Abstract
Environmental reservoirs of glycopeptide-resistant enterococci (GRE) in Norway have been linked to former growth promoting use of the glycopeptide avoparcin in poultry production. We have examined the prevalence of fecal GRE in poultry and poultry farmers 3 to 8 years after the Norwegian avoparcin ban in 1995 and performed molecular analyses of the GRE population. Fecal samples from poultry farmers and their flocks on 29 previously avoparcin-exposed farms were collected on five occasions during the study period (1998 to 2003). All flocks (100%) were GRE positive in 1998. Throughout the study period, 78.5% of the poultry samples were GRE positive. Glycopeptide-resistant Enterococcus faecium (GREF) was isolated from 27.6% of the farmer samples in 1998 and from 27.8% of the samples collected between 1998 and 2003. The prevalence of fecal GRE in poultry declined significantly during the study period, but prevalence in samples from the farmers did not decline. PCR analysis revealed a specific Tn1546-plasmid junction fragment in 93.9% of E. faecium isolates. A putative postsegregation killing (PSK) system linked to Tn1546 was detected in 97.1% of the isolates examined. Multilocus sequence typing of glycopeptide-susceptible (n = 10) and -resistant (n = 10) E. faecium isolates from humans (n = 10) and poultry (n = 10) on two farms displayed 17 different sequence types. The study confirms the continuing persistence of a widespread common plasmid-mediated vanA-pRE25-PSK element within a heterogeneous GRE population on Norwegian poultry farms 8 years after the avoparcin ban. Moreover, it suggests an important role of PSK systems in the maintenance of antimicrobial resistance determinants in reservoirs without apparent antimicrobial selection.
The glycopeptide avoparcin was introduced as a growth-promoting feed additive to the Norwegian poultry industry in 1986. During the 1990s, several studies showed an association between the use of avoparcin and the occurrence of VanA-type glycopeptide-resistant enterococci (GRE) in farm animals (1, 25, 26). To reduce human exposure to GRE, avoparcin was banned as a growth promoter in Denmark and Norway in 1995, in Germany the following year, and in all European Union countries in 1997. It was anticipated that GRE would revert to glycopeptide susceptibility in the absence of antimicrobial selection, due to the cost imposed by the resistance determinant on the fitness of the bacteria. Studies from Denmark, Germany, Italy, and The Netherlands have reported a decrease in the occurrence of GRE in animal production following the avoparcin ban (2, 6, 24, 31, 37). Data from the Norwegian monitoring program for antimicrobial resistance in the veterinary sector (NORM-VET) have shown that the proportion of GRE among enterococci in broiler meat samples and feces from broilers is not higher in Norway than in other European countries (3, 4). However, in a study performed 3 years after the avoparcin ban, GRE were detected in 99% of poultry fecal samples and in 18% of samples from farmers on Norwegian poultry farms previously exposed to avoparcin (8). Thus, although the concentration of GRE seemed to have been reduced, no significant reduction in the prevalence of GRE-positive flocks following the avoparcin ban was observed (8, 10). Another Norwegian study demonstrated that GRE persist in the farm environment even after depopulation and cleaning of the broiler houses (9). Interestingly, similar results have been reported from Denmark when comparable GRE isolation procedures were applied (18, 19). Heuer et al. observed no significant decrease in the proportion of GRE-positive poultry flocks 3 to 5 years after the avoparcin ban (18) and reported persistence of GRE in Danish poultry houses (19).
The avoparcin ban has given us the opportunity to monitor the population dynamics of GRE and the fate of antimicrobial resistance elements when the selective pressure is discontinued. Our recent study of GRE persistence on two Norwegian farms previously exposed to avoparcin concluded that the GRE population in poultry is heterogeneous with a common plasmid-borne Tn1546 element shared by distinct genetic lineages (21). It was suggested that Tn1546 might be stably maintained through physical linkage to a putative pRE25-related postsegregational killing (PSK) system (21). Open reading frames 18 and 19 of the 50-kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis have recently been suggested as structural and functional homologues of the ɛ/ζ PSK system from pSM19035 in Streptococcus pyogenes (29). PSK systems eliminate plasmid-free segregants and may thus promote the stability of linked plasmid-mediated VanA-type resistance.
The present report is an extended postavoparcin study of GRE in Norwegian poultry production. The aims were to investigate the prevalence of GRE colonization in poultry and poultry farmers 3 to 8 years after the avoparcin ban, as well as to study the molecular characteristics of GRE in poultry environments.
MATERIALS AND METHODS
Collection of samples.
Twenty-nine farms with GRE-positive poultry situated in the southeastern part of Norway were selected among the participants in the Norwegian study conducted in 1998 (8). From 1998 to 2003, the poultry farmers submitted fecal samples from themselves, as well as from their present poultry flock on five separate occasions: summer 1998, spring 2000 (poultry only), spring 2001 (humans only), autumn 2001, spring 2002, and winter 2002 to 2003. The poultry fecal samples were fresh feces collected from the floor at several different sites inside the poultry houses. The fecal samples collected in the 1998 study (8) were used as fecal samples from 1998 in the present study, but not enough poultry sample material from poultry was available from farm 399.
A total of 109 poultry fecal samples were included in the study. Thirteen farms had poultry samples included from all five sampling periods, six farms were sampled four times, three farms were sampled three times, four farms were sampled twice, and three farms were sampled only once. Nineteen farmers submitted fecal samples from all five sampling periods, one farmer submitted samples four times, two submitted samples three times, three submitted samples twice, and four farmers submitted a sample only once. Eleven poultry samples and eight human samples were not received due to discontinued poultry production on three farms during the study period. The samples were frozen at −80°C prior to analysis. Collection of human samples was approved by the Regional Committee for Medical Research Ethics, University of Tromsø, Tromsø, Norway.
Isolation, identification, and quantification of enterococci.
Fecal samples from broilers and turkeys were analyzed as described by Borgen et al. (8). Typical enterococcal colonies were counted on Slanetz and Bartley agar plates with or without vancomycin at 32 mg/liter. Viable cell counts were based on mean CFU numbers on two separate agar plates. Six enterococcal-like colonies from the Slanetz and Bartley agar plates with vancomycin of each sample were plated out on blood agar plates, and one of the six colonies was identified and selected for further analysis. The isolation procedure for human samples was optimized for glycopeptide-resistant Enterococcus faecium (GREF), as this is the GRE species of primary public health concern. Human fecal samples were enriched in Enterococcosel broth (Becton Dickinson, New Jersey) with vancomycin at 8 mg/liter at 37°C for 24 to 48 h, due to the low concentration of GRE. Positive cultures wereplated on cephalexin-arabinose-aztreonam agar plates with vancomycin at 8mg/liter as previously described (8). One arabinose-positive colony from each plate was selected for further analysis.
Identification of GRE.
Identification of presumptive GRE was made on the basis of typical colony morphology, Gram stain, absence of catalase production, and a positive pyrrolidonyl-β-naphthylamide hydrolysis test (Oxoid, Basingstoke, Hampshire, England). All isolates were identified to species level and examined for the presence of the vanA gene by PCR (15, 35). Isolates not identified as E. faecium by PCR were classified as Enterococcus spp. and were not selected for further molecular analysis.
Molecular analyses.
Plasmid DNA isolation, Southern blot hybridization, and PCR analyses were performed as previously described (21). Briefly, all 115 GREF isolates of animal and human origins, except 1 isolate lost during the study, were examined for the presence of a previously described Tn1546-plasmid DNA junction fragment and the putative pRE25 postsegregational killing system (21). The 422-bp Tn1546-junction fragment PCR amplicon covers the Tn1546 insertion site, and the 1,044-bp pRE25-PSK PCR product covers parts of open reading frames 18 and 19 of pRE25. Restriction cutting of plasmid DNA with PstI (Promega, Madison, WI) was performed on a selection of 78 isolates. All isolates from farms with at least one PSK-PCR-positive isolate were chosen. Southern blotting and hybridization with a vanY-vanZ probe and a pRE25 PSK probe were performed according to the method described by Sambrook et al. (33). The probes were labeled with the DIG Luminescent Detection kit (Roche Diagnostics, Basel, Switzerland).
To achieve better separation of the bands with a high-molecular-weight, PstI-restricted plasmid, DNA from 15 of the isolates was run on a pulsed-field gel. The samples were mixed with 6× loading dye and loaded as on an ordinary agarose gel, but they were run by using a CHEF-DR II apparatus (Bio-Rad Laboratories, Hercules, Calif.) for 14 h at 200 V, with pulse times increasing from 0.1 to 10 s.
MLST.
Multilocus sequence typing (MLST) was performed on a selection of 10 GRE and 10 glycopeptide-susceptible enterococci representing distinct pulsed-field gel electrophoresis (PFGE) types. MLST was performed as previously described by Homan et al. (20) and included the genes purK, adk, atpA, ddl, gdh, gyd, and pstS. An extended investigation of purK allele polymorphism was performed by sequencing the purK gene in an additional 21 poultry and 12 farmer GREF isolates. PCR products were purified with the QIAGEN PCR Purification kit (QIAGEN GmbH, Hilden, Germany), and sequencing reactions were performed with the BigDye Terminator kit (Applied Biosystems, Foster City, CA) and run on an ABI 3100 Avant machine (Applied Biosystems). Sequences were analyzed with SeqMan II software (DNASTAR, Inc., Madison, WI). The purK sequences were compared to 29 purK allele types in the MLST database at http://www.mlst.net/links/default.asp.
Statistical analyses.
GRE colonization in poultry and farmers was analyzed by the Mantel-Haenszel chi-square test for trends with a general estimating equation model adjusted for repeated measurements. The proportion of GRE among enterococci in poultry fecal samples was analyzed in a generalized linear model adjusted for repeated measurements. All analyses were performed using the SAS software package (SAS Institute, Inc., North Carolina), and statistical significance was defined as P values of <0.05.
RESULTS
Isolation, identification, and quantification of enterococci from poultry fecal samples.
GRE were isolated from 88.1% (96/109) of poultry fecal samples by direct selective plating. The distribution of positive samples is given in Table 1. A statistically significant decline in flock prevalence of GRE in poultry was observed in the period between 1998 and 2003 (for trends, P = 0.0138; unadjusted for repeated measurements) even though the data set was too incomplete for adjustment for repeated measurements. The 13 GRE-negative poultry fecal samples were distributed among nine farms, and >1 GRE-negative sample was obtained from only three farms. A total of 87.4% (83/95) of poultry GRE isolates were identified as E. faecium. The remaining 12 isolates were classified as Enterococcus spp.
TABLE 1.
Distribution and concentration of GRE in fecal samples from poultry and poultry farmers between 1998 and 2003
| Time of sampling | Poultry
|
Humans
|
||||
|---|---|---|---|---|---|---|
| No. of poultry flock samples | No. (%) of GRE-positive poultry flock samples | Mean concn of GRE (CFU/g) | Mean % of GRE among enterococci | No. of farmer samples | No. (%) of GREF-positive farmer samples | |
| Summer 1998 | 28 | 28 (100) | 5.6 × 106 | 3.2 | 29 | 8 (28) |
| Spring 2000 | 22 | 18 (82) | 7.9 × 106 | 4.6 | ||
| Spring 2001 | 22 | 7 (32) | ||||
| Autumn 2001 | 23 | 22 (96) | 3.2 × 106 | 2.2 | 24 | 6 (25) |
| Spring 2002 | 18 | 15 (83) | 3.1 × 106 | 0.8 | 21 | 6 (29) |
| Winter 2002-2003 | 18 | 13 (72) | 4.2 × 106 | 2.0 | 19 | 5 (26) |
Viable cell counts to determine the concentrations of GRE and the proportions of GRE in the entire enterococcal populations were performed for 94 of 95 GRE-positive poultry fecal samples. All farms except three were represented by two or more samples in the viable cell counts. The mean concentrations (CFU per gram) of GRE and the mean proportions of GRE relative to the total enterococcal populations on each occasion are presented in Table 1. There was no significant decline in the proportion of GRE among enterococci over time (for trends, P = 0.0777; adjusted for repeated measurements).
Isolation and identification of GREF from human fecal samples.
A total of 115 human fecal samples were included in the study. The numbers of human samples included from each sampling period are given in Table 1. GREF was isolated from a total of 27.8% (32/115) of farmer fecal samples. At the beginning of the study, 8 of the farmers were GREF positive and 21 were GREF negative. The distribution of GREF-positive samples is given in Table 1. There was no significant change in the prevalence of GREF over time (for trends, P = 0.8658; unadjusted for repeated measurements; P = 0.9931, adjusted for repeated measurements). Only one farmer was GREF positive in all five sampling periods. Three of the eight farmers positive for GREF in 1998 were GREF negative in both 2001 and 2002. On the other hand, GREF-positive fecal samples were obtained in 2001 or 2002 from 8 of the 21 farmers negative for GREF in 1998. Thirteen farmers were GREF negative throughout the study period, and 8 of these were sampled on all five occasions.
GREF-positive farmer samples corresponded to simultaneous GRE-positive poultry fecal samples on the same farms, with only two exceptions.
Molecular analyses of poultry and human GREF.
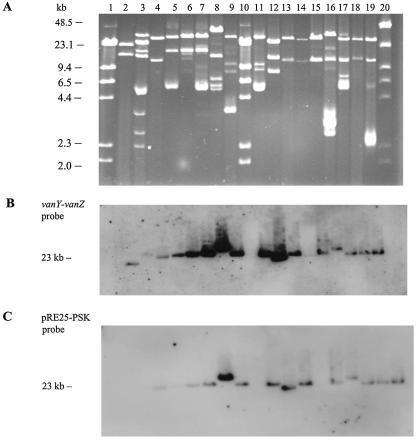
A total of 93.9% (108/115) of GREF isolates were Tn1546-junction fragment PCR positive, with 92.3% (77/83) of poultry and 96.8% (31/32) of farmer GREF isolates being positive. The presence of the putative pRE25-PSK system was shown by PCR in 20.1% (24/115) of GREF isolates in the study, 22.9% (19/83) and 15.6% (5/32) in poultry and farmer fecal isolates, respectively. However, by hybridization 68 of 70 (97.1%) of the selected isolates were PSK positive (Table 2). The two remaining isolates showed inconclusive results. Sixty-nine of the 70 isolates hybridized with a vanY-vanZ probe, and 1 isolate showed an inconclusive result. In 66 of the 68 PSK-positive isolates, both the vanY-vanZ and the PSK probes cohybridized to a fragment of approximately 23 kb, as illustrated in Fig. 1. In one human isolate (399/98/H) and one poultry isolate (31/98/A), the two probes hybridized to fragments of different sizes (data not shown). The hybridization of the pulsed-field gel confirmed that the vanY-vanZ and the PSK probes hybridized to the same fragment of approximately 23 kb.
TABLE 2.
Presence of a Tn1546 junction fragment and a putative PSK system in a selection of glycopeptide-resistant Enterococcus faecium strains isolated from poultry and poultry farmers between 1998 and 2003
| Time of sampling | Poultry
|
Humans
|
||||
|---|---|---|---|---|---|---|
| Tn1546 junction | PSK PCR | PSK hybridizationa | Tn1546 junction | PSK PCR | PSK hybridization | |
| Summer 1998 | 12/13 | 7/13 | 11b/11 | 4/4 | 2/4 | 4/4 |
| Spring 2000 | 11/12 | 2/12 | 11/11 | |||
| Spring 2001 | 5/5 | 0/5 | 3/5c | |||
| Autumn 2001 | 10/13 | 6/13 | 13/13 | 3/4 | 1/4 | 4/4 |
| Spring 2002 | 10/10 | 2/10 | 9/9 | 4/4 | 1/4 | 4/4 |
| Winter 2002-2003 | 10/10 | 1/10 | 6/6 | 3/3 | 1/3 | 3/3 |
| Total | 53/58 | 18/58 | 50/50 | 19/20 | 5/20 | 18/20c |
Eight isolates were not hybridized due to technical problems.
One isolate was hybridized to fragments of different sizes.
Two isolates showed inconclusive results.
FIG. 1.
PFGE of PstI-digested plasmid DNA from a selection of poultry and human GRE isolates (A), vanY-vanZ hybridization analysis (B), and pRE25-PSK system hybridization analysis (C). Lanes 1 and 10, lambda DNA/HindIII ladder (Promega, Madison, WI); lane 20, Low Range PFG Marker (New England Biolabs, Beverly, MA); lane 2, BM4147; lane 3, 399/F98/A4; lane 4, 399/F99/A9; lane 5, 58/S02/H; lane 6, 32/F02/H; lane 7, 58/F02/H; lane 8, 31/F01/H; lane 9, 58/F01/H; lane 11, 58/98/H; lane 12, 356/98/H; lane 13, 14/H02/A; lane 14, empty; lane 15, 14/V02/A; lane 16, 17/98/A; lane 17, 32/98/A; lane 18, 33/H01/A; lane 19, 58/H02/A.
MLST.
MLST showed a variety of different sequence types (STs), and eleven new STs were detected (Table 3). STs 8 and 195 were represented by a single GREF isolate on each farm, and STs 241 and 242 were found in two isolates on the same farm. Interestingly, ST 242 was found in one human sample and one poultry sample at the same sampling time. Most of the loci had one predominant allele. There was a pattern of lower variability of alleles among the resistant isolates than among the susceptible ones. In all GREF typed, four of the seven genes (purK, adk, ddl, and gyd) had identical alleles, whereas none of the genes in the glycopeptide-susceptible enterococci had identical alleles in all isolates. There also seemed to be greater allele variability among human than among poultry isolates, although there were variations in both groups. The DNA sequences of purK determined in the additional 21 poultry and 12 farmer GREF isolates all displayed purK type 6 (33/33).
TABLE 3.
Multilocus sequence types and allele numbers for 20 E. faecium strains isolated from poultry and poultry farmers on two farms in 1998 and 1999
| Farm | ST | Gene
|
Origin | Susceptibilitya | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| purK | adk | atpA | ddl | gdh | gyd | pstS | ||||
| 64 | ST 8 | 6 | 1 | 5 | 2 | 1 | 1 | 7 | Poultry | R |
| 64 | ST 26 | 6 | 1 | 9 | 2 | 1 | 1 | 1 | Human | R |
| 64 | ST 60 | 8 | 6 | 13 | 8 | 8 | 11 | 10 | Human | S |
| 64 | ST 146 | 6 | 1 | 3 | 2 | 6 | 1 | 1 | Poultry | R |
| 64 | ST 195 | 6 | 1 | 9 | 2 | 1 | 1 | 7 | Poultry | R |
| 64 | ST 242 | 6 | 1 | 9 | 2 | 7 | 1 | 16 | Poultry | S |
| 64 | ST 242 | 6 | 1 | 9 | 2 | 7 | 1 | 16 | Human | R |
| 64 | ST 246 | 6 | 1 | 9 | 2 | 1 | 1 | 20 | Human | S |
| 64 | ST 247 | 9 | 15 | 38 | 4 | 12 | 2 | 1 | Human | S |
| 64 | ST 248 | 6 | 1 | 5 | 2 | 13 | 1 | 7 | Poultry | S |
| 399 | ST 8 | 6 | 1 | 5 | 2 | 1 | 1 | 7 | Human | R |
| 399 | ST 9 | 6 | 1 | 5 | 2 | 6 | 1 | 7 | Poultry | R |
| 399 | ST 48 | 6 | 1 | 9 | 2 | 1 | 1 | 16 | Human | S |
| 399 | ST 194 | 6 | 1 | 4 | 2 | 7 | 1 | 7 | Human | R |
| 399 | ST 195 | 6 | 1 | 9 | 2 | 1 | 1 | 7 | Human | R |
| 399 | ST 241 | 6 | 1 | 9 | 2 | 13 | 1 | 7 | Poultry | R |
| 399 | ST 241 | 6 | 1 | 9 | 2 | 13 | 1 | 7 | Poultry | S |
| 399 | ST 243 | 6 | 1 | 9 | 2 | 7 | 7 | 1 | Human | S |
| 399 | ST 244 | 32 | 1 | 4 | 3 | 1 | 1 | 7 | Poultry | S |
| 399 | ST 245 | 6 | 1 | 9 | 2 | 1 | 1 | 36 | Poultry | S |
R, resistant to vancomycin; S, susceptible to vancomycin.
DISCUSSION
The present study demonstrates a high prevalence of GRE-positive poultry flocks and farmers on Norwegian poultry farms 3 to 8 years after the avoparcin ban. GRE were isolated from 88.1% of the poultry samples and GREF was isolated from 27.8% of the farmer samples collected from 29 Norwegian poultry farms formerly identified as having GRE-positive poultry in the period 1998 to 2003. Although still high, the prevalence of GRE at flock level gradually declined during the study period, whereas the prevalence of GREF among farmers was not significantly reduced. However, the results from the present study and the results from a previous study (21) indicate that the same GREF clones do not persist in the farmer samples, which suggests that the poultry GRE are constantly reintroduced in the farmers.
The quantitative GRE analyses showed that high concentrations of GRE persisted in the GRE-positive poultry samples throughout the study period. Mean GRE concentrations were never <1.0 × 106 CFU per g of poultry feces on any occasion, and the mean proportions of GRE relative to the enterococcal populations were between 0.8 and 4.6%. Hence, the proportion of GRE relative to the total enterococcal population has been stable in Norwegian poultry fecal samples between 1998 and 2003 (10, 26). These results are consistent with GRE concentrations and proportions in poultry fecal samples from Denmark and Belgium, 1 and 5 years after the abolishment of avoparcin, respectively (2, 12). In New Zealand, persistence of GRE has also been demonstrated 2 years after the use of avoparcin was discontinued in 2000 (28).
The purK and MLST data support the hypothesis that GREF has been transferred from poultry to farmer. purK type 6 has been associated with poultry GRE reservoirs (7), and this was the dominant allele in our study in both poultry and farmer isolates. Although allele variation was lower among GREF than among glycopeptide-susceptible E. faecium isolates, the results demonstrated several GREF sequence types, indicating that single GRE clones did not dominate on each farm. A previous study based on PFGE concluded that glycopeptide-susceptible E. faecium reservoirs were more heterogeneous than GREF reservoirs on the same farms (21). The present results are in accordance with these earlier findings.
The widespread occurrence of a common Tn1546-plasmid DNA junction fragment has recently been documented in heterogeneous GREF populations on two Norwegian poultry farms studied over a 1-year period (1998 to 1999) (21). In the present study, 93.9% (108/115) of GREF isolates were Tn1546-junction fragment PCR positive, with similar proportions for poultry and human isolates. Tn3-like transposons such as Tn1546 must be integrated on conjugative elements for horizontal transfer to occur; however, a hot spot for integration of Tn1546 has not been revealed (5). The widely disseminated Tn1546-junction fragment may thus represent evidence for successful horizontal spread of a specific plasmid mediated vanA-containing element, as previously suggested (21, 30, 38).
Experimental evidence has recently been presented suggesting segregational stability of vanA-containing plasmids as a mechanism for GREF persistence; a putative PSK system physically linked to Tn1546 may contribute to this effect (21, 22). Presence of the putative pRE25-PSK system was shown by PCR in 20.1% (24/115) of GREF isolates in this study. However, 97.1% (68/70) of the isolates were PSK positive by hybridization. Hybridization is a more robust method than PCR for the detection of variable gene sequences, as single point mutations in primer binding sites may reduce PCR efficiency, whereas probe hybridization is unaffected. The molecular basis for the discrepancy between PSK PCR and hybridization results remains to be determined. The hybridization results are in accordance with the theory that “plasmid addiction” systems may play a role in the persistence of GREF (21). However, parts of PSK genes may be found on plasmids without being functional, and the probes could bind to these partially deleted sequences (29, 34). The Axe-Txe proteic toxin-antitoxin system has been shown to be a functional plasmid segregational cassette in enterococci (16). Experimental studies are needed to determine whether the pRE25-PSK genes are functional in E. faecium.
An increasing body of evidence suggests that clinical E. faecium isolates worldwide belong to a distinct genetic lineage harboring the purK-1 allele (20, 27, 39) as opposed to E. faecium reservoirs of animal origin where other purK alleles predominate (20). We recently demonstrated the presence of purK-6 in 36 of 40 genetically distinct glycopeptide-resistant (n = 11) and -susceptible (n= 29) E. faecium strains isolated from two farmers and their poultry (21). The finding of purK-6 in 33/33 strains from farmers (n = 12) and poultry (n = 21) in the present study is in accordance with these earlier observations. An association between purK-6 and poultry has previously been suggested by Homan et al. (20). The hypothesis of distinct genetic lineages of human clinical isolates and GREF from the agricultural community is further supported by the finding of purK-1 in 80 of 102 Norwegian clinical E. faecium strains (23) and the clustering of 218 Norwegian E. faecium isolates from poultry feces, carcasses, and farm environments in a previously described poultry amplified fragment length polymorphism genogroup (11). Nevertheless, the public health importance of animal GRE reservoirs cannot be discarded, as enterococci are highly promiscuous in terms of horizontal gene transfer (32). Enterococci are considered the most likely source of VanA-type glycopeptide resistance in recently reported vancomycin-resistant Staphylococcus aureus isolates (13, 14, 36). In this context, the demonstrated persistence of glycopeptide resistance on Norwegian poultry farms is worrying, as a source of resistance determinants transferable to species and genetic lineages pathogenic to humans. However, the enterococci most likely to be responsible for the transfer to S. aureus (13, 14, 36) are enterococci of human clinical origin and not of poultry origin.
Implicit in the reasoning behind the European avoparcin ban was the assumption that GRE are less competitive than their glycopeptide-susceptible counterparts, due to the biological costs of harboring the resistance determinant. Removal of the selective antimicrobial pressure was therefore thought to reverse glycopeptide resistance in the agricultural community. However, these expectations seem to be hard to achieve in a short time, as documented by the persistence of a readily detectable GRE reservoir in Norwegian poultry 8 years after the avoparcin ban. Persistence due to a link between resistance to macrolides and glycopeptide resistance has previously been suggested (17), but this is not a likely explanation for the persistence of GRE in Norwegian poultry, since macrolides are not used in Norwegian poultry production.
Thus, the molecular mechanisms responsible for the stable maintenance of the plasmid encoded glycopeptide resistance determinants remain to be elucidated. However, our observational study strongly suggests a significant role of specific PSK systems in the persistence of genetically linked vanA-encoding transposon Tn1546. Plasmid maintenance determinants may also be a more general mechanism, enabling stable distribution and long-term persistence of antimicrobial resistance in reservoirs without apparent antimicrobial selective pressure.
Acknowledgments
We thank Gerhard Schaller for help with the collection of samples and Tom Wilsgaard for help with performance of the statistical analyses.
The work was supported by European Commission contract QLK2-CT-2002-00843 “ARTRADI” and the Helse-Nord Research Foundation.
REFERENCES
- 1.Aarestrup, F. M. 1995. Occurrence of glycopeptide resistance among Enterococcus faecium isolates from conventional and ecological poultry farms. Microb. Drug Resist. 1:255-257. [DOI] [PubMed] [Google Scholar]
- 2.Aarestrup, F. M., A. M. Seyfarth, H. D. Emborg, K. Pedersen, R. S. Hendriksen,and F. Bager. 2001. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 45:2054-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. 2002. NORM/NORM-VET 2001. Consumption of antimicrobial agents and occurrence of antimicrobial resistance in Norway. ISSN 1502-2307. [Online.] http://www.zoonose.no.
- 4.Anonymous. 2003. NORM/NORM-VET 2002. Consumption of antimicrobial agents and occurrence of antimicrobial resistance in Norway. ISSN 1502-2307. [Online.] http://www.zoonose.no.
- 5.Arthur, M., C. Molinas, F. Depardieu, and P. Courvalin. 1993. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 175:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bager, F., F. M. Aarestrup, M. Madsen, and H. C. Wegener. 1999. Glycopeptide resistance in Enterococcus faecium from broilers and pigs following discontinued use of avoparcin. Microb. Drug Resist. 5:53-56. [DOI] [PubMed] [Google Scholar]
- 7.Bonora, M. G., M. Ligozzi, M. De Fatima, L. Bragagnolo, A. Goglio, G. C. Guazzotti, and R. Fontana. 2004. Vancomycin-resistant Enterococcus faecium isolates causing hospital outbreaks in northern Italy belong to the multilocus sequence typing C1 lineage. Microb. Drug Resist. 10:114-123. [DOI] [PubMed] [Google Scholar]
- 8.Borgen, K., G. S. Simonsen, A. Sundsfjord, Y. Wasteson, O. Olsvik, and H. Kruse. 2000. Continuing high prevalence of VanA-type vancomycin-resistant enterococci on Norwegian poultry farms three years after avoparcin was banned. J. Appl. Microbiol. 89:478-485. [DOI] [PubMed] [Google Scholar]
- 9.Borgen, K., M. Sorum, H. Kruse, and Y. Wasteson. 2000. Persistence of vancomycin-resistant enterococci (VRE) on Norwegian broiler farms. FEMS Microbiol. Lett. 191:255-258. [DOI] [PubMed] [Google Scholar]
- 10.Borgen, K., M. Sorum, Y. Wasteson, and H. Kruse. 2001. VanA-type vancomycin-resistant enterococci (VRE) remain prevalent in poultry carcasses 3 years after avoparcin was banned. Int. J. Food Microbiol. 64:89-94. [DOI] [PubMed] [Google Scholar]
- 11.Borgen, K., Y. Wasteson, H. Kruse, and R. J. Willems. 2002. Vancomycin-resistant Enterococcus faecium (VREF) from Norwegian poultry cluster with VREF from poultry from the United Kingdom and The Netherlands in an amplified fragment length polymorphism genogroup. Appl. Environ. Microbiol. 68:3133-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butaye, P., L. A. Devriese, H. Goossens, M. Ieven, and F. Haesebrouck. 1999. Enterococci with acquired vancomycin resistance in pigs and chickens of different age groups. Antimicrob. Agents Chemother. 43:365-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 2004. Vancomycin-resistant Staphylococcus aureus—New York, 2004. Morb. Mortal. Wkly. Rep. 53:322-323. [PubMed] [Google Scholar]
- 14.Chang, S., D. M. Sievert, J. C. Hageman, M. L. Boulton, F. C. Tenover, F. P. Downes, S. Shah, J. T. Rudrik, G. R. Pupp, W. J. Brown, D. Cardo, and S. K. Fridkin. 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342-1347. [DOI] [PubMed] [Google Scholar]
- 15.Dutka-Malen, S., S. Evers, and P. Courvalin. 1995. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J. Clin. Microbiol. 33:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grady, R., and F. Hayes. 2003. Axe-Txe, a broad-spectrum proteic toxin-antitoxin system specified by a multidrug-resistant, clinical isolate of Enterococcus faecium. Mol. Microbiol. 47:1419-1432. [DOI] [PubMed] [Google Scholar]
- 17.Hasman, H., and F. M. Aarestrup. 2002. tcrB, a gene conferring transferable copper resistance in Enterococcus faecium: occurrence, transferability, and linkage to macrolide and glycopeptide resistance. Antimicrob. Agents Chemother. 46:1410-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heuer, O. E., K. Pedersen, J. S. Andersen, and M. Madsen. 2002. Vancomycin-resistant enterococci (VRE) in broiler flocks 5 years after the avoparcin ban. Microb. Drug Resist. 8:133-138. [DOI] [PubMed] [Google Scholar]
- 19.Heuer, O. E., K. Pedersen, L. B. Jensen, M. Madsen, and J. E. Olsen. 2002. Persistence of vancomycin-resistant enterococci (VRE) in broiler houses after the avoparcin ban. Microb. Drug Resist. 8:355-361. [DOI] [PubMed] [Google Scholar]
- 20.Homan, W. L., D. Tribe, S. Poznanski, M. Li, G. Hogg, E. Spalburg, J. D. van Embden, and R. J. Willems. 2002. Multilocus sequence typing scheme for Enterococcus faecium. J. Clin. Microbiol. 40:1963-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnsen, P. J., J. I. Osterhus, H. Sletvold, M. Sorum, H. Kruse, K. Nielsen, G. S. Simonsen, and A. Sundsfjord. 2005. Persistence of animal and human glycopeptide-resistant enterococci on two Norwegian poultry farms formerly exposed to avoparcin is associated with a widespread plasmid-mediated vanA element within a polyclonal Enterococcus faecium population. Appl. Environ. Microbiol. 71:159-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnsen, P. J., G. S. Simonsen, O. Olsvik, T. Midtvedt, and A. Sundsfjord. 2002. Stability, persistence, and evolution of plasmid-encoded VanA glycopeptide resistance in enterococci in the absence of antibiotic selection in vitro and in gnotobiotic mice. Microb. Drug Resist. 8:161-170. [DOI] [PubMed] [Google Scholar]
- 23.Jureen, R., J. Top, S. C. Mohn, S. Harthug, N. Langeland, and R. J. Willems. 2003. Molecular characterization of ampicillin-resistant Enterococcus faecium isolates from hospitalized patients in Norway. J. Clin. Microbiol. 41:2330-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klare, I., D. Badstubner, C. Konstabel, G. Bohme, H. Claus, and W. Witte. 1999. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb. Drug Resist. 5:45-52. [DOI] [PubMed] [Google Scholar]
- 25.Klare, I., H. Heier, H. Claus, R. Reissbrodt, and W. Witte. 1995. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol. Lett. 125:165-171. [DOI] [PubMed] [Google Scholar]
- 26.Kruse, H., B. K. Johansen, L. M. Rorvik, and G. Schaller. 1999. The use of avoparcin as a growth promoter and the occurrence of vancomycin-resistant Enterococcus species in Norwegian poultry and swine production. Microb. Drug Resist. 5:135-139. [DOI] [PubMed] [Google Scholar]
- 27.Leavis, H. L., R. J. Willems, J. Top, E. Spalburg, E. M. Mascini, A. C. Fluit, A. Hoepelman, A. J. de Neeling, and M. J. Bonten. 2003. Epidemic and nonepidemic multidrug-resistant Enterococcus faecium. Emerg. Infect. Dis. 9:1108-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manson, J. M., J. M. Smith, and G. M. Cook. 2004. Persistence of vancomycin-resistant enterococci in New Zealand broilers after discontinuation of avoparcin use. Appl. Environ. Microbiol. 70:5764-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meinhart, A., J. C. Alonso, N. Strater, and W. Saenger. 2003. Crystal structure of the plasmid maintenance system epsilon/zeta: functional mechanism of toxin zeta and inactivation by epsilon 2 zeta 2 complex formation. Proc. Natl. Acad. Sci. USA 100:1661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miele, A., M. Bandera, and B. P. Goldstein. 1995. Use of primers selective for vancomycin resistance genes to determine van genotype in enterococci and to study gene organization in VanA isolates. Antimicrob. Agents Chemother. 39:1772-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pantosti, A., M. Del Grosso, S. Tagliabue, A. Macri, and A. Caprioli. 1999. Decrease of vancomycin-resistant enterococci in poultry meat after avoparcin ban. Lancet 354:741-742. [DOI] [PubMed] [Google Scholar]
- 32.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 34.Schwarz, F. V., V. Perreten, and M. Teuber. 2001. Sequence of the 50-kb conjugative multiresistance plasmid pRE25 from Enterococcus faecalis RE25. Plasmid 46:170-187. [DOI] [PubMed] [Google Scholar]
- 35.Simonsen, G. S., M. R. Myhre, K. H. Dahl, O. Olsvik, and A. Sundsfjord. 2000. Typeability of Tn1546-like elements in vancomycin-resistant enterococci using long-range PCRs and specific analysis of polymorphic regions. Microb. Drug Resist. 6:49-57. [DOI] [PubMed] [Google Scholar]
- 36.Tenover, F. C., L. M. Weigel, P. C. Appelbaum, L. K. McDougal, J. Chaitram, S. McAllister, N. Clark, G. Killgore, C. M. O'Hara, L. Jevitt, J. B. Patel, and B. Bozdogan. 2004. Vancomycin-resistant Staphylococcus aureus isolate from a patient in Pennsylvania. Antimicrob. Agents Chemother. 48:275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van den Bogaard, A. E., N. Bruinsma, and E. E. Stobberingh. 2000. The effect of banning avoparcin on VRE carriage in The Netherlands. J. Antimicrob. Chemother. 46:146-148. [DOI] [PubMed] [Google Scholar]
- 38.Van der Auwera, P., N. Pensart, V. Korten, B. E. Murray, and R. Leclercq. 1996. Influence of oral glycopeptides on the fecal flora of human volunteers: selection of highly glycopeptide-resistant enterococci. J. Infect. Dis. 173:1129-1136. [DOI] [PubMed] [Google Scholar]
- 39.Willems, R. J., W. Homan, J. Top, M. Santen-Verheuvel, D. Tribe, X. Manzioros, C. Gaillard, C. M. Vandenbroucke-Grauls, E. M. Mascini, E. van Kregten, J. D. van Embden, and M. J. Bonten. 2001. Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet 357:853-855. [DOI] [PubMed] [Google Scholar]