Abstract
Throughout alcoholic fermentation, Saccharomyces cerevisiae cells have to cope with several stress conditions that could affect their growth and viability. In addition, the metabolic activity of yeast cells during this process leads to the production of secondary compounds that contribute to the organoleptic properties of the resulting wine. Commercial strains have been selected during the last decades for inoculation into the must to carry out the alcoholic fermentation on the basis of physiological traits, but little is known about the molecular basis of the fermentative behavior of these strains. In this work, we present the first transcriptomic and proteomic comparison between two commercial strains with different fermentative behaviors. Our results indicate that some physiological differences between the fermentative behaviors of these two strains could be related to differences in the mRNA and protein profiles. In this sense, at the level of gene expression, we have found differences related to carbohydrate metabolism, nitrogen catabolite repression, and response to stimuli, among other factors. In addition, we have detected a relative increase in the abundance of proteins involved in stress responses (the heat shock protein Hsp26p, for instance) and in fermentation (in particular, the major cytosolic aldehyde dehydrogenase Ald6p) in the strain with better behavior during vinification. Moreover, in the case of the other strain, higher levels of enzymes required for sulfur metabolism (Cys4p, Hom6p, and Met22p) are observed, which could be related to the production of particular organoleptic compounds or to detoxification processes.
Wine fermentation is a complex microbiological and biochemical process in which the yeast Saccharomyces cerevisiae plays a central role. Nowadays, the usual strategy to carry out wine production involves the inoculation of selected yeast cells into the wine must. This method affords a decrease in the lag phase, a quick and complete fermentation of the must, and an important degree of reproducibility in the final product (6, 20). Of the criteria proposed for the selection of the yeast strain to be inoculated (12, 13, 56, 57), the ability to conduct vigorous fermentation, the production of desirable flavors, and the resistance to stress conditions are among the most important.
Wine flavors result from a complex system of interactions among hundreds of compounds (30), many of them produced by yeast and bacteria in various biosynthetic pathways that are active during alcoholic and malolactic fermentations. The levels and activity of enzymes involved in these metabolic pathways therefore play a crucial role in determining the organoleptic properties of the final product.
Throughout wine production, yeast cells are affected by a plethora of stress conditions (3, 6). To properly carry out the whole process, they must detect and respond to these unfavorable growth conditions without significant viability loss (6). For this purpose, yeast cells have sensor systems to detect variations in the environmental conditions (osmolarity, temperature, pH, nitrogen and carbon starvation, chemical and physical agents, etc.). This sensing step is followed by the activation of signal transduction pathways, which results in changes in gene expression, synthesis of protective molecules, and/or modulation of the protein activity by posttranslational modifications or subcellular localization (16, 25, 40). Ultimately, modifications in the level or activity of yeast proteins will play an important role in the adaptation of the cell to different external conditions.
During the last few years, an important effort has been made to characterize gene expression profiles during vinification. For this purpose, analyses of particular subsets of genes (mainly related to stress response and carbohydrate metabolism) have been carried out (33, 36, 37, 39). The development of global analysis methodologies (DNA microarrays and two-dimensional electrophoresis combined with subsequent identification and characterization by mass spectrometry) has allowed a detailed analysis of changes in gene expression and protein levels at various time points during vinification (4, 31, 42, 50, 51). These studies have been carried out with a particular industrial strain adapted to vinification conditions, able to complete the process without any defect.
In our laboratory, we have been characterizing wine yeast strains from the physiological and molecular points of view. Recently, we demonstrated that some stress response genes in these strains show differential expression patterns depending on the behavior of the strains during vinification (58). In this paper, we present a comparison between the mRNA and protein profiles of two yeast strains with different fermentative behaviors at the time point corresponding to the entry into stationary phase, which correlates with the divergence in the fermentation profiles. Our results indicate changes in the mRNA and protein levels and, probably, posttranslational modifications of several proteins, some of them involved in stress response and metabolism. This information is important for a better understanding of the physiological differences between these strains during vinification and for identification of features that could contribute to the adequate adaptation of yeast cells to fermentation conditions.
MATERIALS AND METHODS
Yeast strains and growth conditions.
Experiments have been carried out with S. cerevisiae strains ICV 16 (Fermicru primeur; DSM) and ICV 27 (UCLM S-377; Springer Oenologie).
Microvinification experiments were carried out using synthetic must at 22°C following the experimental details described by Zuzuarregui and del Olmo (58). This synthetic must contains 200 g/liter of an equimolecular mixture of glucose and fructose and 300 mg/liter of assimilable nitrogen. Cells from overnight cultures were inoculated into the must at 5 × 106 cells/ml. Three independent vinifications were carried out with each strain.
Microvinifications were also carried out with natural musts of Bobal and Sauvignon blanc varieties supplied by the wineries Torre Oria and Carrascal (Requena, Valencia, Spain). Bobal must contained approximately 500 mg/liter of assimilable nitrogen, while in the case of Sauvignon must the amount was 200 mg/liter. In some experiments Sauvignon blanc must was supplemented with 300 mg/liter of nitrogen in the form of diammonium phosphate. For microvinifications with natural musts, active dry yeast cells were rehydrated at a 10% (wt/vol) concentration in 5% (wt/vol) glucose for 20 min at 37°C and were inoculated in a 1:50 proportion in the must, according to the instructions of the yeast manufacturer. This corresponds approximately to 2 × 108 viable cells/ml.
The evolution of the microvinification was determined by measurements of viability, sugar and nitrogen consumption (58), and ethanol production (11).
RNA analysis.
RNA was isolated and quantified as described previously (58).
For microarray analyses, cDNA preparation, labeling, and hybridization were carried out as described by Fazzio et al. (18). For hybridization the Cy3-Cy5 combinations were 16A-27A, 16C-27B, 27B-16B, 27B-16C, and 27C-16A, with A, B, and C representing three independent cultures of each strain. The intensity obtained in each channel for each pair of microarrays was normalized by the Lowess method (10), and the ratios of the five experiments were evaluated by a t test using the statistic package ArrayStat with a significance level of 95% and at least four replicates. The overrepresentation of categories containing functionally related genes in each strain was statistically analyzed using the “Function Associate” tool (http://llama.med.harvard.edu/cgi/func/funcassociate).
The expression of some genes was also analyzed by semiquantitative reverse transcription-PCR (RT-PCR). For cDNA preparation, 5 μg of RNA was incubated with 1 unit of DNase I in a total volume of 8.8 μl at 37°C for 30 min. Afterward, the instructions of the manufacturer of Superscript II were followed. For the amplification of the resulting cDNA, it was 20-fold diluted and 5 μl was used for a PCR carried out in a final volume of 15 to 25 μl containing primers (0.5 mM each), deoxynucleoside triphosphates (0.2 mM each), MgCl2 (3 mM), buffer, and 1 unit of DNA polymerase Biotaq (Bioline) from Thermus aquaticus YT-1. The reaction conditions were as follows: 1 cycle of 3 min at 94°C, 20 to 45 cycles of 1 min at 94°C, 1 min at the optimal primer hybridization temperature in each case, and 1 min at 72°C, and, finally, a 10 min cycle at 72°C. For ACT1, FBA1, ERG10, and AUS1, 20 amplification cycles were used; for SNQ2 and AGP1, 35 cycles were used; and in the case of MUP1 and ADR1, 40 and 45 cycles were used instead. Table 1 includes the sequences of the oligonucleotides used in these amplification reactions.
TABLE 1.
Oligonucleotides used for the RT-PCR analyses
| Oligonucleotide | Sequence |
|---|---|
| ACT1-1 | GGATCTTCTACTACATCAGC |
| ACT1-2 | CACATACCAGAACCGTTATC |
| FBA1-1 | TAAAGAGAAAGACCGGTGTCAT |
| FBA1-2 | GGGAGGAGAATAATGGTTCA |
| ADR1-1 | TCCCGTTGTTGACTTG |
| ADR1-2 | ACTTGACTGAGGATGC |
| MUP1-1 | CGAAAACGCTCCAAGA |
| MUP1-2 | CCTCACCAAAGACAGA |
| AGP1-1 | GTCTCTATACGAACTG |
| AGP1-2 | TCTAGTTCTTGAGCC |
| ERG10-1 | TATCGACTGCCAGAAC |
| ERG10-2 | CATTACAAATGGCGGC |
| AUS1-1 | TTCCTTGCTCACCTGCAA |
| AUS1-2 | CCCATTAAGGCGGTCAAA |
| SNQ2-1 | ACTGTGTACCCAACGT |
| SNQ2-2 | CATTGATCGCCTCTGA |
Protein analysis.
Samples were prepared as described by Hernández et al. (24). Analytical and micropreparative two-dimensional polyacrylamide gel electrophoresis was carried out as reported by Hernández et al. (24) with the following modifications. Volumes of 80 μg of protein for analytical gels and 1 mg for micropreparative gels were loaded. For the first dimension, the program values used were 500 V for 1 h, 500 to 2,000 V for 1 h, and 8,000 V for 6.2 h for analytical gels and 500 V for 1 h, 1,000 V for 1 h, 2,000 V for 1 h, 2,000 to 5,000 V for 2 h, and 8,000 V for 9 h for micropreparative gels. Preparative gels were silver stained according to the protocol described by Mann (45).
Silver-stained gels were digitalized using a computing densitometer (GS-690 imaging densitometer; Bio-Rad) and analyzed with MELANIE 3.0 software (Bio-Rad). By use of MELANIE 3.0 tools, protein spots were enumerated, quantified, and compared between different gels. Nine gels corresponding to three independent cultures of each strain were analyzed to perform the statistical analysis.
Mass spectrometry of protein spots was carried out as previously described (24). Monoisotopic peptide mass fingerprinting data obtained from matrix-assisted laser desorption ionization-time of flight (mass spectrometry) MALDI-TOF (MS) and MS/MS sequence analysis carried out using a MALDI-TOF-TOF mass spectrometer 4700 proteomics analyzer (Applied Biosystems, Framingham, MA) were used to search for protein candidates by use of the programs Mascot and ProFound (prowl.rockefeller.edu) and the SWISS-PROT/TrEMBL nonredundant protein database (www.expasy.ch/sprot).
Ergosterol determination.
Samples were obtained from vinifications carried out in synthetic and Sauvignon blanc musts. A volume of cells representing an optical density at 600 nm of 50 (approximately 5 × 108 cells) was used for each ergosterol extraction, which was carried out according to the method of Quail and Steven (38). For the quantification of the ergosterol present in the extracts, the spectrophotometric measurement at 282 nm was used (38) (the ɛ1% of ergosterol at 282 nm is 290).
RESULTS
Strain selection and behavior during vinification.
Two commercial wine yeast strains were selected for these experiments: ICV 16 and ICV 27. Electrophoretic karyotype, MET2 gene restriction fragment length polymorphism, and mitochondrial DNA analyses have indicated that these strains are different and correspond to S. cerevisiae (data not shown).
Selected parameters of the vinifications carried out with these strains in synthetic must are shown in Table 2. According to previous studies (57, 58), the behaviors of these strains in vinification are quite similar in terms of growth, numbers of viable cells, nitrogen consumption, ethanol production, and glucose consumption up to 6 days after inoculation of the cells into the must. In addition, the expression profiles of their stress response genes during this time are quite similar (58). For these reasons, these strains are very useful for comparisons of gene and protein expression. The most relevant difference between them is related to sugar consumption: after 6 days, an important decrease in the consumption rate was observed in strain ICV 27, which was unable to complete the vinification, leaving approximately 15 g/liter of residual sugar at the end of the process. Sluggish or stuck fermentations represent an important problem in wine production. Nowadays, some sweet wines (sparkling wines, for instance) are produced, but in these cases sweetening is achieved by the addition of sugar or, in most cases, partially fermented or unfermented grape juice (27).
TABLE 2.
Selected parameters of the vinifications carried out with ICV 16 and ICV 27 strains in synthetic must
| Parameter | Value for indicated strain and time pointa
|
|||
|---|---|---|---|---|
| ICV 16 (day 6) | ICV 27 (day 6) | ICV 16 (day 30) | ICV 27 (day 30) | |
| Sugar (g/liter) | 89-94 | 89-92 | 2-3 | 12-15 |
| Assimilable nitrogen (mg/liter) | 168-175 | 158-188 | 162-168 | 153-158 |
| Ethanol (% [vol/vol]) | 5.7-6.3 | 5.7-6 | 9.3-10.3 | 9.3-9.6 |
| CFU · 106/ml | 28-38 | 38-42 | 12-17 | 15-17.9 |
Values shown correspond to the interval found in at least three independent vinifications carried out with each one of the strains. A more detailed description of the variation of these parameters during the process can be found in the work by Zuzuarregui and del Olmo (58).
The transcriptomic and proteomic analyses were carried out using samples obtained in three independent experiments at 6 days after beginning the vinification. This time was selected because of the reduction in the rate of sugar consumption in strain ICV 27 at this point. Moreover, this time is coincident with entry into the stationary phase, which involves significant changes in gene expression. mRNA and protein expression profiles can help us to understand the reasons for or consequences of these changes.
Overall description of genes with differential expression in strains ICV 16 and ICV 27.
The microarray used in these experiments contained DNA fragments corresponding to 6,251 yeast open reading frames. The statistical analysis of the results was carried out using the Array Stat package; the analysis showed that 2,073 genes present statistically significant differences between the strains. Of these, 1,018 showed higher expression in ICV 16 than in ICV 27 and 1,055 were more expressed in ICV 27 than in ICV 16. Of those with levels of expression increased at least twofold, 196 genes were overexpressed in ICV 16 relative to ICV 27 and 227 in ICV 27 relative to ICV 16. This indicates that about 6.8% of the yeast genes show changes higher than twofold in the expression levels among strains.
The application of the “Function Associate” tool to the genes differentially expressed more than twofold for which the biological process is known (Table 3) shows that genes related to alcohol (in particular, carbohydrate) metabolism, telomere maintenance, and cell wall and sterol metabolism appear to be selectively more expressed in the ICV 16 strain. In contrast, a higher number of genes involved in drug response and transport processes was observed to be overexpressed in the ICV 27 strain.
TABLE 3.
Distribution of genes differentially expressed more than twofold in functional categories statistically overrepresented in each strain according to the function associate tool
| Strain and functional gene category | No. of genes expressed more than twofold in the category | Total no. of genes in the category | Pa |
|---|---|---|---|
| ICV 16 | |||
| Alcohol metabolism | 29 | 148 | 2.8e-16 |
| Glycolysis | 10 | 18 | 1.9e-11 |
| Telomerase-independent telomere maintenance | 7 | 13 | 3.1e-08 |
| Cell wall | 15 | 109 | 8.1e-07 |
| Sterol metabolism | 8 | 34 | 5.7e-06 |
| ICV 27 | |||
| ATPase activity coupled to ion transmembrane movement | 12 | 58 | 5.5e-07 |
| Sodium transport | 3 | 3 | 4.3e-05 |
| Drug response | 7 | 29 | 4.8e-05 |
| Amine transport | 8 | 42 | 8.5e-05 |
Single hypothesis one-sided P value of the association between the total number of genes and the genes expressed more than twofold in each category.
A detailed description of the genes with differential expression is shown in Table 4, in which more-specific categories are considered. For the remainder of the present study we chose to focus on a subset of interesting categories shown in this table: carbohydrate metabolism, nitrogen metabolism and transport, sterol metabolism and transport, and stimulus response.
TABLE 4.
Distribution detailed in categories of the genes differentially expressed more than twofold in strains ICV 16 and ICV 27
| Functional category | Gene (fold expression increase)a
|
|
|---|---|---|
| ICV 16 | ICV 27 | |
| Carbohydrate metabolism | ||
| Transport | HXT3 (7.1), HXT4 (2.4) | HXT5 (3.4) |
| Fermentation, glycolysis, and gluconeogenesis | PFK27 (4.9), ENO1 (3), ENO2 (3), FBA1 (2.8), ADH1 (2.8), TDH3 (2.5), ADH2 (2.5), TDH2 (2.4), TDH1 (2.3), GPM1 (2.3), PGI1 (2.3), PFK2 (2.1), ADH6 (2) | ADR1 (3.6), SIP4 (3.3), GUT2 (3.2), CAT8 (2.8), GPM3 (2.2), PCK1 (2.1) |
| Organic compound oxidation and respiration | ||
| Krebs cycle | LSC2 (2.6), CIT1 (2.5) | YJL045W (7.5) |
| Acetyl-CoA metabolism and β oxidation | ADR1 (3.6), ACS1 (3.1), CRC1 (3), PEX10 (2.6), CTA1 (2.5), AGP2 (2.1) | |
| ATP synthase | NCA3 (2.6), NCA2 (2.1) | |
| NADH oxidation | NDE2 (3.7), GUT2 (3.2) | |
| Others | ISF1 (2.6), BCS1 (2.1), HAP1 (2) | |
| Pentose phosphate pathway | TKL1 (3.2) | |
| Others | GRE3 (2.1), DOG2 (2.1), PGM2 (2.1) | |
| Nitrogen | ||
| Transport | TPO2 (3.5), PTR2 (3.1) | MEP1 (14.7), AGP1 (9.8), MUP1 (4.1), SAM3 (3.6), GAT1 (3.3), GNP1 (3.3), PTK1 (2.7), DUR3 (2.3), AGP2 (2.1), AVT6 (2) |
| Metabolism | SFA1 (13), YMR226C (2.3), SAH1 (2), BAT1 (2), LEU2 (2) | GAT1 (3.3), GLT1 (3.1), DUR1,2 (2.3) |
| Esterols | ||
| Transport | AUS1 (3.1), DAN1 (2.4), PDR11 (2) | |
| Metabolism | CYB5 (6.2), IDI1 (2.5), ERG10 (2.5), ERG13 (2.3), ERG20 (2.3), ERG26 (2.3), ARE2 (2.2) | |
| Genes related to oxygen presence/absence | ANB1 (3.9), HYP2 (3.6), ROX1 (3.5), AHP1 (2.8), TSA1 (2) | SRX1 (7.8), CTA1 (4.2), SNQ2 (4.1), YAP1 (2.1), HAP1 (2) |
| Ion transport and cellular homeostasis | FTR1 (3.4), PMA1 (3.2), VCX1 (2.9), AHP1 (2.8), CUP9 (2.7), TSA1 (2) | ENA2 (6.9), ENA5 (6.3), ENA1 (6.2) |
| Stimulus response | ||
| Drugs | RDS1 (4.1), PDR16 (2.2) | PDR10 (8.5), SNQ2 (4.1), PDR5 (3), PDR15 (2.7), YOR1 (2.5), SNG1 (2.5), PDR1 (2.1) |
| Stress | SSA4 (3.9), UBC4 (2.8), YHB1 (2.1), MSN4 (2.4), RVS161 (2.3), HSP26 (2.3) | XBP1 (4.5), SIP18 (3.8), SSA3 (3.3), RDH54 (2.9), RAD55 (2.5) |
| Temperature | SPL2 (3) | |
| Pheromone | HBT1 (5.1), CSN9 (3.2), FUS3 (3.2) | |
| Cellular signalling | FUS3 (3.2), TPK1 (2.7), IRA2 (3.3) | |
| Oxidation | SRX1 (7.8), CTA1 (4.2), SNQ2 (4.1), OYE3 (2.7), GSH1 (2.3), YAP1 (2.1) | |
| DNA metabolism and changes | YCL074W (4.8), YER138C (2.5), YML039W (2.4), YFL002W-A (2.4), YRF1 (2.4-2.5), YMR050C (2.2) | |
| Meiosis, mitosis, and cell wall organization and biogenesis | MUC1 (6.1), PIR3 (2.8), GAS1 (2.7), PIR2 (2.5), TIP1 (2.5) | FMP45 (6.8), FLO9 (5.9), FLO5 (5.1), ZIP2 (4.5), ECM12 (4.1), MPS2 (3.9), MCM16 (3.7), FLO1 (3.4), SPS22 (3.3), ECM5 (3.1), RDH54 (2.9), SPC24 (2.9), CSM2 (2.6), RAD55 (2.5), KTR7 (2.5), NRG2 (2.5), ECM8 (2.4), SPO22 (2.2), YBL009W (2.2), CTF19 (2.2), SMK1 (2.2), RCK2 (2.1), DST1 (2) |
| Others | ||
| Proteolysis, autophagy, and vacuolar processes | YPS1 (3), UBC4 (2.8) | ATG15 (2.7), CVT17 (2.7), MON1 (2.6) |
| Nucleic acid biosynthesis | SRL1 (2.5), AAH1 (2.4), NPT1 (2.3), BNA6 (2.3), URA10 (2.2) | URA2 (3.9) |
| RNA processing and modification | REX3 (3.2) | TAD2 (2.8), PTA1 (2.6) |
| Fatty acid metabolism and transport | SAC1 (2.6), ELO1 (2.5), DPL1 (2.5), SCS7 (2.3), YPC1 (2.1) | ADR1 (3.6), SFH5 (3.1), CRC1 (3), PEX10 (2.6), PEX10 (2.6), CTA1 (2.5), PXA1 (2.4), AGP2 (2.1) |
| Protein modification | SDS3 (3.4), MNN5 (2.8), KNS1 (2.5), SMK1 (2.2), RCK2 (2.1) | |
| Others | PRM7 (6), SCM4 (3.2), CLN3 (2.5) | SLY41 (3.6) |
The average level obtained from at least four different experiments is shown in parentheses.
Comparison of the expression of genes involved in carbohydrate metabolism between strains ICV 16 and ICV 27.
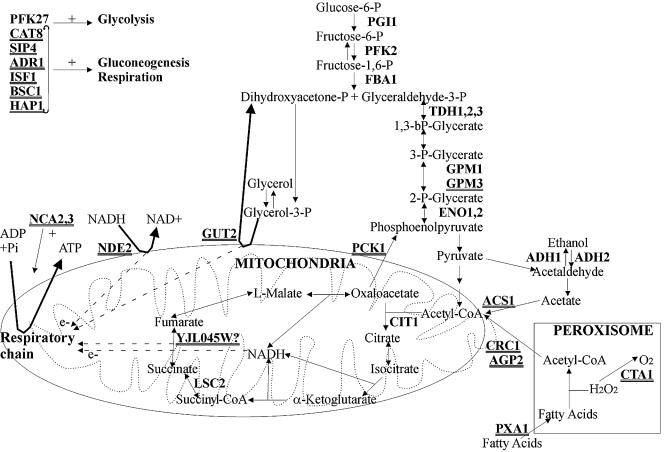
According to the information shown in Table 4, a large proportion of genes related to alcohol and carbohydrate metabolism are more expressed in strain ICV 16, whereas the opposite occurs in the case of some genes that participate in the oxidation of organic compounds and in gluconeogenesis (Fig. 1).
FIG. 1.
Overall scheme of the metabolism with indication of the genes with differential severalfold expression levels higher than 2 in strains ICV 16 and ICV 27 (underlined).
Almost all genes involved in the glycolytic pathway that appear in our analysis are more highly expressed in ICV 16. Among them, we find genes encoding fructose bisphosphate aldolase, enolase, gliceraldehyde-3-phosphate dehydrogenase, phosphoglycerate mutase, phosphoglucose isomerase, the β subunit of phosphofructokinase, and 6-phosphofructo-2-kinase, with differential severalfold expression levels ranging between 2.1 and 4.9. Although some of these enzymes catalyze reversible steps in glycolysis, the detection of activators of this pathway and the genes ADH1, ADH2, and ADH6, involved in fermentation, could indicate a better disposition for a fermentative metabolism in strain ICV 16, at least in terms of gene expression.
In the case of ICV 27, higher mRNA levels of genes that act as transcription activators of the gluconeogenic pathway (CAT8, SIP4, and ADR1) were observed. In addition, the expression of the genes encoding the enzymes that catalyze the two irreversible steps in this pathway (PCK1 and FBP1) shows levels respectively 2.1 and 1.4 times higher in this strain (data not shown). On the other hand, several genes related to the tricarboxylic acid cycle are more highly expressed in this strain. Interestingly, genes YJL045W (similar to succinate dehydrogenase gene SDH1) and SDH2 show respective expression levels 7.5 and 1.7 times higher than those seen with ICV 16. It has been reported that during fermentation this cycle is not active, but two branches are functional (one oxidative from pyruvate to at least α-cetoglutarate and one reductive in the opposite sense), and there is a complete inhibition of the succinate dehydrogenase complex (8). Moreover, in ICV 27 there are higher mRNA levels of genes involved in the mechanisms for directing cytosolic NADH to the mitochondrial respiratory chain (NDE2 and GUT2) and, although to a lesser extent, in acetyl-coenzyme A (CoA) metabolism and transport, β oxidation, regulation of ATP synthase activity, and cytochrome organization.
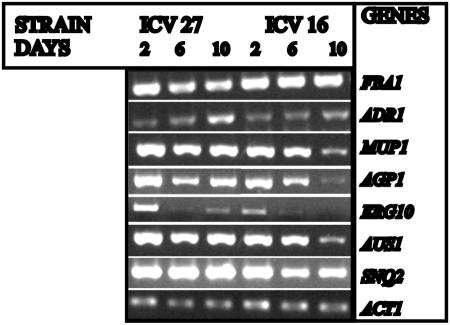
To confirm the results obtained with microarray analysis by an alternative molecular approach, semiquantitative RT-PCR was carried out for the FBA1 (glycolytic) gene and ADR1 gene (involved in regulation of gluconeogenesis and respiration). In this experiment we also included other time points during vinification to understand the changes in the expression of these genes throughout the process. The results (Fig. 2) are consistent with the microarray data obtained at day 6. Moreover, the expression profiles throughout vinification shown in Fig. 2 indicate that the expression of the FBA1 gene is lower in strain ICV 27 at about 6 days of vinification, when cells enter into stationary phase, than at 2 days. In the case of ADR1, the mRNA levels slightly increase at 6 days in strain ICV 27 and this is followed by a stronger change on the following days, whereas in ICV 16, an increase is only detected after 10 days of vinification.
FIG. 2.
Semiquantitative RT-PCR. The experiment was carried out by amplification of the cDNA obtained at the time points during vinification indicated in the figure with the specific oligonucleotides for each gene listed in Table 1. ACT1 was used as control. Experimental details are described in Materials and Methods.
Differential expression of genes involved in transport and metabolism of nitrogen compounds.
A statistically significant category overexpressed in ICV 27 corresponds to genes involved in amine, polyamine, and amino acid transport. We detected higher expression levels in this strain at the point of vinification considered for the genes encoding Agp1p (a wide-range permease), Mup1p (a high-affinity methionine permease), Gnp1p (a high-affinity glutamine permease), Sam3p (an S-adenosyl-methionine permease), Mep1p (an ammonia permease), and Gat1p, a regulator that controls the transcriptional activity of genes regulated by nitrogen catabolite repression. In fact, many of the genes of this category are controlled by this regulatory mechanism.
In the case of ICV 16, only a few genes involved in particular steps of the metabolism of several amino acids appear to be upregulated compared to strain ICV 27 results.
Semiquantitative PCR with MUP1 and AGP1 (Fig. 2), two genes more highly expressed in strain ICV 27, confirms the results from the microarray analysis. Besides, expression analysis of MUP1 throughout the vinification indicates that mRNA levels of this gene in both strains are similar at the beginning of vinification (day 2) but decrease in strain ICV 16 as the fermentation progresses. In the case of AGP1, the mRNA levels decrease in both strains during vinification, but this effect is stronger in ICV 16.
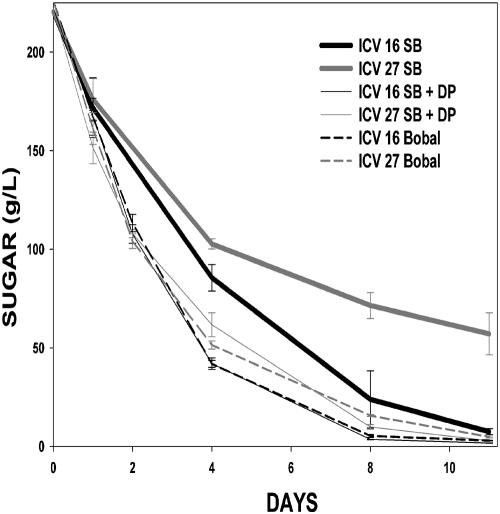
The higher expression of genes involved in nitrogen transport regulated by nitrogen catabolite repression in ICV 27 suggests that this strain is sensing nitrogen limitation at this point. However, according to the evolution of nitrogen consumption during the vinification, nitrogen is not completely consumed (Table 1) and 6 days after inoculation the amount of total nitrogen is still about 168 mg/liter. In fact, experiments carried out with natural musts containing different amounts of nitrogen (Fig. 3) indicate that when the available nitrogen amounts are similar to those found in the synthetic must used in our experiments (Sauvignon blanc must), strains ICV 16 and ICV 27 show the differences in fermentative behavior described so far. In spite of this, when musts with significantly higher nitrogen amounts are used (Bobal must or supplemented Sauvignon must) both strains behave in the same way and are capable of completing the vinification without significant differences.
FIG. 3.
Vinifications were carried out in natural musts (Bobal, Sauvignon blanc [SB], and Sauvignon blanc supplemented with 300 mg N/liter as diammonium phosphate [SB + DP]) inoculated with rehydrated dry active yeast cells according to manufacturer's instructions. Vinifications were carried out at 22°C as described in Materials and Methods. Bobal must contained 500 mg/liter of assimilable nitrogen and Sauvignon must 200 mg/liter.
Differential expression of genes involved in sterol transport and metabolism.
Many of the genes encoding enzymes that participate in ergosterol biosynthesis show higher mRNA levels in strain ICV 16. Some of them (ERG10 and IDI1) appear in Table 4 because the differences in levels between strains exceed twofold, but some others (ERG13, ERG20, ERG26, ERG9, and ERG4) show severalfold variations ranging between 1.4 and 2. Ergosterol is the main sterol in the S. cerevisiae plasma membrane. It has been shown that fermentative efficiency and ethanol resistance are usually related to the increase in the ratio of ergosterol to phospholipids and to the decrease in the fatty acid saturation index (9, 44). Previous results obtained with these strains indicate that, at least under some growth conditions, ICV 16 displays higher ethanol resistance than ICV 27 (57).
In strain ICV 27, however, a few genes related to sterol transport, namely, AUS1, DAN1, and PDR11, are overexpressed, with severalfold differences of 3.1, 2.4, and 2 relative to ICV16 results.
Semiquantitative RT-PCR was carried out with ERG10 (ergosterol biosynthesis) and AUS1 (ergosterol transport) and confirmed the information from the microarray analysis. As shown in Fig. 2, ICV 27 shows higher ERG10 mRNA levels at day 2, but the mRNA is almost undetectable at day 6, whereas in ICV 16 some amounts are still found. Regarding AUS1, there is also a decrease in the expression level between 2 and 6 days and, especially in ICV16, between days 6 and 10.
Determinations of ergosterol levels in samples obtained from these vinifications indicate differences among strains that correlate with the gene expression data (Table 5). This correlation is more significant when we consider the results at the beginning of the vinification, when ergosterol (Table 5; 6 h) and mRNA levels of genes involved in the metabolism of this lipid (ERG10 in Fig. 2) are higher. Similar results were obtained in vinifications with Sauvignon blanc natural must inoculated with active dry yeast cells at the time point of vinification corresponding to the entry into stationary phase (data not shown).
TABLE 5.
Ergosterol determination in vinifications carried out in synthetic must
| Strain | μg/OD600 unit at indicated time pointa
|
||
|---|---|---|---|
| 6 h | Day 6 | End | |
| ICV 16 | 1.6 ± 0.26 | 0.67 ± 0.07 | 0.5 ± 0.07 |
| ICV 27 | 1.023 ± 0.05 | 0.52 ± 0.06 | 0.55 ± 0.01 |
Values were obtained from at least three independent experiments. Standard deviation values are included.
Differential expression of genes related to stimulus response.
An important number of genes involved in stimulus response show differences in mRNA levels among these strains, and in most cases the levels are higher in strain ICV 27. We find, for instance, differential expression of some genes (PDR10, PDR5, PDR15, SNQ2, YOR1) that participate not only in multidrug resistance (54) but also in other physiological functions, such as the transport of weak acids or glutathione-sulfur compound conjugates (55).
In the subcategory of stress response, higher expression levels are found in strain ICV 27 for some genes involved in DNA repair (RAD55 and RDH54), desiccation and osmotic stress (SIP18), transcriptional regulation (XBP1), and temperature response (SSA3 and SPL2). However some stress response genes display higher mRNA levels in strain ICV 16; among them we detect some genes involved in protein degradation (UBC4) and others encoding heat shock proteins (SSA4 and HSP26).
One interesting subcategory of genes overexpressed in strain ICV 27 contains a large number of oxidative stress response genes (SRX1, CTA1, SNQ2, OYE3, and YAP1 with differential severalfold expression levels higher than 2 but many others—including SKN7, the other main regulator of the response to this form of stress—with values ranging between 1.5 and 2). Besides, HAP1 and HAP2 genes, the principal regulators of the yeast response to oxygen, show levels 2- and 1.9-fold higher (data not shown), respectively, in ICV 27. It is difficult to understand the variations in the expression of these genes under vinification conditions, but this result may have a link with the higher expression in ICV 27 of genes that participate in organic compound oxidation and respiration.
Taken together, our data suggest that, at this point of vinification, strain ICV 27 may be developing mechanisms of detoxification, such as the multidrug response or the destruction of reactive oxygen species. Semiquantitative PCR experiments with the SNQ2 gene (involved in response to oxidative stress and in multidrug resistance) confirm the results obtained in the microarray analysis (Fig. 2) and indicate that in ICV 27, the maximal expression is detected at day 6. In the case of strain ICV 16, the expression levels of this gene are lower at days 6 and 10.
Proteomic analysis reveals that some proteins related to carbohydrate and sulfur metabolism and stress response display differential levels in strains ICV 16 and ICV 27.
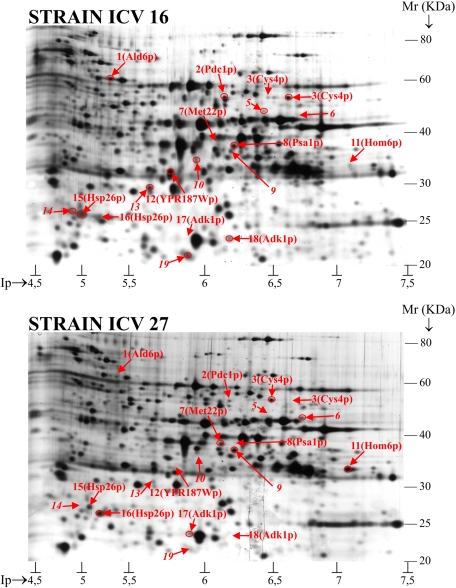
In order to investigate whether the differences observed between these strains regarding gene expression are reflected in protein levels, whole-cell protein extracts were prepared from the same samples and analyzed by two-dimensional gel electrophoresis, as described in the Materials and Methods section. Fig. 4 shows the reference two-dimensional gel and the protein profile for each of the strains. Each protein sample was analyzed in at least three independent gels. Spots for which differential expression was detected in at least eight gels are indicated with a number.
FIG. 4.
Two-dimensional gel (immobilized pH gradient strip [pH 3 to 10], 10% total acrylamide concentration, silver staining) showing the 19 differentially expressed spots in two strains of S.cerevisiae at the entry of stationary phase during vinification. Panel A shows the reference gel of strain ICV 16 and Panel B the corresponding gel of strain ICV 27. The identified proteins are annotated in the gel by their protein names according to the Saccharomyces genome database. Nonidentified proteins are indicated solely with an italic number. Spots representing higher expression levels in one strain are circled. Mr, molecular mass.
A total of 19 spots with consistent differential expression levels between strains were found. Of these spots, 13 were identified by MALDI-TOF-TOF (MS) and correspond to 10 different proteins. This number represents about 4% of the total number of spots found in the gels (approximately 450). Table 6 contains information about the spots with differential expression among strains.
TABLE 6.
List of spots representing differential expression results for strains ICV 16 and ICV 27 at the entry of stationary phase during vinification
| IDa | Proteinb | Expt Mr/Lit Mr (kDa)c | Expt pI/Lit pIc | % Covd | No. of peptidese | Differential expression (fold)f | Locationg | Posttranslational modification(s)h | Function |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ald6 | 60/54 | 5.2/5.3 | 22 | 10 | +16 | C, N, M, V | Ac | Cytosolic aldehyde dehydrogenase |
| 2 | Pdc1 | 54/61 | 6.2/6 | MS/MS | +16 | C, N | Ac+, Carb+ | Pyruvate carboxylase | |
| 3 | Cys4 | 54/56 | 6.6/6.5 | 15 | 8 | ++27 | C, M | Cystathionine-β-synthase | |
| 4 | Cys4 | 54 | 6.7 | 23 | 13 | 16 | C, M | Cystathionine-β-synthase | |
| 5 | NI | 54 | 6.6 | 16 | |||||
| 6 | NI | 47 | 6.8 | 27 | |||||
| 7 | Met22 | 37/39 | 6.1/6 | 37 | 14 | 27 | C, N | Ac | 3′-(2′),5′-Bisphosphate nucleosidase |
| 8 | Psa1 | 37/40 | 6.3/6.1 | 34 | 11 | 16 | C | Carb+ | Mannose-1-phosphate guanilyl transferase |
| 9 | Qcr2 | 36/39 | 6.2/6.2 | 26 | 10 | +27 | M | Carb+ | Ubiquinol-cytochrome c reductase |
| 10 | NI | 34 | 6.0 | 16 | |||||
| 11 | Hom6 | 33/38 | 7.1/7.2 | 18 | 7 | 27 | C, N, M | Ac | Homoserine dehydrogenase |
| 12 | YPR127w | 32/38 | 5.8/5.8 | 44 | 19 | 16 | C, N | Ac | Similar to S. pombe piridoxal reductase |
| 13 | NI | 30 | 5.6 | 16 | |||||
| 14 | NI | 25 | 5.0 | 16 | |||||
| 15 | Hsp26 | 25/24 | 5.1/5.4 | 42 | 8 | ++16 | C, N | Ac+, P+ | Molecular chaperone involved in stress response |
| 16 | Hsp26 | 25 | 5.2 | 42 | 7 | +27 | C, N | Ac+, P+ | Molecular chaperone involved in stress response |
| 17 | Adk1 | 23/24* | 5.9/6* | 55 | 14 | 16 | C, M | Ac+ | Adenylate kinase |
| 18 | Adk1 | 23/24 | 6.2/6.2 | MS/MS | 27 | C, M | Ac+ | Adenylate kinase | |
| 19 | NI | 21 | 5.9 | +16 |
ID, identification number of the spots in two-dimensional gel.
Protein name according to the Saccharomyces genome database (www.yeastgenome.org). NI, protein not identified.
Mr, molecular mass; Expt, value obtained experimentally (melanie 3.0); Lit, value obtained from the literature (Proteome package). An asterisk (*) indicates the values of the mature form (after the N-terminal methionine excision, if it occurs). In the case of Adk1p, the values corresponding to both immature and mature form (after Met-Ser excision and N-terminal acetylation) are shown.
Percentage of the amino acid sequence coverage for the identified proteins. MS/MS, proteins were analyzed by tandem mass spectrometry using MALDI-TOF/TOF MS.
Numbers of peptide masses matching the top hit from MS-Fit protein motive force are shown.
Differential severalfold expression of the spots in the strains. Relative values from the comparison of five different gels are indicated by + and ++ (+ indicates levels of protein approximately between two- and fivefold greater in one strain than in the other. ++ indicates differential severalfold expression levels approximately higher than 5). When no symbol is shown the spot only appears in the strain indicated.
Subcellular location. M, mitochondria; C, cytoplasm; N, nucleus; V, vacuol. Data obtained from the Proteome package.
Posttranslational modifications. Ac, N-terminal acetylation; Carb, carbonylation; P, phosphorylation. A superscript plus indicates that the modifications were reported in the literature. Data obtained from the Proteome package.
Several proteins involved in carbohydrate metabolism are differentially expressed in the strains ICV 16 and ICV 27: Ald6p (the major cytosolic aldehyde dehydrogenase enzyme, largely responsible for acetate formation from glucose during alcoholic fermentation) (53), Pdc1p (involved in the alcoholic fermentation and cytosolic acetyl-CoA generation), and Qcr2p (ubiquinol-cytochrome c reductase core protein 2, component of the cytochrome bc1 complex in the mitochondrial respiratory chain). In the case of Ald6p and Pdc1p the levels are higher in strain ICV 16, suggesting a more active fermentation in this strain. As described above, at 6 days, this strain continues metabolizing glucose actively, while in the case of strain ICV 27 the rate of glucose consumption decreases at this time point. In the case of Pdc1p, the relevance of this finding is not clear, as the isoform detected corresponds to a minor form of this protein, probably due to vacuolar degradation (50). In the case of Qcr2p, the higher levels found in strain ICV 27 could be indicative of a more active respiratory metabolism in this strain.
In this work, we have found three protein species related to sulfur amino acid metabolism (49) that appear more abundant in strain ICV 27. The first one is Cys4p, cystathionine-β-synthase, which catalyzes an intermediate step in the synthesis of cysteine and the conversion of serine and homocysteine in cystathionine. The second one is Met22p (Hal2p), involved in methionine biosynthesis, whose activity with respect to nucleotide phosphatase [3′-(2′),5′-bisphosphate nucleosidase] allows fine tuning of 3′-phospho-5′-adenylylsulfate (PAPS), an extremely toxic intermediate in the formation of sulfite from sulfate (43). Finally, Hom6p encodes homoserine dehydrogenase, an enzyme involved in the biosynthesis of homoserine, a precursor of homocysteine. It is worth mentioning that Cys4p requires pyridoxal phosphate for its activity, and another protein identified in our studies, YPR127w, shows similarity to the Schizosaccharomyces pombe pyridoxal reductase, thus establishing a possible link between these two proteins. In the case of Cys4p, two isoforms have been detected in this work, one more abundant in strain ICV 27 and the other present only in strain ICV 16 (Table 6). As this protein is located in both the mitochondria and cytosol, these two isoforms could be related to the different subcellular locations of the protein species. Taken together, these results suggest that the pathway of sulfur assimilation into homoserine, homocysteine, cysteine, and methionine (Fig. 5) is more active in strain ICV 27.
FIG. 5.
Schematic diagram of the sulfur fixation and sulfur amino acids metabolism in S. cerevisiae showing the involvement of several proteins with differential expression in the strains studied in this work. Data were obtained from the Kegg pathway database (www.genome.ad.jp/kegg/pathway.html).
Two stress-related proteins (Hsp26p and the product of the gene YPR127w) were identified as differentially expressed in strains ICV 16 and ICV 27. Hsp26p is a molecular chaperone (23) induced under many sets of stress conditions (7, 14, 22, 34, 37, 46). In our experiments, two protein species of Hsp26p with different isoelectric points appear differentially expressed and each is more abundant in a different strain. Several posttranslational modifications that may affect protein activity determine changes in the isoelectric point of proteins. As phosphorylation sites have been previously detected in Hsp26p (19) and as phosphorylation is predicted to lower the isoelectric point, the isoform more abundant in ICV 16 could correspond to a protein species with a higher degree of phosphorylation. This difference could be important in the regulation of signal transduction pathways (26). Considering together the two isoforms differentially expressed, higher levels of this protein are detected in strain ICV 16, which is in accordance with the differences previously reported between these strains (58) in the steady-state mRNA levels observed for this gene at the time point of the vinification considered. The YPR127w gene encodes an uncharacterized protein sharing a certain similarity (approximately 25% identity) with the products of YPL088w (putative aryl-alcohol dehydrogenase) and YJR096w (similar to aldo-keto reductases). Global expression analyses have indicated an induction in the expression of this gene by nitrogen starvation and entry into stationary phase (21), although no further evidence about the relevance of this protein in stress responses is available.
Finally, two other proteins with differential levels among strains have been detected. One of them is Psa1p (mannose-1-phosphate guanilyl transferase), involved in glycosylation and cell wall biogenesis, which is only detected in strain ICV 16. The other one is Adk1p. Each strain contains one isoform of this protein, which acts as an adenylate kinase (GTP-AMP phosphotransferase). This enzyme performs the important function of catalyzing the rapid return of the adenine nucleotide pool to equilibrium, by the reutilization of AMP for the synthesis of ADP, and mutants lacking the ADK1 gene product are considerably impaired in growth (5, 29). The two protein species detected could correspond to allelic variations, as shown previously for various strains (1), to different subcellular location, or to the presence or absence of N-terminal acetylation, a modification that has been described for this protein and may be important for the activity or stability of some proteins under particular conditions (32, 35, 47, 48).
DISCUSSION
Global analyses of gene expression and protein profiles have become a powerful tool in understanding how cells respond to changing environments. Wine fermentation is clearly one example of a process in which yeast cells have to adapt to significant variations throughout the whole process.
In this work, global gene expression and protein profiles of two wine yeast strains fermenting synthetic must have been considered. These two strains were selected because of their similarities but also for the different fermentative behaviors that they display from the time of entry into stationary phase (6 days after inoculation). For this reason this time point of the vinification was considered. Up to now, only one proteomic analysis with wine yeast cells under conditions that try to mimic vinification has been carried out (50). Although some transcriptomic studies with different purposes have been published (4, 31, 42, 51), our study is the first one in which strains that do not behave in the same way during vinification are considered in an integrated transcriptomic-proteomic analysis.
The comparison of the gene expression data obtained from this study and a previous analysis of stress response genes (58) with the protein profiles indicates agreement in some cases (for instance, for HSP26). For other genes, however, the relationship is not so straightforward, and several aspects contribute to this. First of all, it is important to consider that the protein levels are affected not only by gene expression but also by translation regulation. On the other hand, several isoforms of proteins are present and with the approach followed in this work only the spots with different levels of abundance were characterized (other electrophoretic forms of the proteins may be present in similar amounts).
Strain ICV 16, but not ICV 27, is able to complete the fermentation. Our gene expression and protein data indicate that aspects related to carbohydrate and sulfur metabolism, nitrogen transport, stimulus response, and sterol biosynthesis and transport may explain some of the differences in fermentative behavior among these strains and could help to identify desirable traits of wine yeast cells.
Regarding carbohydrate metabolism, several genes that encode enzymes involved in glycolysis and fermentation (FBA1, ENO1, ENO2, PFK27, TDH1, TDH2, TDH3, GPM1, PGI1, PFK2, ADH1, ADH2, ADH6) are more expressed in strain ICV16. In addition, this strain shows higher protein levels of the aldehyde dehydrogenase Ald6p. In ICV 27, higher gene expression of gluconeogenic regulators and enzymes involved in the tricarboxylic acid cycle and ATP synthase and NADH oxidation was found (genes CAT8, SIP4, PCK1, FBP1, ADR1, GUT2, YJL045W, SDH2, NCA3, NCA2, NDE3). Besides, higher levels of the chain-respiratory protein Qcr2p are also detected in this strain. These results and the data obtained by semiquantitative PCR during the vinification favor the possibility that strain ICV 27 could show—at the time of entry into stationary phase—a decrease in the fermentation-respiration balance, while in ICV 16 this metabolic change would take place later on. The possibility of higher respiratory activity (or lower fermentative metabolism) in strain ICV 27 does not seem very appropriate for an accurate process and could explain lower glucose consumption later on.
Analyzing the results obtained regarding carbohydrate and nitrogen metabolism—in particular, the expression profiles during fermentation of some genes involved in these processes, such FBA1 and ADR1—it seems that the changes in gene expression detected in strain ICV 27 at early stationary phase correspond to those found for strain ICV 16 and for other commercial yeast strains with appropriate fermentative behavior (42, 51) in late stationary phase. Interestingly, nitrogen limitation is known to induce entry into the stationary phase, and in this strain, despite the nitrogen available at this point, we find higher mRNA levels of genes regulated by nitrogen catabolite repression (many of them involved in the transport of nitrogen compounds) and of the transcription factor Gat1p. These data also suggest that the nitrogen transport capacity of ICV 27 is somehow affected, and this could be one of the reasons for the fermentative problems detected in some musts.
Another aspect relevant during wine fermentation is stress response. Within the category of stimulus (and in particular stress) response, we find two aspects of great interest. First, HSP26 mRNA and protein levels are higher in strain ICV 16. Another protein induced by stress (encoded by YPR127w) shows similar differences, and the expression of other stress genes (SSA4, UBC4, MSN4) also displays this kind of behavior. Recent data indicate that the expression of Hsp26p is probably important for maintaining the viability of cells during fermentative conditions. In this sense, proteomic analysis of a wild-type wine yeast strain has revealed an important increase in the level of this protein when glucose is exhausted and yeast cells enter into stationary phase (50). Moreover, the levels of this and other stress proteins are up-regulated during the first generation in fermentations carried out by brewing yeast strains, and these levels are maintained during subsequent generations (28). According to the points stated in the introduction, the higher abundance of those proteins involved in stress response in strain ICV 16, especially Hsp26p, may be indicative of a better adaptation or response to the stationary-phase conditions and, hence, an improvement of the capability of this strain of fully completing the vinification process.
On the other hand, in strain ICV 27 some indications of oxidative stress are found; in fact, higher levels of expression of genes involved in the metabolism of reactive oxygen species (SRX7, CTA1, OYE3, GSH1, SKN7, and YAP1 among others) have been detected. Taking these results and those related to carbohydrate metabolism together, it is possible that in ICV 27 the relative deviation of metabolism with respect to processes such as gluconeogenesis, organic compound oxidation, and respiration would require a more active metabolism of reactive oxygen species.
In strain ICV 27, some proteins involved in the sulfur assimilation pathway (Cys4p, Met22p, and Hom6p) appear to overaccumulate. As sulfur compounds contribute to the organoleptic properties of the wine (30), the data obtained in this analysis suggest that differences could exist in the sulfur compound profiles generated in the wine by these two strains. In fact, the ICV 27 strain is commercially very interesting for the aromatic profile in the resulting wine (A. Briones, personal communication). According to other proteomic and transcriptomic data published, it cannot be ruled out that the higher activity of the sulfur assimilation pathway in strain ICV 27 could be related to other processes, for instance, detoxification. A connection between glutathione biosynthesis and cadmium detoxification in yeast cells has been shown (15, 52). Moreover, recent work from our research group (2) has revealed that high concentrations of acetaldehyde also elicit a transcriptional induction of most genes involved in the pathways of sulfur amino acid metabolism and also of Tpo transporters. It is interesting to mention in this sense that our microarray data indicate higher expression levels in ICV 27 of PDR10, SNQ2, PDR5, PDR15, YOR1, and PDR1 genes. Interestingly, the mRNA levels of GSH1, a gene involved in glutathione biosynthesis, are also higher in ICV 27.
Other genes with differential expression levels are involved in ergosterol biosynthesis or transport. Yeast cells cannot produce ergosterol in the absence of oxygen, but, in agreement with our data, several reports (42, 51) indicate that many genes encoding proteins involved in ergosterol biosynthesis are expressed at midexponential and early stationary phases and are down-regulated at the end of fermentation. The reported increase in cellular viability and fermentation rate after punctual additions of oxygen during the stationary phase in vinification (41) could have a relationship to a change in the proportion of ergosterol to phospholipids and hence in the protection against ethanol (9, 44). The lower ergosterol content observed for strain ICV 27 could explain, at least partially, lower ethanol resistance and hence a greater effect of this compound on the transport of glucose and nitrogen across the membrane.
The information obtained in this work validates the potential application of not only transcriptomic—as suggested for other authors (17)—but also proteomic approaches for the identification of the molecular bases of traits that can be used for the prediction of environmental phenotypic variation. In particular, these approaches can be useful for the understanding of the process of wine fermentation and the physiological differences between strains. Importantly, our results indicate that these techniques can be applied to industrial S. cerevisiae strains, which differ in many genetic and physiological aspects from the laboratory strains previously characterized.
Acknowledgments
We thank Lola Guitérrez and M. Luisa Hernaez from the “Centro de Genómica y Proteómica” of the “Universidad Complutense” for excellent technical support and Lynne Yenush for critically reading the manuscript and revision of the English text. We are indebted to Jeffrey Dellrow for his collaboration on the microarray analysis.
This work was supported by grants AGL2002-01109 from the “Ministerio de Ciencia y Tecnología” and GRUPOS03/012 from the “Generalitat Valenciana.” A.Z. was a fellow of the “Generalitat Valenciana.”
REFERENCES
- 1.Andlid, T., L. Blomberg, L. Gustafsson, and A. Blomberg. 1999. Characterization of Saccharomyces cerevisiae CBS 7764 isolated from rainbow trout intestine. Syst. Appl. Microbiol. 22:145-155. [DOI] [PubMed] [Google Scholar]
- 2.Aranda, A., and M. del Olmo. 2004. Exposure of Saccharomyces cerevisiae to acetaldehyde induces sulfur amino acid metabolism and polyamine transporter genes, which depend on Met4p and Haa1p transcription factors, respectively. Appl. Environ. Microbiol. 70:1913-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attfield, P. V. 1997. Stress tolerance: the key to effective strains of industrial baker's yeast. Nat. Biotech. 15:1351-1357. [DOI] [PubMed] [Google Scholar]
- 4.Backhus, L. E., J. DeRisi, P. O. Brown, and L. F. Bisson. 2001. Functional genomic analysis of a commercial wine strain of Saccharomyces cerevisiae under differing nitrogen conditions. FEMS Yeast Res. 1:111-125. [DOI] [PubMed] [Google Scholar]
- 5.Bandlow, W., G. Strobel, C. Zoglowek, U. Oechsner, and V. Magdolen. 1988. Yeast adenylate kinase is active simultaneously in mitochondria and cytoplasm and is required for non-fermentative growth. Eur. J. Biochem. 178:451-457. [DOI] [PubMed] [Google Scholar]
- 6.Bauer, F. F., and I. S. Pretorius. 2000. Yeast stress response and fermentation efficiency: how to survive the making of wine. S. Afr. J. Enol. Vitic. 21:27-51. [Google Scholar]
- 7.Blomberg, A. 1997. Osmoresponsive proteins and functional assessment strategies in Saccharomyces cerevisiae. Electrophoresis 18:1429-1440. [DOI] [PubMed] [Google Scholar]
- 8.Camarasa, C., J. P. Grivet, and S. Dequin. 2003. Investigation by 13C-NMR and tricarboxylic acid (TCA) deletion mutant analysis of pathways for succinate formation in Saccharomyces cerevisiae during anaerobic fermentation. Microbiology 149:2669-2678. [DOI] [PubMed] [Google Scholar]
- 9.Chi, Z., and N. Arneborg. 1999. Relationship between lipid composition, frequency of ethanol-induced respiratory deficient mutants, and ethanol tolerance in Saccharomyces cerevisiae. J. Appl. Microbiol. 86:1047-1052. [DOI] [PubMed] [Google Scholar]
- 10.Cleveland, W. S. 1979. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74:829-836. [Google Scholar]
- 11.Cornell, N. W., and R. L. Veech. 1983. Enzymatic measurement of ethanol or NAD in acid extracts of biological samples. Anal. Biochem. 132:418-423. [DOI] [PubMed] [Google Scholar]
- 12.Darriet, P., T. Tominaga, V. Lavigne, J. N. Boidron, and D. Dubourdieu. 1995. Identification of a powerful aromatic component of Vitis vinifera L. var. Sauvignon wines: 4-mercapto-4-methylpentan-2-one. Flavour Fragr. J. 10:385-392. [Google Scholar]
- 13.Degré, R. 1993. Selection and commercial cultivation of wine yeast and bacteria, p. 421-447. In G. H. Fleet (ed.), Wine microbiology and biotechnology. Harwood Academic Publishers, Chur, Switzerland.
- 14.de Nobel, H., L. Lawrie, S. Brul, F. Klis, M. Davis, H. Alloush, and P. Coote. 2001. Parallel and comparative analysis of the proteome and transcriptome of sorbic acid-stressed Saccharomyces cerevisiae. Yeast 18:1413-1428. [DOI] [PubMed] [Google Scholar]
- 15.Dormer, U. H., J. Westwater, N. F. McLaren, N. A. Kent, J. Mellor, and D. J. Jamieson. 2000. Cadmium-inducible expression of the yeast GSH1 gene requires a functional sulfur-amino acid regulatory network. J. Biol. Chem. 275:32611-32616. [DOI] [PubMed] [Google Scholar]
- 16.Estruch, F. 2000. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 24:469-486. [DOI] [PubMed] [Google Scholar]
- 17.Fay, J., H. McCullough, P. Sniegowski, and M. Eisen. 2004. Population genetic variation in gene expression is associated with phenotypic variation in Saccharomyces cerevisiae. Genome Biol. 5:R26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fazzio, T. G., C. Kooperberg, J. P. Goldmark, C. Neal, R. Basom, J. Delrow, and T. Tsukiyama. 2001. Widespread collaboration of Isw2 and Sin3-Rpd3 chromatin remodeling complexes in transcriptional repression. Mol. Cell. Biol. 21:6450-6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ficarro, S. B., M. L. McCleland, P. T. Stukenberg, D. J. Burke, M. M. Ross, J. Shabanowitz, D. F. Hunt, and F. M. White. 2002. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 20:301-305. [DOI] [PubMed] [Google Scholar]
- 20.Fleet, G. H., and G. M. Heard. 1993. Yeasts—growth during fermentation, p. 42-43. In G. H. Fleet (ed.), Wine microbiology and biotechnology. Harwood Academic Publishers, Chur, Switzerland.
- 21.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godon, C., G. Lagniel, J. Lee, J. M. Buhler, S. Kieffer, M. Perrot, H. Boucherie, M. B. Toledano, and J. Labarre. 1998. The H2O2 stimulon in Saccharomyces cerevisiae. J. Biol. Chem. 273:22480-22489. [DOI] [PubMed] [Google Scholar]
- 23.Haslbeck, M., S. Walke, T. Stromer, M. Ehrnsperger, H. E. White, S. Chen, H. R. Saibil, and J. Buchner. 1999. Hsp26: a temperature-regulated chaperone. EMBO J. 18:6744-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernández, R., C. Nombela, R. Diez-Orejas, and C. Gil. 2004. Two-dimensional reference map of Candida albicans hyphal forms. Proteomics 4:374-382. [DOI] [PubMed] [Google Scholar]
- 25.Hohmann, S., and W. H. Mager. 2003. Yeast stress responses. Springer-Verlag, Berlin, Germany.
- 26.Hubbard, M. J., and P. Cohen. 1993. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem. Sci. 18:172-177. [DOI] [PubMed] [Google Scholar]
- 27.Jackson, R. S. 1994. Wine science. Principles and applications. Academic Press, San Diego, Calif.
- 28.Kobi, D., S. Zugmeyer, S. Potier, and L. Jaquet-Gutfreund. 2004. Two-dimensional protein map of an “ale”-brewing yeast strain: proteome dynamics during fermentation. FEMS Yeast Res. 5:213-230. [DOI] [PubMed] [Google Scholar]
- 29.Konrad, M. 1988. Analysis and in vivo disruption of the gene coding for adenylate kinase (ADK1) in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 263:19468-19474. [PubMed] [Google Scholar]
- 30.Lambrechts, M. G., and I. S. Pretorius. 2000. Yeast and its importance to wine aroma—a review. S. Afr. J. Enol. Vitic. 21:97-129. [Google Scholar]
- 31.Marks, V. D., G. K. van der Merwe, and H. J. J. van Vuuren. 2003. Transcriptional profiling of wine yeast in fermenting grape juice: regulatory effect of diammonium phosphate. FEMS Yeast Res. 3:269-287. [DOI] [PubMed] [Google Scholar]
- 32.Mullen, J. R., P. S. Kayne, R. P. Moerschell, S. Tsunasawa, M. Gribskov, M. Colavito-Shepanski, M. Grunstein, F. Sherman, and R. Sternglanz. 1989. Identification and characterization of genes and mutants for an N-terminal acetyltransferase from yeast. EMBO J. 8:2067-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Torrado, R., P. Carrasco, A. Aranda, J. Gimeno-Alcaniz, J. E. Perez-Ortin, E. Matallana, and M. L. del Olmo. 2002. Study of the first hours of microvinification by the use of osmotic stress-response genes as probes. Syst. Appl. Microbiol. 25:153-161. [DOI] [PubMed] [Google Scholar]
- 34.Piper, P. W., K. Talreja, B. Panaretou, P. Moradas-Ferreira, K. Byrne, U. M. Praekelt, P. Meacock, M. Recnacq, and H. Boucherie. 1994. Induction of major heat-shock proteins of Saccharomyces cerevisiae, including plasma membrane Hsp30, by ethanol levels above a critical threshold. Microbiology 140:3031-3038. [DOI] [PubMed] [Google Scholar]
- 35.Polevoda, B., J. Norbeck, H. Takakura, A. Blomberg, and F. Sherman. 1999. Identification and specificities of N-terminal acetyltransferases from Saccharomyces cerevisiae. EMBO J. 18:6155-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puig, S., and J. E. Pérez-Ortín. 2000. Expression levels and patterns of glycolytic yeast genes during wine fermentation. Syst. Appl. Microbiol. 23:300-303. [DOI] [PubMed] [Google Scholar]
- 37.Puig, S., and J. E. Pérez-Ortín. 2000. Stress response and expression patterns in wine fermentations of yeast genes induced at the diauxic shift. Yeast 16:139-148. [DOI] [PubMed] [Google Scholar]
- 38.Quail, M. A., and L. K. Steven. 1993. The extraction and analysis of sterols from yeasts, p. 123-131. In I. Evans (ed.), Methods in molecular biology: yeast protocols, vol. 53. Humana Press Inc., Totowa, N.J. [DOI] [PubMed] [Google Scholar]
- 39.Riou, C., J. M. Nicaud, P. Barre, and C. Gaillardin. 1997. Stationary-phase gene expression in Saccharomyces cerevisiae during wine fermentation. Yeast 13:903-915. [DOI] [PubMed] [Google Scholar]
- 40.Rodrigues-Pousada, C. A., T. Nevitt, R. Menezes, D. Azevedo, J. Pereira, and C. Amaral. 2004. Yeast activator proteins and stress response: an overview. FEBS Lett. 567:80-85. [DOI] [PubMed] [Google Scholar]
- 41.Rosenfeld, E., B. Beauvoit, B. Blondin, and J. M. Salmon. 2003. Oxygen consumption by anaerobic Saccharomyces cerevisiae under enological conditions: effect on fermentation kinetics. Appl. Environ. Microbiol. 69:113-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossignol, T., L. Dulau, A. Julien, and B. Blondin. 2003. Genome-wide monitoring of wine yeast gene expression during alcoholic fermentation. Yeast 20:1369-1385. [DOI] [PubMed] [Google Scholar]
- 43.Russel, M., P. Model, and A. Holmgren. 1990. Thioredoxin or glutaredoxin in Escherichia coli is essential for sulfate reduction but not for deoxyribonucleotide synthesis. J. Bacteriol. 172:1923-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sajbidor, J., Z. Ciesarova, and D. Smogrovicova. 1995. Influence of ethanol on the lipid content and fatty acid composition of Saccharomyces cerevisiae. Folia Microbiol. (Prague) 40:508-510. (In Czech.) [DOI] [PubMed] [Google Scholar]
- 45.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 46.Susek, R. E., and S. Lindquist. 1990. Transcriptional derepression of the Saccharomyces cerevisiae HSP26 gene during heat shock. Mol. Cell. Biol. 10:6362-6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tercero, J. C., J. D. Dinman, and R. B. Wickner. 1993. Yeast MAK3 N-acetyltransferase recognizes the N-terminal four amino acids of the major coat protein (gag) of the L-A double-stranded RNA virus. J. Bacteriol. 175:3192-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tercero, J. C., L. E. Riles, and R. B. Wickner. 1992. Localized mutagenesis and evidence for post-transcriptional regulation of MAK3. A putative N-acetyltransferase required for double-stranded RNA virus propagation in Saccharomyces cerevisiae. J. Biol. Chem. 267:20270-20276. [PubMed] [Google Scholar]
- 49.Thomas, D., and Y. Surdin-Kerjan. 1997. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 61:503-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trabalzini, L., A. Paffetti, A. Scaloni, F. Talamo, E. Ferro, G. Coratza, L. Bovalini, P. Lusini, P. Martelli, and A. Santucci. 2003. Proteomic response to physiological fermentation stresses in a wild-type wine strain of Saccharomyces cerevisiae. Biochem. J. 370:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varela, C., J. Cárdenas, F. Melo, and E. Agosin. 2005. Quantitative analysis of wine yeast gene expression profiles under winemaking conditions. Yeast 22:369-383. [DOI] [PubMed] [Google Scholar]
- 52.Vido, K., D. Spector, G. Lagniel, S. Lopez, M. B. Toledano, and J. Labarre. 2001. A proteome analysis of the cadmium response in Saccharomyces cerevisiae. J. Biol. Chem. 276:8469-8474. [DOI] [PubMed] [Google Scholar]
- 53.Wang, X. P., C. J. Mann, Y. L. Bai, L. Ni, and H. Weiner. 1998. Molecular cloning, characterization, and potential roles of cytosolic and mitochondrial aldehyde dehydrogenases in ethanol metabolism in Saccharomyces cerevisiae. J. Bacteriol. 180:822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilcox, L. J., D. A. Balderes, B. Wharton, A. H. Tinkelenberg, G. Rao, and S. L. Sturley. 2002. Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast. J. Biol. Chem. 277:32466-32472. [DOI] [PubMed] [Google Scholar]
- 55.Wolfger, H., Y. M. Mamnun, and K. Kuchler. 2001. Fungal ABC proteins: pleiotropic drug resistance, stress response and cellular detoxification. Res. Microbiol. 152:375-389. [DOI] [PubMed] [Google Scholar]
- 56.Zaworsky, P. G., and R. C. Heimsch. 1987. The isolation and characterization of flocculent yeast. In G. G. Hiebsch (ed.), Biological research on industrial yeast III. CRC Press Inc., Boca Raton, Fla.
- 57.Zuzuarregui, A., and M. del Olmo. 2004. Analyses of stress resistance under laboratory conditions constitute a suitable criterion for wine yeast selection. Antonie Leewenhoek 85:271-280. [DOI] [PubMed] [Google Scholar]
- 58.Zuzuarregui, A., and M. del Olmo. 2004. Expression of stress response genes in wine strains with different fermentative behavior. FEMS Yeast Res. 4:699-710. [DOI] [PubMed] [Google Scholar]