formed by the dehydratase are reduced to the corresponding alcohols by an excess of yeast alcohol dehydrogenase. The assay mixture contained 0.03 M (NH4)2SO4, 0.1 M 1,2-propanediol, 0.1 M potassium carbonate buffer, pH 7.0, 2 mM DTT, and 10 mM NADH. Coenzyme B12 (10 μM) or S-adenosyl-methionine (4 mM) was added to or omitted from this reaction mixture. NADH consumption was followed continuously at 340 nm.
Glyceraldehyde-3-phosphate dehydrogenase activity was measured according to the method of Lovitt et al. (20). The assay mixture contained 100 mM Tricine-NaOH (pH 8.1), 2 mM DTT, 5 mM KH2PO4, 20 mM KH2AsO4, 10 mM fructose 1,6-diphosphate, 2 mM NAD+, and 2 U aldolase.
Lactate dehydrogenase activity was determined by following NADH oxidation at 340 nm according to the method of Freier and Gottschalk (8). The assay mixture contained 50 mM succinate-NaOH (pH 5.4), 0.6 mM NADH, 4.5 mM sodium pyruvate, and 3 mM fructose 1,6-diphosphate.
Pyruvate formate-lyase activity was determined with a procedure adopted from Abbe et al. (1). The assay mixture contained 100 mM potassium phosphate (pH 7.4), 1 mM NAD+, 2 mM DTT, 0.1 mM coenzyme A-SH, 14 U malate dehydrogenase, 10 U citrate synthase, 2 mM sodium pyruvate, and 0.6 mM malate.
Determination of NAD+ and NADH pools.
All nucleotide pool assays were performed in triplicate with two different culture extracts (between each sampling, 2.5 to 5 residence time periods were allowed). The results given are the average values of six measurements. NADH and NAD+ levels were determined after extraction of a culture broth sample as described by Vasconcelos et al. (37) and fluorimetric enzyme assays as described by Klingerberg (17).
Chemicals.
Enzymes and coenzymes were purchased from Sigma Chemical Company (St. Louis, Mo.). All gases used (carbon dioxide, nitrogen, and hydrogen) were purchased from GASIN (Matosinhos, Portugal). All other chemicals were of analytical grade.
RESULTS
Engineering of C. acetobutylicum DG1 for the production of 1,3-propanediol.
The conversion of glycerol to 1,3-propanediol in C. butyricum occurs in two steps. First, glycerol is dehydrated to 3-hydroxipropionaldehyde in a reaction catalyzed by the B12-independent glycerol dehydratase. Next, 3-hydroxipropionaldehyde is reduced to 1,3-propanediol by 1,3-propanediol dehydrogenase, consuming 1 mole of NADH. Both the pSPD5 plasmid (27) carrying the 1,3-propanediol operon from C. butyricum and the control pIMP1 plasmid were introduced into the C. acetobutylicum DG1 mutant, which is cured of the pSOL1 megaplasmid and is thus unable to produce solvents and to sporulate (6, 24). While C. acetobutylicum DG1(pIMP1) was unable to grow on glycerol, C. acetobutylicum DG1(pSPD5) could grow and consume glycerol to produce 1,3-propanediol as the main fermentation product (11).
Metabolism and energetics of C. acetobutylicum DG1(pSPD5) and C. butyricum VPI 3266 grown on glycerol.
In order to compare the metabolic fluxes of the recombinant C. acetobutylicum DG1(pSPD5) strain and the 1,3-propanediol natural producer C. butyricum VPI 3266, specific rates of product formation and substrate consumption rates were analyzed in chemostat cultures at a dilution rate of 0.05 h−1 and a glycerol feed concentration of 650 mM.
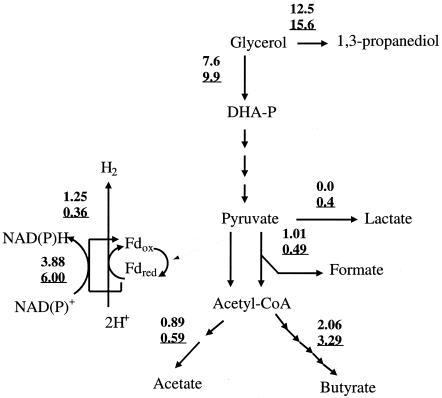
Differences in specific product formation rates and specific substrate consumption rates of the two strains are shown in Fig. 1. Due to a lower cell concentration, most of the specific rates were higher for C. acetobutylicum DG1(pSPD5) than for C. butyricum VPI 3266. For both strains, all glycerol was consumed and 1,3-propanediol became the main fermentation product (412 mM), leading to a molar yield of 0.64. Small amounts of hydrogen were also produced. The acetate-specific formation rate in cultures of C. acetobutylicum DG1(pSPD5) was much lower than that in cultures of C. butyricum VPI 3266, and lactate was only produced by C. acetobutylicum DG1(pSPD5). The formate-specific formation rate was twofold higher in C. butyricum VPI 3266 than in cultures of C. acetobutylicum DG1(pSPD5).
FIG. 1.
Quantitative flow schemes (mmol h−1 g dry weight−1) for growth of C. acetobutylicum DG1(pSPD5) (underlined values) and C. butyricum VPI 3266 on glycerol (D = 0.05 h−1; 650 mM feed glycerol, pH 6.5; 35°C). Fdred, reduced ferredoxin; Fdox, oxidized ferredoxin; DHA-P, dihydroxyacetone phosphate.
Enzymatic activities of C. acetobutylicum DG1(pSPD5) and C. butyricum VPI 3266 grown on glycerol.
In order to understand the regulation taking place in both strains, an enzymatic analysis of the key metabolic activities was undertaken with chemostat cultures grown at a dilution rate of 0.05 h−1, a glycerol feed concentration of 650 mM, and a pH of 6.5.
(i) Enzymes responsible for glycerol metabolism and 1,3-propanediol formation.
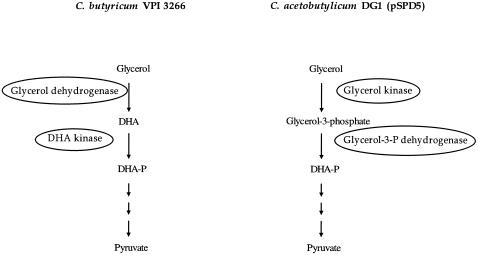
Enzymatic activities involved in glycerol assimilation in C. acetobutylicum DG1(pSPD5) and C. butyricum VPI 3266 were determined (Table 2). We confirmed that glycerol dehydrogenase and dihydroxyacetone phosphate kinase were the only enzymes responsible for glycerol oxidation in C. butyricum (31). In contrast, no glycerol dehydrogenase and DHA kinase activities were detected in cultures of C. acetobutylicum DG1(pSPD5). In this strain, the oxidation of glycerol proceeds through the glycerol kinase and glycerol-3-phosphate dehydrogenase (Fig. 2).
TABLE 2.
Enzymatic activities of C. butyricum VPI 3266 and C. acetobutylicum DG1(pSPD5) in chemostat cultures (0.05 h−1, 650 mM glycerol, pH 6.5, 35°C)
| Enzyme type | Enzymea | Activity (U/mg protein [SD])b
|
|
|---|---|---|---|
| C. butyricum VPI 3266 | C. acetobutylicum DG1 (pSPD5) | ||
| Hydrogenase and coupling enzymes | Hydrogenase (H2 evolution) | 0.045 (0.03) | 0.43 (0.05) |
| Fd-NAD+ reductase | 0.03 (0.01) | 0.25 (0.08) | |
| NADH-Fd reductase | 0.33 (0.005) | 0.034 (0.01) | |
| Glycerol oxidation enzymes | Glycerol dehydrogenase | 0.26 (0.05) | <0.005 |
| DHA kinase | 1.1 (0.1) | <0.002 | |
| Glycerol kinase | <0.002 | 0.14 (0.03) | |
| Glycerol 3-P dehydrogenase | <0.002 | 0.52 (0.05) | |
| Glycerol reduction enzymes | 1,3-Pdiol dehydrogenase | 0.37 (0.05) | 1.2 (0.2) |
| Glycerol dehydratase | 0.42 (0.15) | 1.7 (0.4) | |
| Acidogenic enzymes | Phosphotransacetylase | 0.30 (0.03) | 0.25 (0.03) |
| Acetate kinase | 0.56 (0.03) | 0.32 (0.04) | |
| Phosphotransbutyrylase | 8.04 (0.53) | 6.95 (0.35) | |
| Butyrate kinase | 1.26 (0.20) | 1.46 (0.18) | |
| Lactate dehydrogenase | <0.002 | 0.19 (0.01) | |
| Central axis enzymes | Pyruvate-Fd oxidoreductase | 4.0 (0.8) | 4.5 (0.2) |
| Pyruvate formate lyase | 0.15 (0.03) | 0.12 (0.02) | |
| Glyceraldehyde 3-P dehydrogenase | 5.58 (0.88) | 4.96 (0.05) | |
Fd, ferredoxin; DHA, dihydroxyacetone; 1,3-Pdiol, 1,3-propanediol.
Data in parentheses are standard deviation values from four to six determinations.
FIG. 2.
Glycerol oxidation pathways in C. butyricum VPI 3266 and C. acetobutylicum DG1(pSPD5). DHA, dihydroxyacetone; DHA-P, dihydroxyacetone phosphate; glycerol-3-P dehydrogenase, glycerol-3-phosphate dehydrogenase.
1,3-Propanediol dehydrogenase and glycerol dehydratase activities were three- and fourfold higher, respectively, in C. acetobutylicum DG1(pSPD5) than in C. butyricum VPI 3266.
(ii) Hydrogenase and coupling enzymes.
For C. acetobutylicum DG1(pSPD5), the in vitro hydrogenase activity (H2 evolution) was 10-fold higher than that in C. butyricum VPI 3266 (Table 2). While the NADH-ferredoxin reductase activity was high and the ferredoxin-NAD reductase activity was low in C. butyricum VPI 3266, the reverse was observed for C. acetobutylicum DG1(pSPD5) (Table 2).
(iii) Enzymes associated with acid formation.
The levels of phosphotransacetylase, acetate kinase, phosphotransbutyrylase, and butyrate kinase were relatively high and not significantly different between C. butyricum VPI 3266 and C. acetobutylicum DG1(pSPD5). On the other hand, lactate dehydrogenase activity was measured in cultures of C. acetobutylicum DG1(pSPD5), while this enzyme activity was not detected in cultures of C. butyricum VPI 3266.
(iv) Central axis enzymes.
The activities of glyceraldehyde-3-phosphate dehydrogenase, pyruvate-ferredoxin oxidoreductase, and pyruvate formate lyase were not significantly different between C. butyricum VPI 3266 and C. acetobutylicum DG1(pSPD5) (Table 2).
Intracellular NAD+ and NADH levels of C. acetobutylicum DG1(pSPD5) and C. butyricum VPI 3266 grown on glycerol.
Both C. butyricum VPI 3266 and C. acetobutylicum DG1(pSPD5) presented similar and very high NADH/NAD+ ratios when grown on glycerol (Table 3).
TABLE 3.
Nucleotide pools of C. butyricum VPI 3266 and C. acetobutylicum DG1(pSPD5) cells grown in steady-state continuous glycerol fermentations (0.05 h−1, 650 mM glycerol, pH 6.5, 35°C)
| Strain | Nucleotide concn (μmol/g dry cell wt)a
|
NADH/NAD+ ratio | |
|---|---|---|---|
| NAD+ | NADH | ||
| C. butyricum VPI3266 | 10.5 (0.58) | 16.8 (2.50) | 1.60 |
| C. acetobutylicum DG1(pSPD5) | 6.90 (0.36) | 12.5 (0.14) | 1.81 |
Data in parentheses are standard deviation values from six determinations.
DISCUSSION
C. acetobutylicum is not able to grow on glycerol as the sole carbon source since it cannot reoxidize the excess of NADH generated by glycerol catabolism (10, 37). Nevertheless, when the NADH-consuming 1,3-propanediol pathway from C. butyricum VPI 3266 (26) was introduced into C. acetobutylicum DG1, growth on glycerol was achieved and 1,3-propanediol was the main fermentation product.
In order to compare the physiological behavior of the recombinant strain C. acetobutylicum DG1(pSPD5) to that of the 1,3-propanediol natural producer C. butyricum VPI 3266, both strains were grown in chemostat cultures with glycerol as the sole carbon source. A simple metabolic flux comparison showed surprisingly similar “global behaviors” of both strains: 1,3-propanediol was the main fermentation product, and qH2 flux was very low. In the presence of an excess of reducing equivalents generated by glycerol catabolism and confirmed by the high NADH/NAD+ ratios, one might expect a high qH2 flux, but surprisingly the reverse was observed, as most of the reduced ferredoxin produced by the decarboxylation of pyruvate was used to generate NADH (qNADH from Fd, >0), leading to low hydrogen production in both strains. The physiological explanation for this phenomenon was different for each strain. In the case of C. butyricum VPI 3266, as already demonstrated by Saint-Amans et al. (31) with glucose-glycerol mixtures, the low qH2 flux was mainly due to a low hydrogenase level, with the accumulation of reduced ferredoxin being used by the reversible NADH-ferredoxin oxidoreductase to generate NADH. For C. acetobutylicum DG1(pSPD5), on the other hand, the level of hydrogenase remained high, but the high level of ferredoxin-NAD+ reductase efficiently competed for the consumption of reduced ferredoxin (probably due to a higher catalytic efficiency than that of the hydrogenase), which explains the high qNADH from Fd flux and the low qH2 flux. A major difference between both strains also concerns the pathway for glycerol oxidation. While C. butyricum VPI 3266 exclusively uses a glycerol dehydrogenase and dihydroxyacetone kinase, as already demonstrated by Saint-Amans et al. (31) for glucose-glycerol-grown chemostat cultures, Clostridium acetobutylicum DG1(pSPD5) uses only a glycerol kinase and glycerol-3-phosphate dehydrogenase to oxidize glycerol (Fig. 2). A similar glycerol oxidation pathway has been described before for K. pneumoniae and Escherichia coli K-12 (19). However, this pathway is normally used under aerobic conditions. Our results are in agreement with the genome sequence of C. acetobutylicum reported by Nölling et al. (25), where glycerol kinase and glycerol-3-phosphate dehydrogenase genes were reported but no glycerol dehydrogenase or dihydroxyacetone kinase genes were found.
The production of 1,3-propanediol in C. butyricum VPI 3266 is associated with the induction of a B12-independent glycerol dehydratase and an NADH-dependent 1,3-propanediol dehydrogenase (31), and the encoding genes have recently been cloned, sequenced, and characterized (27). When these genes were expressed in Clostridium acetobutylicum DG1 from a synthetic operon carried on the pSPD5 plasmid, a high expression level was obtained. These results demonstrate that DhaB1 was activated in C. acetobutylicum and that DhaB2, the activase (26), had then found an appropriate redox partner to carry the activation reaction. In agreement with the multiple copies of pSPD5, the glycerol dehydratase and 1,3-propanediol dehydrogenase levels were fourfold and threefold higher, respectively, in the recombinant strain than in the natural 1,3-propanediol producer. This indicates that in chemostat culture, the molar yield (equal to 0.64 for both cultures) of 1,3-propanediol production is not controlled by the levels of glycerol dehydratase and 1,3-propanediol dehydrogenase.
Regarding the production of acetate and butyrate, the levels of phosphotransacetylase, acetate kinase, phosphotransbutyrylase, and butyrate kinase were relatively high and not significantly different between both cultures. Furthermore, they were also not very different from previously published data for glucose-grown cultures (at neutral pH) of C. butyricum VPI 3266 (31) and C. acetobutylicum ATCC 824 (37), which have much higher fluxes of acetate and butyrate production. This clearly indicates that the control of acid formation occurs upstream in the glycolytic pathway. It was previously demonstrated for C. acetobutylicum (9) that when glycerol was used as a cosubstrate, the glycolytic pathway was controlled at the level of the glyceraldehyde-3-phosphate dehydrogenase by the NADH/NAD+ ratio, a key factor affecting the in vitro activity of this enzyme. With regard to the high NADH/NAD+ ratios observed for both cultures and their similar glyceraldehyde-3-phosphate dehydrogenase levels, the same control probably exists in both strains.
Regarding flux distribution at the pyruvate node, both strains mainly produced acetyl-coenzyme A by the pyruvate-ferredoxin oxidoreductase due to the higher level of this enzyme than of the pyruvate formate lyase. On the other hand, C. butyricum and C. acetobutylicum DG1(pSPD5) clearly differ regarding lactate production, as the former does not express a lactate dehydrogenase and consequently does not produce lactate.
In conclusion, this work demonstrates that although C. butyricum VPI 3266 and C. acetobutylicum DG1(pSPD5) show similar “global behavior” and share common regulatory features in glycerol-grown chemostat cultures, these strains also exhibit significant differences in terms of (i) regulation of their electron flow and (ii) their pathways for glycerol oxidation. In another paper, we demonstrate that C. acetobutylicum DG1(pSPD5) can be used for the continuous industrial production of 1,3-propanediol, with high yields, titers, and productivities, using raw glycerol from a biodiesel production plant as the carbon source (11).
Acknowledgments
This work was financially supported by the European project (contract no. QLRT-1999-01364) and the Agence de l'Environnement et de la Maitrise de l'Energie (contract no. 00 01 027). M. González-Pajuelo was supported by PRAXIS XXI (Ph.D. grant BD/16036/98).
REFERENCES
- 1.Abbe, K., S. Takahashi, and T. Yamada. 1982. Involvement of oxygen-sensitive pyruvate-lyase in mixed-acid fermentation by Streptococcus mutans under strictly anaerobic conditions. J. Bacteriol. 152:175-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biebl, H., S. Marten, H. Hippe, and W. D. Deckwer. 1992. Glycerol conversion to 1,3-propanediol by newly isolated clostridia. Appl. Microbiol. Biotechnol. 36:592-597. [Google Scholar]
- 3.Blomberg, A., and L. Adler. 1989. Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. J. Bacteriol. 171:1087-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford, M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Cornillot, E., and P. Soucaille. 1996. Solvent-forming genes in clostridia. Nature 380:489. [Google Scholar]
- 6.Cornillot, E., R. V. Nair, E. T. Papoutsakis, and P. Soucaille. 1997. The genes for butanol and acetone formation in Clostridium acetobutylicum ATCC 824 reside on a large plasmid whose loss leads to degeneration of the strain. J. Bacteriol. 179:5442-5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabrock, B., H. Bahl, and G. Gottschalk. 1992. Parameters affecting solvent production by Clostridium pasteurianum. Appl. Environ. Microbiol. 58:1233-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freier, D., and G. Gottschalk. 1987. l(+)-Lactate dehydrogenase of Clostridium acetobutylicum is activated by fructose-1,6-biphosphate. FEMS Microbiol. Lett. 43:229-233. [Google Scholar]
- 9.Girbal, L., and P. Soucaille. 1994. Regulation of Clostridium acetobutylicum metabolism as revealed by mixed-substrate steady-state continuous cultures: role of NADH/NAD ratio and ATP pool. J. Bacteriol. 176:6433-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girbal, L., C. Croux, I. Vasconcelos, and P. Soucaille. 1995. Metabolic shifts in Clostridium acetobutylicum. FEMS Microbiol. Rev. 17:287-297. [Google Scholar]
- 11.González-Pajuelo, M., I. Meynial-Salles, F. Mendes, J. C. Andrade, P. Soucaille, and I. Vasconcelos. Metabolic engineering of Clostridium acetobutylicum for the industrial production of 1,3-propanediol from glycerol. Metab. Eng., in press. [DOI] [PubMed]
- 12.Green, E. M., Z. L. Boyton, L. M. Harris, F. B. Rudolph, E. T. Papoutsakis, and G. N. Bennett. 1996. Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079-2086. [DOI] [PubMed] [Google Scholar]
- 13.Heyndrickx, M., P. de Vos, M. Vancanneyt, and J. de Ley. 1991. The fermentation of glycerol by Clostridium butyricum LMG 1212t2 and 1213t1 and Clostridium pasteurianum LMG 3285. Appl. Microbiol. Biotechnol. 34:637-642. [Google Scholar]
- 14.Homann, T., C. Tag, H. Biebl, W.-D. Deckwer, and B. Schink. 1990. Fermentation of glycerol to 1,3-propanediol by Klebsiella and Citrobacter strains. Appl. Microbiol. Biotechnol. 33:121-126. [Google Scholar]
- 15.Humphreys, F. B. 1924. Formation of acrolein by Bacillus welchii. Infect. Dis. 35:282-290. [Google Scholar]
- 16.Johnson, E. A., S. K. Burke, R. G. Forage, and E. C. C. Lin. 1984. Purification and properties of dihydroxyacetone kinase from Klebsiella pneumoniae. J. Bacteriol. 160:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klingenberg, M. 1965. Nicotinamide-adenine dinucleotides (NAD+, NADP, NADH, NADPH). Spectrophotometric and fluorimetric methods, p. 2045-2059. In H. U. Bergmeyer (ed.), Methods of enzymatic analysis, 2nd ed., vol. 4. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 18.Kremer, D. R., and T. A. Hansen. 1987. Glycerol and dihydroxyacetone in Desulfovibrio strains. Arch. Microbiol. 147:249-256. [Google Scholar]
- 19.Lin, E. C. C. 1976. Glycerol dissimilation and its regulation in bacteria. Annu. Rev. Microbiol. 30:535-578. [DOI] [PubMed] [Google Scholar]
- 20.Lovitt, R. W., G. J. Shen, and J. G. Zeikus. 1988. Ethanol production by thermophilic bacteria: biochemical basis for ethanol and hydrogen tolerance in Clostridium thermohydrosulfuricum. J. Bacteriol. 170:2809-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mermelstein, L. D., and E. T. Papoutsakis. 1993. In vivo methylation in Escherichia coli by Bacillus subtilis phage F 3T methyltransferase to protect plasmids from restriction upon transformation of Clostridium acetobutylicum ATCC 824. Appl. Environ. Microbiol. 59:1077-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mermelstein, L. D., N. E. Welker, G. G. Bennett, and E. T. Papoutsakis. 1992. Expression of cloned homologous fermentative genes in Clostridium acetobutylicum ATCC 824. Biotechnology 10:190-195. [DOI] [PubMed] [Google Scholar]
- 23.Miller, H. 2000. Major fiber producers hop on corterra bandwagon. Int. Fiber J. 15:14-16. [Google Scholar]
- 24.Nair, R. 1995. Molecular characterisation and regulation of a multifunctional aldehyde/alcohol dehydrogenase gene from and its use for genetic engineering of Clostridium acetobutylicum ATCC 824. Ph.D. thesis. Northwestern University, Evanston, Ill.
- 25.Nölling, J., G. Breton, M. V. Omelchenko, K. S. Makarova, Q. Zeng, R. Gibson, H. M. Lee, J. Dubois, D. Qui, J. Hitti, GTC Sequencing Center Production, Finishing, and Bioinformatics Teams, Y. I. Wolf, R. L. Tatusov, F. Sabathe, L. Doucette-Stamm, P. Soucaille, M. J. Daly, G. N. Bennett, E. V. Koonin, and D. R. Smith. 2001. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183:4823-4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien, J. R., C. Raynaud, C. Croux, L. Girbal, P. Soucaille, and W. N. Lanzilotta. 2004. Insight into the mechanism of the B12-independent glycerol dehydratase from C. butyricum; preliminary and structural characterization. Biochemistry 43:4635-4645. [DOI] [PubMed] [Google Scholar]
- 27.Raynaud, C., P. Sarçabal, I. Meynial-Salles, C. Croux, and P. Soucaille. 2003. Molecular characterization of the 1,3-propanediol operon of Clostridium butyricum encoding a novel coenzyme B12 independent glycerol dehydratase and a 1,3 propanediol dehydrogenase. Proc. Natl. Acad. Sci. USA 100:5010-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruch, J. R., E. C. C. Lin, J. D. Kowit, C. T. Tang, and A. L. Goldberg. 1980. In vivo inactivation of glycerol dehydrogenase in Klebsiella aerogenes: properties of active and inactivated proteins. J. Bacteriol. 141:1077-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudie, R. 2000. Cargill Dow sows seeds of future fibers; will build $300 million PLA polymer plant. Int. Fiber J. 15:8-12. [Google Scholar]
- 30.Saint-Amans, S., P. Perlot, G. Goma, and P. Soucaille. 1994. High production of 1,3-propanediol from glycerol by Clostridium butyricum VPI 3266 in a simply controlled fed-batch system. Biotech. Lett. 16:831-836. [Google Scholar]
- 31.Saint-Amans, S., L. Girbal, J. Andrade, K. Ahrens, and P. Soucaille. 2001. Regulation of carbon and electron flow in Clostridium butyricum VPI 3266 grown on glucose-glycerol mixtures. J. Bacteriol. 183:1748-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schütz, H., and F. Radler. 1984. Anaerobic reduction of glycerol to propanediol-1,3 by Lactobacillus brevis and Lactobacillus buchneri. Syst. Appl. Microbiol. 5:169-178. [Google Scholar]
- 33.Solobov, M., and K. L. Smiley. 1960. Metabolism of glycerol by an acrolein-forming lactobacillus. J. Bacteriol. 79:261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Streekstra, H., M. J. Teixeira de Mattos, O. M. Neijssel, and D. W. Tempest. 1987. Overflow metabolism during anaerobic growth of Klebsiella aerogenes NCTC 418 on glycerol and dihydroxyacetone in chemostat culture. Arch. Microbiol. 147:268-275. [Google Scholar]
- 35.Sullivan, C. J. 1993. Propanediols, p. 163-171. In Ullmann's encyclopedia of industrial chemistry, vol. 22. VCH, Weinheim, Germany. [Google Scholar]
- 36.Toraya, T., E. Krodel, A. S. Mildvan, and R. H. Abeles. 1979. Role of peripheral side chains of vitamin B12 coenzymes in the reaction catalyzed by dioldehydrase. Biochemistry 18:417-426. [DOI] [PubMed] [Google Scholar]
- 37.Vasconcelos, I., L. Girbal, and P. Soucaille. 1994. Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J. Bacteriol. 176:1443-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]