Abstract
Burkholderia sp. strain PsJN stimulates root growth of potato explants compared to uninoculated controls under gnotobiotic conditions. In order to determine the mechanism by which this growth stimulation occurs, we used Tn5 mutagenesis to produce a mutant, H41, which exhibited no growth-promoting activity but was able to colonize potato plants as well as the wild-type strain. The gene associated with the loss of growth promotion in H41 was shown to exhibit 65% identity at the amino acid level to the nadC gene encoding quinolinate phosphoribosyltransferase (QAPRTase) in Ralstonia solanacearum. Complementation of H41 with QAPRTase restored growth promotion of potato explants by this mutant. Expression of the gene identified in Escherichia coli yielded a protein with QAPRTase activities that catalyzed the de novo formation of nicotinic acid mononucleotide (NaMN). Two other genes involved in the same enzymatic pathway, nadA and nadB, were physically linked to nadC. The nadA gene was cotranscribed with nadC as an operon in wild-type strain PsJN, while the nadB gene was located downstream of the nadA-nadC operon. Growth promotion by H41 was fully restored by addition of NaMN to the tissue culture medium. These data suggested that QAPRTase may play a role in the signal pathway for promotion of plant growth by PsJN.
The soil immediately adjacent to roots harbors microorganisms whose activity and diversity differs from the activity and diversity of the microorganisms in the bulk soil (26). This population of microorganisms is dependent on the release from plant roots of various soluble exudates, mucilage, shed cells, or cell wall material (26). Bacteria in the rhizosphere, termed rhizobacteria, have been shown to dramatically affect plant health, development, and environmental adaptation in a variety of ways (2, 6). Rhizobacteria that stimulate the growth of plants are referred to as plant growth-promoting rhizobacteria (PGPR) (24).
The mechanisms of plant growth promotion associated with PGPR can be classified into two major groups. The mechanisms in the first group involve direct promotion of plant growth by production of phytohormones, such as auxins, cytokinins, and gibberellins (3, 32, 43), and increase in the levels of nutrients, such as nitrogen and phosphorus, as well as micronutrients in the rhizosphere (27, 37). For example, many rhizobacteria, such as Pseudomonas and Azospirillum, produce indole-3-acetic acid predominantly by tryptophan-dependent pathways (4). Biofertilization accounts for approximately 65% of the nitrogen supplied to crops worldwide and 20 to 50% of the total soil organic phosphorus (2, 36). Indirect beneficial effects are derived from activities related to plant protection. Many rhizosphere organisms produce antibiotics that limit the activities of pathogens directly or allow beneficial microbes to be more competitive in root colonization. Some may act by stimulating plant resistance (34, 48).
There is an enormous volume of literature on the use of bacteria for improvement of plant performance, but few bacteria have been developed as commercial products due to difficulties in obtaining successful formulations and insufficient knowledge of the basic molecular principles of the actions of the microorganisms (2). In the past two decades, many studies have focused on the mode of action of PGPR for plant growth promotion, and significant progress has been made in gene identification and regulation associated with improving plant development (33, 35). Different regulated tryptophan-dependent pathways for indole-3-acetic acid synthesis and the ipdC gene encoding indole-3-pyruvate decarboxylase that promote plant growth have been found in Azospirillum and Pseudomonas (4, 31, 32).
A Burkholderia sp. strain (designated PsJN) that was originally isolated from surface-sterilized onion roots is an excellent candidate for potential development as a soil inoculant to increase crop yields. Under tissue culture conditions, potato nodal explants treated with PsJN showed significant growth stimulation, which resulted in 6- to 10-fold increases in root dry weight (5, 11, 12, 25). In addition, bacterized potato plantlets had a higher survival rate than nonbacterized controls after they were transplanted from tissue culture conditions to the field and produced more tubers, leading to increased potato yield in growth chamber, greenhouse, and field conditions (25). The mechanism by which plant growth is stimulated by PsJN is not known.
The objectives of this study were to identify and characterize the genes in PsJN involved in promotion of potato growth by creating non-growth-promoting mutants using Tn5 mutagenesis, screening a genomic DNA library, subcloning, and performing complementation tests. The proteins encoded by the genes identified were overexpressed in Escherichia coli and purified, and their enzymatic activities were examined. The product of the reaction catalyzed by the enzymes was used to restore growth-promoting activity in a non-growth-promoting mutant.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. A Burkholderia sp. strain, strain PsJN, which was originally isolated from surface-sterilized onion (Allium cepa L.) roots, is a wild-type strain that significantly promotes growth of potato nodal explants under gnotobiotic culture conditions (29). PsJN was grown in nutrient broth yeast (NBY) medium (49) or King's B (KB) medium (23) at 30°C. A rifampin-resistant PsJN mutant, PsJN(Rifr), which exhibited the same plant growth-promoting activity as PsJN, was developed by successively transferring PsJN to KB medium supplemented with rifampin (20 μg/ml). Mutant PsJN(Rifr) was used as the recipient for transposon mutagenesis. E. coli strains were cultured in solid or liquid Luria-Bertani (LB) medium at 37°C. When necessary, antibiotics were added at the following concentrations: rifampin, 20 or 100 μg/ml; kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; and tetracycline, 12.5 or 25 μg/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Pseudomonas strains | ||
| PsJN | Wild type, growth-promoting activity | 27 |
| PsJN(Rifr) | Spontaneous Rifr derivative of PsJN | This study |
| H41 | Mutant, non growth-promoting activity, Rifr, Kmr | This study |
| E. coli strains | ||
| DH5α | F−recA1 endA1 gyrA1 thi-1 hsdR17 supE44 relA1 Φ80dlacIqZΔM15 λ− Δ(lacZ-argF)U169 | Stratagene |
| XL1-Blue | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 λ−lac [F′proAB lacIqZΔM15 Tn10(tet)] | Stratagene |
| BL21 | F−ompT hsdS (rB− mB−) gal | Amersham |
| LE392 | F−hsdR514 supE44 supF58 lacY1 galK2 galT22 metB1 trpR55 λ− | Promega |
| WA803 | met thi | 46 |
| Plasmids | ||
| pGS9 | Suicide vector, Cmr, Tn5, Kmr | 41 |
| pBluescript | pBluescript II SK(+) cloning vector, Apr | Stratagene |
| pRK415 | Inc PI (RK2 replicon), Tcr, plac and polylinker of pUC19 | 20 |
| pGEX-5X-3 | GST fusion protein expression vector, Apr, lacIq, factor Xa cleavage site | Amersham |
Tn5 mutagenesis of PsJN(Rifr).
The rifampin-resistant strain PsJN(Rifr) was used for all mutation experiments. Transposon Tn5 was introduced into the genome of PsJN(Rifr) by conjugation with donor strain E. coli WA803 carrying suicide plasmid pGS9 (pGS9::Tn5) (39, 50). Exponentially grown E. coli WA803 cells grown at 30°C in LB broth and PsJN(Rifr) cells grown at 30°C in NBY medium to the stationary phase were mixed and dispensed onto HAWP membrane filters (pore size, 0.45 μm; Millipore Corp.). The filters were placed on an NBY plate and incubated at 30°C for 18 h. The bacteria were then washed off the filters into 10 mM potassium phosphate buffer (pH 7.2), plated onto minimal growth medium (42) supplemented with 0.3% (wt/vol) glucose in the presence of kanamycin and rifampin, and incubated at 30°C for 3 to 4 days.
Screening of non-growth-promoting mutants.
The plant growth-promoting ability of Tn5 transconjugants was tested using the gnotobiotic assay with potato nodal explants. Transconjugants of PsJN(Rifr) were grown on KB medium at 30°C for 3 days. Five colonies were transferred into potassium phosphate buffer (1 ml) and mixed briefly. Nodal cuttings from a potato tissue culture (cultivar Kennebec) were dipped in the bacterial suspension for 1 min, dried on sterilized paper towels, and transferred into tissue culture tubes containing Murashige minimal organic medium (Sigma) at pH 6.0. Plants inoculated with wild-type strain PsJN and with potassium phosphate buffer alone were used as controls. The plantlets were grown in a growth chamber at 22°C with a 16-h photoperiod.
Comparison of root colonization by PsJN(Rifr) and mutant H41.
Potato nodes (cultivar Kennebec) were inoculated with PsJN(Rifr), mutant H41, and other mutants as previously described. Plants were grown under gnotobiotic conditions for 5 weeks, and then roots were removed and surface sterilized for 1 min using calcium hypochlorite (3.25% active ingredient) plus 0.1% sodium bicarbonate. The roots were rinsed twice for 1 min in sterile water, placed on sterile paper towels to remove excess water, and placed in a cylinder containing a ball bearing plus 6.5 ml of an ascorbic acid solution (0.05 g/liter), and the tissue was homogenized using a Kleco 2000 tissue homogenizer for 40 s. Serial dilutions were prepared using 10 mM phosphate buffer and plated on KB medium for PsJN(Rifr) and on KB medium plus rifampin and kanamycin for H41 and the other mutants.
Construction of genomic libraries of PsJN and mutant H41.
The genomic DNA libraries of wild-type strain PsJN and mutant H41, which showed a loss of growth-promoting activity, were constructed with the vector lambda GEM-11 (Promega, Madison, WI). The genomic DNAs of PsJN and H41 were isolated using a QIAGEN Blood & Cell Culture DNA midi kit (QIAGEN Inc., Mississauga, ON, Canada) and were partially digested with Sau3AI. The DNA fragments in the size range from 15 to 23 kb were isolated by fractionation of the digests on 10 to 40% sucrose gradients. After ligation of the purified fragments into the LamdaGEM-11 BamHI arms that were prepared by BamHI digestion and dephosphorylation with alkaline phosphatase, the ligation mixture was packaged using Packagene extract (Promega, Madison, WI), followed by transfection into E. coli LE 392 as described by the manufacturer.
DNA manipulations.
DNA electrophoresis, lambda and plasmid DNA isolation, restriction endonuclease digestion, ligation reactions, and subcloning were carried out using standard protocols (38). Nested deletion was performed with an ExoIII/S1 deletion kit (Fermentas, Burlington, ON, Canada) by following the manufacturer's instructions. Transformation of E. coli DH5α was performed by the method of Hanahan (16). Plasmid pRK415 was used as the vector for all subcloning steps.
Screening of lambda clones carrying Tn5-inserted region from H41 and the corresponding region from PsJN.
The Tn5 DNA fragment was excised from plasmid pGS9::Tn5 by HindIII digestion and was purified from a low-melting-point agarose gel using a Prep-A-Gene kit (Bio-Rad, Richmond, CA). The Tn5 probes were prepared by random primer extension using a digoxigenin (DIG) labeling kit (Boehringer Mannheim, Quebec, Canada) according to the manufacturer's instructions. The clones carrying a Tn5-inserted region from mutant H41 were screened by hybridization of DIG-labeled Tn5 probes. Briefly, the plaques were transferred to nitrocellulose filters that were then denatured with alkali and dried. The DNA on the membranes was cross-linked by UV irradiation and then hybridized with DIG-labeled Tn5 probes at 38°C overnight. The membrane was washed at 68°C using 0.5× SSC buffer supplemented with 0.1% sodium dodecyl sulfate (SDS) (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate).
The lambda clones carrying the Tn5 insert region were identified by restriction enzyme mapping and Southern hybridization. Lambda DNA was extracted using the Wizard Lambda Preps DNA purification system (Promega Madison, WI). The Tn5 flanking regions were excised with restriction enzyme BamHI, isolated from the gel, and used for probe preparation with the digoxigenin labeling kit for screening the lambda clones carrying the putative genes involved in plant growth promotion, using the procedure described above.
Complementation test.
Competent cells of the H41 mutant were prepared as previously described (7). The plasmids containing the DNA inserts in subclones were isolated from E. coli DH5α and transformed into mutant H41 by electroporation under the following conditions: 18 to 20 kV/cm, 25 μF, 200 Ω. The organisms were then plated on KB medium supplemented with tetracycline and kanamycin. The clones selected were transferred to fresh KB medium, grown at 30°C for 4 days, and used for inoculation of potato nodal cuttings in order to assess the restoration of plant growth-promoting activity as described above, using mutant H41, PsJN, and potassium phosphate buffer as controls.
DNA sequencing and analysis.
Sequence templates were generated by a combination of restriction digestion and nested deletion strategies. Deletion clones with overlapping regions of DNA were selected to isolate plasmids for DNA sequencing using rapid plasmid purification systems (Marligen Bioscience Inc., Ljamsville, MD). DNA sequencing was performed using a BigDye Terminator v3.1 cycle sequencing kit and an ABI PRISM 3100 genetic analyzer (Applied Biosystems, Foster City, CA). The Lasergene package (DNASTAR Inc., Madison, Wis.) was used to assemble DNA sequences and to identify putative open reading frames (ORFs) and their directions of transcription. The similarity of nucleotide sequences and deduced amino acid sequences was analyzed by using the BLAST program to search the databases maintained at the National Center for Biotechnology Information, the European Molecular Biology Laboratory, and Swiss-Prot.
Reverse transcription-PCR.
Wild-type strain PsJN was grown in tryptic soy broth at 30°C and harvested at the exponential growth phase. Total RNA was isolated with an RNeasy mini kit (QIAGEN) according to the manufacturer's instructions. RNase-free DNase I was used for column digestion to remove any contaminating DNA. Reverse transcription (RT)-PCR was carried by using a two-step protocol. Reverse transcription of the nadA-nadC operon was performed by using primer nadC2 (5′-TCAGACGATTCGCATCGAGTAA-3′) complementary to the 3′ end of nadC and a QIAGEN OneStep RT-PCR kit under the following conditions: 50 μg of total RNA and 20 pmol of primer in a 50-μl (final volume) reaction mixture. Samples were incubated for 30 min at 50°C and then heated at 95°C for 5 min to destroy the reverse transcriptase. Therefore, only the RT reaction was carried out, although this kit is ordinarily used for one-step RT-PCR. A 1-μl aliquot of the synthesized cDNA was then used for PCR amplification of nadA, nadC, and nadA-nadC using primer pairs nadA1 (5′-ATGGATCAGCAGGCGATCAG-3′)-nadA2 (5′-TTAAGCCGCCCCCACGTTC-3′), nadC2-nadC1 (5′-ATGGGCGCGCCAGAAGGCA-3′), and nadA1-nadC2, respectively. The RNA sample was used as a control in PCR to verify the absence of DNA contamination.
Protein expression and purification.
The quinolinate phosphoribosyltransferase (QAPRTase) encoded by nadC was expressed and purified by using the glutathione S-transferase (GST) gene fusion system (Amersham Pharmacia). The nadC coding sequence was amplified by PCR using primers nadCF (5′-CCGGGATCCTAATAGAAGGTCGTATGGGCGCGCCAGAAGGCA-3′) and nadCR (5′-GGCTTGGCGGCCGCTCAGACGATTCGCATCGAGTAA-3′) containing BamHI and NotI restriction sites (underlined), respectively. The nucleotide sequence (boldface type) encoding the recognition sequence of protease Factor Xa was added immediately upstream of the initiation codon of nadC to remove any amino acid contamination from the target proteins after Factor Xa cleavage of the fusion protein for purification of the nadC-encoded protein. The PCR product was digested and ligated into pGEX-5X-3 digested with BamHI and NotI. The pGST-nadC construct was transferred to host strain E. coli BL21. The fusion protein was expressed after sequence confirmation. Isopropyl-d-thiogalactoside-induced E. coli BL21 cells containing pGST-nadC were collected by centrifugation, resuspended in 1× phosphate-buffered saline (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4; pH 7.3), and lysed by ultrasonication. The cell extracts were applied to a GSTrap FF affinity column for purification of the GST fusion protein. The fusion protein was cleaved by protease Factor Xa through on-column cleavage, and protease Factor Xa was removed using benzamidine Sepharose FF (high sub) as recommended by Amersham Biosciences. On the basis of the deduced amino acid sequence of QAPRTase, the purified protein concentration was calculated by assuming that 1 A280 unit was equivalent to 1.57 mg/ml.
QAPRTase assay.
The activity of purified QAPRTase was measured by using a modification of the method of Bhatia and Calvo (1). The assay mixture (total volume, 550 μl) contained 50 mM phosphate buffer (pH 7.2), 5 mM MgCl2, 4 mM quinolinic acid (QA), and 1 mM 5-phosphoribosyl-1-pyrophosphate (PRPP). About 4 μg of the purified protein solution was added to the reaction mixture, which was then incubated for 15 min at room temperature. The reaction was stopped by addition of 550 μl of 2 M sodium cyanide and was allowed to stand at 30°C for an additional 15 min, followed by measurement of the absorbance at 315 nm against a blank consisting of the solution described above without QA. The enzyme kinetics of QAPRTase were further examined by using a procedure similar to that mentioned above. The reaction mixture (total volume, 250 μl) contained 2 μg of purified protein, and the reaction was terminated at 1-min intervals. The absorbance at 315 nm was measured against an identical mixture for the zero-time reaction. The relationship between the concentration of nicotinic acid mononucleotide (NaMN) and the absorbance at 315 nm was determined by using the following conditions: 50 mM potassium phosphate (pH 7.2), 4 mM MgCl2, 1 M sodium cyanide, and a variable amount of NaMN.
Chemical restoration of growth promotion by mutant H41.
The biosynthetic product NaMN from the reaction catalyzed by QAPRTase was used to investigate the effect restoration of growth promotion with mutant H41. The minimal organic agar medium was supplemented with NaMN at final concentrations of 10, 50, and 100 μM. Potato nodal explants that were inoculated with H41 or PsJN or were not inoculated were grown on both amended and unamended media. Six weeks after inoculation, plant roots were washed out of the medium and air dried for 5 days at room temperature. Root dry weight data were subjected to an analysis of variance with Duncan's test at α = 0.01. Each data point represented at least five replicates, and standard deviations were determined.
Nucleotide sequence accession number.
The DNA sequence determined in this study has been deposited in the GenBank database under accession no. DQ207953.
RESULTS
Tn5 transposon mutagenesis.
A rifampin-resistant strain, PsJN(Rifr), had the same phenotype and the same ability to induce plant growth as wild-type strain PsJN. The stability of growth promotion by PsJN or PsJN(Rifr) has been demonstrated over a 10-year period in our laboratory. To search for the genes involved in plant growth promotion in PsJN, non-growth-promoting mutants of PsJN(Rifr) were created by Tn5 random insertion and were screened by the potato tissue culture bioassay. Of 2,400 Tn5 mutants screened, 7 consistently exhibited loss of growth-promoting activity. One mutant, H41, was found after extensive tests to be devoid of any growth-promoting activity. In colonization tests, the numbers of bacteria inside the roots after 5 weeks of growth were 2.2 × 108 CFU/g of root tissue for PsJN(Rifr) and 8.0 × 107 CFU/g for H41. A mutant designated E24 was found to promote growth as well as PsJN(Rifr) and sometimes promoted growth even better, but it was also found to have smaller populations (6.3 × 107 CFU/g of root tissue) in root tissue than PsJN(Rifr) or the wild-type strain. Mutant H41 was stable over many generations and was used for further study. Southern blot analysis of EcoRI- and BamHI-digested H41 genomic DNA using the internal HindIII fragment of Tn5 as a probe revealed one EcoRI fragment and two BamHI fragments (data not shown). This indicated that a single copy of Tn5 was present in the genome of mutant H41 as Tn5 does not contain an EcoRI recognition site but has one BamHI site in the middle of the transposon.
Cloning of Tn5-containing DNA fragments from mutant H41.
To identify the genes interrupted by the Tn5 insertion, a genomic library of mutant H41 was constructed in the lambda Gem-11 vector. Five lambda clones designated H41λ1, H41λ2, H41λ3, H41λ4, and H41λ5 containing the Tn5 insert were identified by hybridization with DIG-labeled Tn5 probes. Since the Tn5 transposon contains a single BamHI restriction site in the middle of the transposon but not an EcoRI restriction site, we used these two endonucleases to identify the fragments containing the Tn5 transposon and the flanking sequences. A restriction analysis of each clone performed with EcoRI and BamHI revealed that Tn5 was present in a 10-kb EcoRI DNA fragment, which was consistent with the results of a Southern blot analysis of H41 genomic DNA (data not shown). Lambda clone H41λ2 contained only a partial Tn5 transposon, while the other four lambda clones contained the whole Tn5 transposon. A 6-kb BamHI/EcoRI fragment containing the partial sequence of the Tn5 transposon and one flanking sequence was isolated from lambda clone H41λ3 and cloned into pBluescript II SK(+), resulting in clone pBL141.
Identification of PsJN genomic clones that restore plant growth-promoting activity.
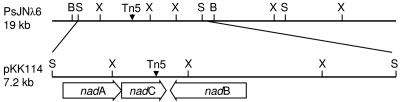
A DIG-labeled 6-kb BamHI/EcoRI fragment from H41λ3 was used to screen the genomic library of wild-type strain PsJN to isolate the intact region complementary to the mutated sequence in H41. Eight lambda clones that hybridized to the probe were identified and designated PsJNλ2, PsJNλ3, PsJNλ4, PsJNλ5, PsJNλ6, PsJNλ7, PsJNλ9, and PsJNλ11. Restriction analysis of the clones revealed overlapping sequences matching the Tn5-interrupted region of mutant H41. The restriction fragments identified by Southern hybridization were subcloned into broad-host-range plasmid pRK415. A complementation test performed by inoculation of potato nodal plants with the transformed mutant H41 showed that the clones containing a 7.2-kb SstI fragment fully restored growth promotion in mutant H41, suggesting that the 7.2-kb DNA fragment conferred growth-promoting activity. In addition, the ability to restore growth-promoting activity in mutant H41 by both DNA inserts in either direction in pRK415 suggested that the encoded genes had their own promoters as the Plac and tet R promoters in plasmid pRK415 were in the same direction adjacent to the HindIII site (8, 22). The clone carrying a 7.2-kb DNA fragment from PsJNλ9 was designated pKK114 (Fig. 1). Subsequent subcloning of pKK114 by nested deletion and testing of complemented strains using the tissue culture bioassay narrowed the region to a 2.4-kb DNA fragment in clone pKK114-19-4 which could fully restore promotion of growth of potato by H41.
FIG. 1.
Physical relationship of clones containing PsJN plant growth-promoting activity and schematic map of genes identified by sequence analysis. PsJN λ6 is an approximately 19-kb Sau3AI fragment in lambda GEM-11. pKK114 contains a 7.2-kb SstI fragment from PsJN λ6 cloned into pRK415. The arrows indicate the translation orientations of the genes. Restriction sites: B, BamHI; S, SstI; X, XhoI.
DNA sequence analysis.
The sequence analysis revealed that the 7.2-kb DNA fragment had an overall G+C content of 61.57% and contained three putative ORFs with AUG as the start codon (Fig. 1). ORF1 consists of 1,140 nucleotides that encode a 379-amino-acid polypeptide with a deduced Mr of 41,001. The G+C content of ORF1 is 63.77%, and there is significant bias in codon usage at three nucleotide positions. The G+C contents at the three nucleotide positions are 67.3% at position 1 (GC1), 44.3% at position 2 (GC2), and 80.2% at position 3 (GC3). ORF2 contains 900 nucleotides that encode a 299-amino-acid polypeptide with a deduced Mr of 32,312. The G+C content of ORF2 is 63.4%, and the G+C contents at nucleotide positions GC1, GC2, and GC3 are 69%, 46.7%, and 74.3%, respectively. ORF1 and ORF2 are closely linked. The putative initiation codon of ORF2 (ATG) overlaps the stop codon (TGA) of ORF1. The insertion of transposon Tn5 was identified between nucleotides 867 and 868 of ORF2 by comparative analysis of the nucleotide sequences of the Tn5-inserted region in mutant H41 and the corresponding DNA sequence in PsJN. A putative ribosome-binding site (RBS) (5′-GAAGG-3′) of ORF1 is located 11 nucleotides 5′ of the start codon (AUG), while a putative RBS (5′-GAACGTG-3′) of ORF2 is located in the 3′ terminus of ORF1, 11 nucleotides 5′ of the start codon (AUG). A probable RNA polymerase binding site was found 173 bp upstream of ORF1 and was shared by ORF1 and ORF2. ORF3, which is located 92 bp downstream of ORF2 and has the opposite transcriptional direction, has 1,599 nucleotides that encode a 532-amino-acid polypeptide with a deduced Mr of 58,339. The overall G+C content of ORF3 is 63.6%, and the G+C contents of GC1, GC2, and GC3 are 67.5%, 46.5%, and 76.8%, respectively. A putative RBS (5′-GGAGA-3′) is located 8 nucleotides 5′ of the start codon (AUG).
BLASTX search analyses revealed that the deduced amino acid sequences encoded by ORF1 and ORF3 exhibited 98.7% and 99.1% identity to the quinolinate synthetase A protein (nadA) and l-aspartate oxidase (nadB), respectively, from Burkholderia fungorum (accession no. ZP_00281208). Therefore, ORF1 and ORF3 in PsJN were putative nadA and nadB genes, respectively. The PsJN ORF1 and ORF3 exhibited 85.4 to 91.6% and 86.7 to 88.3% identity to nadA and nadB, respectively, of three other Burkholderia species, Burkholderia cepacia (accession no. ZP_00214043), Burkholderia pseudomallei (YP_107541), and Burkholderia mallei (YP_103797), and 59.4 to 77.4% identity to genes of bacterial species belonging to other genera, such as Ralstonia solanacearum (CAD16154), Ralstonia metallidurans CH34 (ZP_00272923), Ralstonia eutropha JMP134 (ZP_00168566), Salmonella enterica serovar Typhimurium LT2 (NP_461576), and E. coli K-12 (NP_417069). In E. coli, S. enterica serovar Typhimurium, and many other eubacteria, quinolinate synthetase A and l-aspartate oxidase are two components of an enzyme complex, the so-called “quinolinate synthetase complex” that catalyzes the formation of quinolinic acid. l-Aspartate oxidase catalyzes the oxidation of l-aspartate to iminoaspartate, which is subsequently converted to quinolinic acid by quinolinate synthetase A in the presence of dihydroxyacetone phosphate (9, 15, 45, 47). ORF2 exhibited 91% identity at the amino acid level to the probable gene nadC encoding nicotinate-nucleotide pyrophosphorylase in B. fungorum and 80.2 to 81.6% identity to B. cepacia, B. pseudomallei, and B. mallei genes. ORF2 also exhibited 77.4 to 78.9% identity at the amino acid level to putative nicotinate-nucleotide pyrophosphorylase genes in R. solanacearum, R. metallidurans CH34, and R. eutropha JMP134 and 59.4 to 60.2% identity to the nadC gene or the pncB gene encoding nicotinate phosphoribosyltransferase in E. coli K-12 and S. enterica serovar Typhimurium LT2 (Fig. 2). QAPRTase is a key enzyme for de novo NAD biosynthesis in both prokaryotes and eukaryotes that catalyzes the formation of nicotinic NaMN from QA and PRPP, while nicotinate phosphoribosyltransferase has the same function but uses nicotinic acid as instead of QA as a substrate.
FIG. 2.
Alignment of amino acid sequences of nadC from PsJN and other bacteria. The shaded areas indicate the amino acids encoded by nadC from PsJN that are identical to the amino acids in all other bacteria examined in this study. The accession numbers of nadC in other bacteria are as follows: B. fungorum, ZP_00281209; B. cepacia, ZP_00220882; B. pseudomallei, YP_107540; B. mallei, YP_103799; R. solanacearum, CAD16155; R. metallidurans CH34, ZP_00272924; R. eutropha JMP134, ZP_00351024; S. enterica serovar Typhimurium LT2, NP_459150; and E. coli K-12, NP_414651.
ORF2 encodes quinolinate phosphoribosyltransferase.
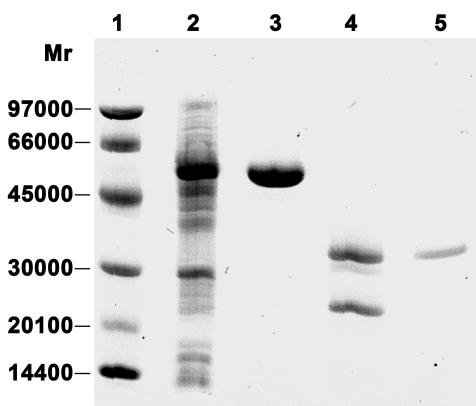
Since the deduced amino acid sequence encoded by ORF2 exhibited 78.9% identity to the sequence of the QAPRTase encoded by nadC in R. solanacearum, we examined whether the ORF2 gene product showed activity similar to that of QAPRTase. The functional protein encoded by ORF2 was expressed and purified by using the GST gene fusion system (Fig. 3). SDS-polyacrylamide gel electrophoresis analysis showed one band at 58 kDa for the purified fusion protein and one band at 32 kDa for the purified protein encoded by ORF2 that was free of any amino acid contamination due to cleavage of the fusion protein using protease Factor Xa. The purified ORF2-encoded protein was subjected to an analysis of enzymatic activity by measuring the formation of NaMN from QA and PRPP. The results demonstrated that the ORF2-encoded protein could catalyze the reaction that converts QA and PRPP to NaMN in the presence of Mg2+. Further analysis of the enzymatic kinetics showed that the activity of the ORF2-encoded enzyme was 0.89 μmol/min/mg under the conditions used, indicating that the purified ORF2-encoded enzyme had high enzymatic activity.
FIG. 3.
SDS-polyacrylamide (12.5%) gel analysis of various samples using PhastSystem (Amersham Pharmacia Biotech) during QAPRTase protein purification from overexpression of nadC in E. coli. The gel was stained with Coomassie blue. Lane 1, low-molecular-weight protein marker; lane 2, crude proteins; lane 3, purified GST-QAPRTase fusion protein; lane 4, fusion protein cleaved by protease factor Xa; lane 5, purified QAPRTase protein.
nadA and nadC genes comprise one operon.
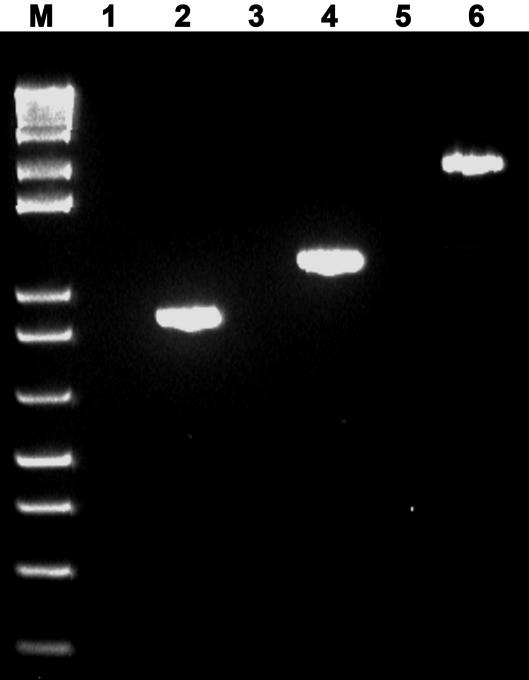
On the basis of the sequence analysis, nadA and nadC in PsJN may comprise one operon. To examine this hypothesis, we used two-step RT-PCR instead of one-step RT-PCR to eliminate DNA contamination. Total RNA samples were prepared from an exponential culture of wild-type strain PsJN and used to synthesize cDNA fragments by using a primer specific for the terminal region of nadC. By using the specific primers designed for amplification of nadA (1,140 bp), nadC (900 bp), and nadA-nadC (2,039 bp), the two genes and the cluster were amplified with the expected sizes from the same RT reaction mixture but not from the total RNA control (Fig. 4), indicating that the two genes comprise one operon.
FIG. 4.
RT-PCR analysis of the nadA-nadC operon. Lane M, 1-kb plus DNA marker; lanes 1, 3, and 5, total RNA as control for each PCR; lanes 2, 4, and 6, PCR amplification of nadC, nadA, nadA-nadC, respectively, using the RT reaction mixture as the template.
Physical evidence that NaMN is involved in growth promotion by PsJN.
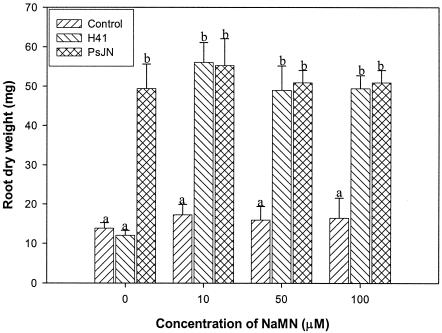
To further confirm that NaMN was deficient in mutant H41 and is involved in the signal pathway for growth promotion in PsJN, commercially available NaMN at different concentrations was directly added to the tissue culture medium for complementation tests. The results indicated that the growth-promoting activity of H41 was fully restored by addition of NaMN at concentrations ranging from 10 to 100 μM (Fig. 5). Statistical analysis showed that the root dry weights of potato plantlets inoculated with mutant H41 when the medium was supplemented with NaMN at concentrations of 10 to 100 μM were not significantly different from the root dry weights of potato plantlets inoculated with wild-type strain PsJN but were significantly greater than the root dry weights of the control and the plantlets inoculated with H41 without NaMN supplementation after culture for 6 weeks (Fig. 6). These data strongly indicated that NaMN plays an essential role that is associated with stimulation of plant growth in PsJN.
FIG. 5.
Effect of nicotinic acid mononucleotide on restoration of promotion of growth of potato plants by mutant H41 6 weeks after inoculation. The plants were cultured under growth chamber conditions.
FIG. 6.
Effect of NaMN on restoration of promotion of potato plant growth by mutant H41 6 weeks after inoculation. A multiple comparison was performed by using the method of Duncan at α = 0.01. The error bars indicate standard deviations. Different letters indicate a significant difference at α = 0.01.
DISCUSSION
The results of this study provide solid evidence that phosphoribosyltransferase is involved in plant growth-promoting activity in PsJN. This was demonstrated by disabling the plant growth-promoting activity using Tn5 mutagenesis and restoring activity by genetic complementation and supplementation of the products of the enzymes. The primary enzymatic activity of QAPRTase is involved in the de novo biosynthesis of NAD by catalyzing the formation of NaMN from QA and PRPP in the presence of Mg2+ (10, 14). Recently, it has been found that QAPRTase also plays an important role in the secondary metabolism involved in plant defense (41), human disease infection by the pathogen Mycobacterium tuberculosis (40), and neurodegenerative disease (13). Genes encoding QAPRTase have been identified in mammal liver and brain (13, 30), plants (41), and more than a dozen microbes, including S. enterica serovar Typhimurium (20) and E. coli (1). The QAPRTase enzyme catalyzes the entry step in the pyridine nucleotide cycle, which involves at least seven enzymes and produces at least five primary metabolites in addition to the major product, NAD. Some of these metabolites have been reported to be substrates for secondary metabolism. For example, nicotinic acid is a substrate for the synthesis of defensive pyridine alkaloids in Nicotiana sp. (41). NAD, except for the de novo synthesis of NADP, also is a substrate in reactions that produce poly(ADP-ribose), which has great importance in both eukaryotic and prokaryotic cells (44). Given that such products play essential roles in many metabolic activities, it is not possible at this time to assign a direct role to this pathway in growth promotion.
Nicotinamide was first isolated from rice hulls and was considered a plant growth regulator based on evidence showing stimulation of plant growth, development, differentiation, and fruiting of plants such as dwarf rice, pea, tomato, and radish (17, 18, 46). In this study, the metabolites from the pyridine nucleotide pathway, nicotinic acid mononucleotide, nicotinamide, and nicotinic acid or nicotinamide mononucleotide, fully restored the growth-promoting activity of mutant H41. However, the metabolites by themselves did not promote the growth of nodal explants, suggesting that they are not directly involved in growth stimulation by PsJN but rather indirectly regulate this activity.
We surmised that perhaps a deficiency in this QAPRTase enzyme may reduce the ability of the mutant to colonize plants, may reduce its survival, and may decrease its growth rate. Interestingly, H41 grew at a rate similar to that of PsJN(Rifr) in KB medium, as indicated by comparison of the slopes of the growth rates. H41, however, grew more slowly in minimal growth medium and Murashige minimal organic medium supplemented with 0.3% glucose, succinate, and sugar (Sigma). The populations of H41 were about 0.5 to 1 log smaller than those of PsJN(Rifr) in homogenates of surface-sterilized roots, but they were similar to those of the E24 mutant of PsJN(Rifr), which promoted growth as well as the wild type and often better. This indicated that H41 retained the ability to invade potato roots and colonize the vascular tissues. In addition, supplementation of the minimal growth medium with NAD did not affect the growth rate of H41 but did fully restore the growth-promoting activity. The data thus suggest that the loss of growth-promoting activity by H41 is more likely due to termination of the metabolic pathway involved in growth stimulation. Alternatively, growth promotion might be positively regulated by the pyridine nucleotide cycle. Further analyses are needed to clearly understand how QAPRTase participates in the growth promotion signal pathway.
The nadA, nadB, and nadC genes are normally far apart in the genomes of bacteria such as E. coli and S. enterica serovar Typhimurium (10). Transcription of the nadA and nadB genes was repressively regulated by the nadI gene product when cells were grown in the presence of exogenous NAD precursors, while nadC is not known to be under any form of genetic regulation (21). In PsJN, however, the nadA, nadB, and nadC genes were found to be physically linked as a gene cluster. The nadB gene in PsJN was located 92 bp downstream of the nadA and nadC genes and had the opposite transcription direction, while nadA and nadC in PsJN had the same promoter and were transcribed together as one operon on the basis of RT-PCR analysis. Physical linkage of these three genes was also observed in other Burkholderia species, including B. fungorum, B. cepacia, B. pseudomallei, and B. mallei (19, 28). Since a putative RNA polymerase binding site was about 173 bp from the translation initiation codon of nadA, we assume that the nadA and nadC genes are transcriptionally regulated. Further analysis of the regulatory sequence should increase our understanding of the nadA-nadC gene regulation mechanism and the signal pathway for growth-promoting activity in PsJN.
We have produced several other transposon insertions that yielded non-growth-promoting mutants of PsJN. These mutants have shown that the process of plant growth promotion involves a number of synthetic steps and hence a number of genes, none of which have been characterized. This study provides a foundation for developing an understanding of the full pathway involved in plant growth promotion in PsJN and other PGPR. Analyses of more mutants are being performed.
Acknowledgments
This work was supported in part by the NSERC Biocontrol Network, by Commercial Alcohols and Agriculture, and by the Agri-Food Canada Matching Fund Initiative.
The technical assistance of Brian Weselowski throughout this project is greatly appreciated. We thank Kelly Jo Bates and Kuflum Kuflu for their work in generation of and screening of the mutants. The suggestions and help in the early phases of the project provided by Shengwu Ma are greatly appreciated.
REFERENCES
- 1.Bhatia, R., and K. C. Calvo. 1996. The sequencing, expression, purification, and steady-state kinetic analysis of quinolinate phosphoribosyl transferase from Escherichia coli. Arch. Bichem. Biophys. 325:270-278. [DOI] [PubMed] [Google Scholar]
- 2.Bloemberg, G. V., and B. J. J. Lugtenberg. 2001. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 4:343:350. [DOI] [PubMed] [Google Scholar]
- 3.Broek, A. C., M. Lambrecht, K. Eggermont, and J. Vanderleyden. 1999. Auxins upregulate expression of the indole-3-pyruvate decarboxylase gene in Azospirillum brasilense. J. Bacteriol. 181:1338-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carreño-Lopez, R., N. Campos-Reales, C. Elmerich, and B. E. Baca. 2000. Physiological evidence for differently regulated tryptophan-dependent pathways for indole-3-acetic acid synthesis in Azospirillum brasilense. Mol. Gen. Genet. 264:521-530. [DOI] [PubMed] [Google Scholar]
- 5.Conn, K. L., J. Nowak, and G. Lazarovits. 1997. A gnotobiotic bioassay for studying interactions between potatoes and plant growth-promoting rhizobacteria. Can. J. Microbiol. 43:801-808. [Google Scholar]
- 6.Curl, E. A. 1982. The rhizosphere: relation to pathogen behaviour and root disease. Plant Dis. 66:624-630. [Google Scholar]
- 7.Dennis, J. J., and P. A. Sokol. 1995. Electrotransformation of Pseudomonas, p. 125-133. In J. A. Nickoloff (ed.), Methods in molecular biology: electroporation protocols for microorganisms. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 8.Ditta, G., T. Schmidhauser, E. Yakobson, P. Lu, X. W. Liang, D. R. Finlay, D. Guiney, and D. R. Helinski. 1985. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid 13:149-153. [DOI] [PubMed] [Google Scholar]
- 9.Flachmann, R., N. Kunz, J. Seifert, M. Gutlich, F. J. Wientjes, A. Laufer, and G. Gassen. 1988. Molecular biology of pyridine nucleotide biosynthesis in Escherichia coli: cloning and characterization of quinolinate synthesis gene nadA and nadB. Eur. J. Biochem. 175:221-228. [DOI] [PubMed] [Google Scholar]
- 10.Foster, J. W., and A. G. Moat. 1980. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol. Rev. 44:83-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frommel, M. I., J. Nowak, and G. Lazarovits. 1991. Growth enhancement and developmental modification in in vitro grown potato (Solanum tuberosum ssp. tuberosum) as affected by a non-fluorescent Pseudomonas sp. Plant Physiol. 96:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frommel, M. I., J. Nowak, and G. Lazarovits. 1993. Treatment of potato tubers with a growth promoting Pseudomonas sp.: plant growth responses and bacterium distribution in the rhizosphere. Plant Soil 150:51-60. [Google Scholar]
- 13.Fukuoka, S. I., C. M. Nyaruhucha, and K. Shibata. 1998. Characterization and functional expression of the cDNA encoding human brain quinolinate phosphoribosyltransferase. Biochim. Biophys. Acta 1395:192-201. [DOI] [PubMed] [Google Scholar]
- 14.Gholson, R., I. Ueda, and N. Ogaswara. 1964. The enzymatic conversion of quinolinate to nicotinic acid mononucleotide in mammalian liver. J. Biol. Chem. 239:1208-1214. [PubMed] [Google Scholar]
- 15.Griffith, G. R., J. L. R. Chandler, and R. K. Gholson. 1975. Studies on the de novo biosynthesis of nicotinamide adenine dinucleotide in Escherichia coli: the separation of the nadB gene product and its purification. Eur. J. Biochem. 54:239-245. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 17.Hathout, T. A., S. A. Sheteawi, and S. M. Khallal. 1993. Effect of mode of application of some growth regulators on the physiology of tomato plants. I. Effect of indole-acetic acid (IAA) on morphology, growth, metabolism and productivity Egypt. J. Physiol. Sci. 17:17-43. [Google Scholar]
- 18.Hathout, T. A., S. A. Sheteawi, and S. M. Khallal. 1993. Effect of mode of application of some growth regulators on the physiology of tomato plants. II. Effect of IAA on the endogenous hormonal contents. Egypt. J. Physiol. Sci. 17:45-62. [Google Scholar]
- 19.Holden, M. T., R. W. Titball, S. J. Peacock, A. M. Cerdeno-Tarraga, T. Atkins, L. C. Crossman, T. Pitt, C. Churcher, K. Mungall, S. D. Bentley, M. Sebaihia, N. R. Thomson, N. Bason, I. R. Beacham, K. Brooks, K. A. Brown, N. F. Brown, G. L. Challis, I. Cherevach, T. Chillingworth, A. Cronin, B. Crossett, P. Davis, D. DeShazer, T. Feltwell, A. Fraser, Z. Hance, H. Hauser, S. Holroyd, K. Jagels, K. E. Keith, M. Maddison, S. Moule, C. Price, M. A. Quail, E. Rabbinowitsch, K. Rutherford, M. Sanders, M. Simmonds, S. Songsivilai, K. Stevens, S. Tumapa, M. Vesaratchavest, S. Whitehead, C. Yeats, B. G. Barrell, P. C. Oyston, and J. Parkhill. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. USA 101:14240-14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes, K. T., A. Dessen, J. P. Gray, and C. Grubmeyer. 1993. The Salmonella typhimurium nadC gene: sequence determination by use of Mud-P22 and purification of quinolinate phosphoribosyltransferase. J. Bacteriol. 175:479-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes, K. T., J. R. Roth, and B. M. Olivera. 1991. A genetic characterization of the nadC gene of Salmonella typhimurium. Genetics 127:657-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 23.King, E. E., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of phycocynin and fluorescein. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 24.Kloepper, J. W., J. Leong, M. Teintze, and M. M. Schroth. 1980. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885-886. [Google Scholar]
- 25.Lazarovits, G., and J. Nowak. 1996. Rhizobacteria for improvement of plant growth and establishment. Hortscience 32:188-192. [Google Scholar]
- 26.Lynch, J. M., and J. M. Whipps. 1991. Substrate flow in the rhizosphere, p. 15-25. In D. L. Kleister and P. B. Cregan (ed.), The rhizosphere and plant growth. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 27.Nautiyal, C. S., S. Bhadauria, P. Kumar, H. Lal, R. Mondal, and D. Verma. 2000. Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol. Lett. 182:291-296. [DOI] [PubMed] [Google Scholar]
- 28.Nierman,W. C., D. DeShazer, H. S. Kim, H. Tettelin, K. E. Nelson, T. Feldblyum, R. L. Ulrich, C. M. Ronning, L. M. Brinkac, S. C. Daugherty, T. D. Davidsen, R. T. Deboy, G. Dimitrov, R. J. Dodson, A. S. Durkin, M. L. Gwinn, D. H. Haft, H. Khouri, J. F. Kolonay, R. Madupu, Y. Mohammoud, W. C. Nelson, D. Radune, C. M. Romero, S. Sarria, J. Selengut, C. Shamblin, S. A. Sullivan, O. White, Y. Yu, N. Zafar, L. Zhou, and C. M. Fraser. 2004. Structural flexibility in the Burkholderia mallei genome. Proc. Natl. Acad. Sci. USA 101:14246-14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowak, J., S. K. Asiedu, G. Lazarovits, V. Pillay, A. Stewart, C. Smith, and Z. Liu. 1995. Enhancement of in vitro growth and transplant stress tolerance of potato and vegetable plantlets co-cultured with a plant growth promoting pseudomonad bacterium, p. 173-180. In F. Carre and P. Chagvardieff (ed.), Proceedings of an International Symposium on Ecophysiology and Photosynthetic In Vitro Cultures. CAE, Aix-en-Provence, France.
- 30.Ohuno, E., and R. Schwartz. 1985. Purification of quinolinic acid phosphoribosyl-transferase from rat liver and brain. Biochim. Biophys. Acta 841:112-119. [DOI] [PubMed] [Google Scholar]
- 31.Patten, C. L., and B. R. Glick. 2002. Regulation of indoleacetic acid production in Pseudomonas putida GR12-2 by tryptophan and the stationary-phase sigma factor RpoS. Can. J. Microbiol. 48:635-642. [DOI] [PubMed] [Google Scholar]
- 32.Patten, C. L., and B. R. Glick. 2002. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 68:3795-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persello-Cartieaux, F., L. Nussaume, and C. Robaglia. 2003. Tales from the underground: molecular plant-rhizobacteria interactions. Plant Cell Environ. 26:189-199. [Google Scholar]
- 34.Pieterse, C. M. J., J. A. Van Pelt, S. C. M. Van Wees, J. Ton, K. M. Léon-Kloosterziel, J. J. B. Keurentjes, B. W. M. Verhagen, M. Knoester, I. Van der Sluis, P. A. H. M. Bakkerand, and L. C. Van Loon. 2001. Rhizobacteria-mediated induced systemic resistance: triggering, signaling and expression. Eur. J. Plant Pathol. 107:51-61. [Google Scholar]
- 35.Ping, L., and W. Boland. 2004. Signals from the underground: bacterial volatiles promote growth in Arabidopsis. Trends Plant Sci. 9:263-266. [DOI] [PubMed] [Google Scholar]
- 36.Richardson, A. E., P. A. Hadobas, and J. E. Hayes. 2001. Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. Plant J. 25:641-649. [DOI] [PubMed] [Google Scholar]
- 37.Rozycki, H., H. Dahm, E. Strzelczyk, and C. Y. Li. 1999. Diazotrophic bacteria in root-free soil and in the root zone of pine (Pinus sylvestris L.) and oak (Quercus robur L.). Appl. Soil Ecol. 12:239-250. [Google Scholar]
- 38.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Selvaraj, G., and V. N. Iyer. 1983. Suicide plasmid vehicles for insertion mutagenesis in Rhizobium meliloti and related bacteria. J. Bacteriol. 156:1292-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma, V., C. Grubmeyer, and J. C. Sacchettini. 1998. Crystal structure of quinolinic acid phosphoribosyltransferase from Mycobacterium tuberculosis: a potential drug target. Structure 6:1587-1599. [DOI] [PubMed] [Google Scholar]
- 41.Sinclair, S. J., K. J. Murphy, C. D. Birch, and J. D. Hamill. 2000. Molecular characterization of quinolinate phosphoribosyltransferase (QPRTase) in Nicotiana. Plant Mol. Biol. 44:603-617. [DOI] [PubMed] [Google Scholar]
- 42.Stanier, R. Y., N. J. Palleroni, and M. Duodoroff. 1966. The aerobic pseudomonads: a taxonomic study. J. Gen. Microbiol. 43:159-271. [DOI] [PubMed] [Google Scholar]
- 43.Steenhoudt, O., and J. Vanderleyden. 2000. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic. Biochemical and ecological aspects. FEMS Microbiol. Rev. 24:487-506. [DOI] [PubMed] [Google Scholar]
- 44.Sugimura, T. 1973. Poly(adenosine diphosphate robose). Prog. Nucleic Acid Res. Mol. Biol. 13:127-151. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki, N., J. Carlson, G. Griffith, and R. K. Gholson. 1973. Studies on the de novo biosynthesis of nicotinamide adenine dinucleotide in Escherichia coli: properties of the quinolinic acid synthetase system. Biochim. Biophys. Acta 304:309-315. [DOI] [PubMed] [Google Scholar]
- 46.Takeuchi, S. Y. Kono, A. Kawarada, Y. Ota, and M. Nakayama. 1975. Nicotinamide as a plant growth regulator isolated from rice hulls. Agric. Biol. Chem. 39:859-861. [Google Scholar]
- 47.Tritz, G. J. 1987. Nicotinamide adenine dinucleotide biosynthesis and recycling, p. 557-563. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, DC.
- 48.Van Loon, L. C., P. A. H. M. Baker, and M. J. Pieterse. 1998. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 36:453-483. [DOI] [PubMed] [Google Scholar]
- 49.Vidaver, A. K. 1967. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl. Microbiol. 15:1523-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood, W. B. 1966. Host specificity of DNA produced by Escherichia coli: bacteria mutations affecting the restriction and modification of DNA. J. Mol. Biol. 16:118-133. [DOI] [PubMed] [Google Scholar]