Abstract
In the surface waters of sulfidic springs near Regensburg, Bavaria, Germany, the SM1 euryarchaeon, together with filamentous bacteria, forms the recently described unique string-of-pearls community. In addition to naturally occurring string-of-pearls communities, the growth of these communities was also observed on polyethylene nets provided as an artificial attachment material in the streamlets of springs. In order to learn more about the distribution and origin of the SM1 euryarchaeon and its possible occurrence in the subsurface, polyethylene nets were incubated as deeply as possible in different spring holes. After a short residence time, slime-like, milky drops, almost completely composed of SM1 euryarchaeon, were attached to the nets, indicating that this organism grows independent of a partner in deeper earth layers. A newly designed in situ biofilm trapping system allowed the quantitative harvesting of organisms exhibiting this newly discovered lifestyle of the SM1 euryarchaeon for detailed biological studies. The discovery of naturally occurring archaeal biofilms extends our knowledge of the biology and ecological significance of archaea in their environments.
Sulfidic springs are fairly common around the world and have been the focus of (micro)biological work for more than 150 years (29, 42). They were classified as areas of high bioactivity, as visible by the development of extended (white) mats representing vast populations of various genera of filamentous sulfur bacteria (23). As the main focus for our detailed investigations of microbial communities in nongeothermal environments, we have chosen a sulfurous marsh, the “Sippenauer Moor” near Regensburg, Germany (32). In this area, cold water (∼10°C) from deeper earth layers reaches the surface and emerges from the ground between rocks and tree roots, forming small streamlets that merge in a large pond. The intake of atmospheric oxygen causes the appearance of white microbial mats in the streamlets, indicating high bioactivity. In these sulfidic streamlets, growth of a unique microbial community occurs, with microbes forming a string-of-pearls-like, macroscopically visible structure: single, whitish pearls with diameters of up to 3 mm are connected to each other by thin, white-colored threads. In the pearl interior, the nonmethanogenic SM1 euryarchaeon is predominant, representing a deep phylogenetic branch within the 16S rRNA tree (32). The exteriors of the pearls and the connecting threads are mainly composed of a single phylotype, the filamentous sulfide-oxidizing bacterium Thiothrix sp. (21).
During a recent microbial survey of cold sulfidic springs in Bavaria, Germany, a second type of microbial string-of-pearls community was discovered in the streamlet of the “Islinger Mühlbach” (Regensburg, Southern Germany) (31). The SM1 euryarchaeon was again predominant in the pearl interior, while its partner was represented by the filamentous so-called IMB1 ɛ-proteobacterium, most probably outcompeting Thiothrix at the low in situ oxygen concentrations. The constant coexistence of the SM1 euryarchaeon with specific bacterial partners suggested a syntrophic or even symbiotic relationship with a sulfur cycle or specific nutrient exchange occurring within single pearls (21, 31).
A novel in situ cultivation technique was designed and successfully applied to obtain larger quantities of microbial string-of-pearls communities, using their cold biotopes as a “natural chemostat” (22, 31). Polyethylene nets were placed in cold, sulfidic streamlets, and within 2 to 3 days, numerous whitish pearls had developed on the nets. The interior of each pearl was dominated by an SM1 euryarchaeal microcolony in which the cells were arranged three-dimensionally at defined distances from each other. Within 1 week of incubation, the pearls had enlarged to about 12 mm, and the SM1 euryarchaeal population became randomly distributed. From these microbial net communities, the SM1 euryarchaeon was purified in vitro from the bacterial fraction by gentle physical methods (22). This selective separation enabled the study of the lifestyle of the low-temperature SM1 euryarchaeon and led to the discovery of a novel cell surface appendage with nano-grappling hooks and a nano-barbed-wire structure. For this previously undescribed prokaryotic appendage, the name “hamus” (plural “hami”) was proposed (20, 22). The monophyletic nature of the SM1 euryarchaeon in native pearls and net populations of the different sulfidic biotopes at the “Sippenauer Moor” marsh and in the “Islinger Mühlbach” streamlet was shown by various molecular methods, including immunofluorescence in situ hybridization (immuno-FISH) studies and sequencing of the 16S rRNA genes and of the 16S-23S archaeal intergenic spacer region (22, 31, 32).
Thus far, the SM1 euryarchaeon had only been detected in euryarchaeal-bacterial string-of-pearls communities that were found thriving in the cold surface waters of sulfidic springs. Since this water originates at depth, it was of interest to investigate the possible occurrence of the SM1 euryarchaeon (and its specific partners) in subsurface water, thereby providing insights into its distribution, abundance, and origin.
MATERIALS AND METHODS
Determination of environmental parameters.
Water temperature, oxygen concentration, pH, and other environmental parameters were determined as described previously (31). To estimate the flow rate of water from the spring, emerging water was collected over a certain period of time (10 s) by plugging the drill hole with a funnel and attached tube (Fig. 1A).
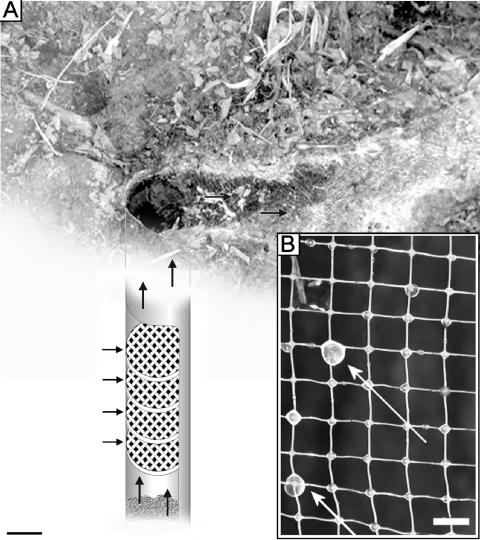
FIG. 1.
(A) Water collector. The funnel with attached tube was used to plug the drill hole, collect the emerging water, and estimate the flow rate of the springwater. Bar, 10 cm. (B) Biofilm trapping system. Frames covered with polyethylene nets (arrow A) were arranged within a plastic tube (diameter, 17 cm; length, 35 cm) (B) and fixed with a screwed-frame construction (arrow C). Bar, 10 cm.
In situ biofilm trapping system.
Nets of polyethylene threads at 0.5-cm-square mesh were treated with 70% (vol/vol) ethanol before exposure to the environment. Initially, single polyethylene nets were clamped into circular plastic frames (diameter, 17 cm) and submersed into the drill hole as deeply as possible (up to 75 cm). For more quantitative trapping of biofilm material, up to 12 frames, each covered with five polyethylene nets, were arranged within a plastic tube (diameter, 17 cm; length, 35 cm). This tube was then placed within the drill hole so that all welling springwater passed through the polyethylene nets (Fig. 1 and 2). At various time intervals (5 min up to 7 days), the tube was carefully lifted and the nets were visually examined for attached biomaterial.
FIG. 2.
(A) Biofilm trapping system (scheme) within the drilling hole of the Islinger Mühlbach spring. Horizontal arrows point to plastic rings covered with polyethylene nets. The other arrows indicate the water flow. Bar, 10 cm. (B) Small, slime-like biomaterial droplets with a milky appearance (white arrows) sticking strongly to polyethylene nets after a short incubation time in the sulfidic water flow (see Fig. 1A). Bar, 0.5 cm.
Collection and preparation of samples.
Prior to biological sampling, all equipment was rendered nucleic acid-free by treatment with 5% HClO4, followed by double-distilled water washes. Slime-like macroscopically visible microbial samples were retrieved using sterile 1-ml syringes. Samples were processed, transported, and stored in fresh springwater at 10°C. Samples taken for FISH were immediately transferred to a 3% (wt/vol) paraformaldehyde solution and processed as described earlier (32).
Phase-contrast, epifluorescence, and electron microscopy.
Phase-contrast and epifluorescence microscopy were performed as previously described (32). Light micrographs were taken with a digital camera (Coolpix 990; Nikon Corporation, Tokyo, Japan). Samples for electron microscopy were treated with 2% (wt/vol) glutaraldehyde, applied to carbon-coated grids, air dried, and negatively stained with uranyl acetate (2% [wt/vol]; pH 4.5). Electron microscopy was conducted using a Philips CM12 transmission electron microscope (FEI Co., Eindhoven, The Netherlands) at 120 keV, and images were recorded with a slow-scan charge-coupled device camera (Tietz GmbH, Gauting, Germany).
FISH and oligonucleotide probes.
For domain-specific FISH, three bacterium-specific probes (EUB338/I [2], EUB 338/II, and EUB 338/III [10]) and three archaeon-specific probes (ARCH915 [34], ARCH344, and ARCH1060 [22]) were used as mixtures for hybridization reactions (EUBmix and ARCHmix, respectively; for details, see reference 31). Furthermore, the SM1 euryarchaeon-specific probe SMARCH714 (22) and the bacterial sulfate reducer-specific probe SRB385 (2) were used. All oligonucleotides were labeled with rhodamine green or Cy3. Whole-cell hybridizations were carried out as described recently (32). After the FISH procedure, each sample was stained with 10 μl of DAPI (4′,6′-diamidino-2-phenylindole; 2 mg/liter, prepared in washing buffer) (32).
DNA isolation, PCR, cloning, RFLP, sequencing, and phylogenetic analyses.
Cell lysis and bulk DNA extraction were performed as described previously (32). Extracted genomic DNAs were used as templates for PCR amplification of archaeal 16S rRNA gene sequences with the archaeon-specific forward primer 344aF (8) and the universal reverse primer 1512uR (16). PCR, cloning, restriction fragment length polymorphism (RFLP) analysis, and sequencing were performed as described previously (32). One to three recombinants for each unique RFLP pattern were chosen for sequencing. Phylogenetic analyses were done according to the method of Rudolph et al. (32).
For amplification of the 16S-23S rRNA intergenic spacer region, the primers 1044aF (8) and 64R (23S) (35) were used (for details, see reference 31). The obtained PCR products were cloned, 30 recombinants per sample were analyzed by RFLP analysis, and those with unique restriction patterns were sequenced and compared using the alignment program ClustalX 1.81 (14).
All centrifugation steps to concentrate collected biofilm material were performed at 14,000 rpm and 10°C, using an Eppendorf 5810R centrifuge with an F45-30-11 rotor.
Live/dead staining, cell counting, degradation and analysis of EPS, and determination of G+C contents.
Live/dead Baclight staining was performed following the manufacturer's instructions (Molecular Probes, Eugene, OR). The cell concentration within the biofilm was determined after a gentle treatment with proteinase K (Merck KGAG, Darmstadt, Germany; 15 U/0.1 g of SM1 biofilm, 20°C, 60 min). During this procedure, the enzyme partially degraded the extracellular polymeric substances (EPS), and the released cells were counted using a Thoma counting chamber.
When necessary for other studies (e.g., pulsed-field gel electrophoresis [PFGE]), a single washing step with the original springwater was added. The pH in the biofilm's interior was determined by dipping pH indicator strips (Merck) into the sampled biofilm mass. Extraction and analysis of EPS and determinations of the G+C content were performed as described elsewhere (4, 19).
Determination of the genome size of the SM1 euryarchaeon.
The biofilm material was washed thrice with KPH buffer (20). Genome size determination was performed by pulsed-field gel electrophoresis as described previously (6), with the following modifications: euryarchaeal SM1 biofilms were treated with proteinase K (see above) to remove the EPS, and the released cells were concentrated by centrifugation. Approximately 5 × 108 cells in 50 μl KPH buffer were mixed with 50 μl InCert agarose (BMA; 1.6% [wt/vol], 37°C). After solidification of the plugs, 1 volume ATL buffer (QIAGEN GmbH, Hilden, Germany) was added, followed by a tough proteinase K treatment (2.5 mg/ml; 50°C) to lyse the cells. The plugs were washed thrice in 15 ml TE buffer (10 mM Tris-HCl, pH 7.5, 1 mM EDTA) at 37°C for 1 h. During the second washing step, 200 μl of phenylmethylsulfonyl fluoride (7 mg/ml; Roth, Karlsruhe, Germany) was added. Gel electrophoresis was carried out as described elsewhere (0.8% FastLane agarose gel; FMC, Rockland, ME) (6). Where required, the following restriction enzymes were used as recommended by the manufacturer: BssHII, NotI, AscI, SacII, EcoRV, HincII, DraIII, XcmI, HpaI, SfiI, KpnI, FseI, BglII, KpnI, and AscI (New England Biolabs GmbH, Frankfurt am Main, Germany).
CLSM.
The SM1 biofilms were either stained with live/dead Baclight (Molecular Probes, Eugene, OR) or hybridized in suspension with archaeon- and bacterium-specific probes as follows (physical and chemical perturbation was avoided during processing). Small amounts of the biofilm were gently harvested using tweezers and fixed in 3% paraformaldehyde (wt/vol). Dehydration steps were omitted. Samples were preincubated at 46°C in 50 μl of final hybridization buffer (0.9 M NaCl, 0.15 M Na3 citrate, 0.01% sodium dodecyl sulfate [wt/vol], 20% [vol/vol] formamide). After 15 min, hybridization probes were added (4 to 10 μg/ml), and samples were incubated for 180 min. After hybridization, the biofilms were immersed twice for 10 min each time in 2 ml of washing buffer (0.9 M NaCl, 0.15 M Na3 citrate, 0.01% sodium dodecyl sulfate) at 48°C. Following cooling on ice, the biofilm material was applied to glass slides, mounted in Citifluor AF-1 (Citifluor Ltd., London, United Kingdom), and gently covered with a cover slide which had previously been decorated with distance pieces. Confocal laser scanning microscopy (CLSM) was performed using an LSM5 Pa microscope (Carl Zeiss, Oberkochen, Germany).
RESULTS
Description of study site.
Experiments were carried out in the sulfidic water of the “Islinger Mühlbach,” Regensburg, southern Germany (31). The site consists of a single, cold sulfidic spring with water (10°C; flow rate, about 5.50 m3/h) emerging from the subsurface at ground level through a metal-lined drill hole with a diameter of 18.5 cm. At a depth of approximately 75 cm, the hole is partially filled with gravel and small rocks (Fig. 2A). Unfortunately, we were unable to obtain detailed information on the drilling (e.g., total depth of the drill hole or date of drilling).
The springwater emanates without turbulence and forms a small streamlet. Differently colored streamers and microbial mats first appear about 30 cm from the rim of the drill hole and are visible in the stream channel for approximately 10 m, indicating high bioactivity (Fig. 2A). This coincides with an increase in dissolved oxygen concentration from 0.13 mg/liter at the bore hole to 0.37 mg/liter 30 cm from the rim. In addition, microbial assemblages with a string-of-pearls-like morphology are floating in the water current. The springwater at the bore hole is characterized by a neutral pH, a low salinity (conductivity, 685 μS/cm), and sulfide and oxygen concentrations of 0.5 mg and 0.13 mg/liter, respectively. A detailed site analysis has been published recently (31).
In situ attachment and growth experiments with microbial communities on polyethylene nets.
Over a period of about 1 year, polyethylene nets were exposed to the sulfidic water at different positions within the spring and streamlet. Rapid (3 to 4 days) and consistent growth of microbial string-of-pearls communities was observed on nets placed in the oxygen-containing zone near white microbial mats at different positions along the sulfidic streamlet (31). However, no growth of microbial string-of-pearls communities was observed when nets were placed in the clear sulfidic water, either in the oxygen-poor zone up to 10 cm from the rim along the stream channel or as deeply as possible (up to 75 cm) in the drill hole itself (Fig. 2A). In contrast, within a short period of time slime-like clumps with diameters of up to 5 mm and a milky appearance were observed on the nets (Fig. 2B). Macroscopic droplets (0.5 to 1 mm in diameter) were already attached to the nets after exposure in the drill hole for 10 min. Incubation for up to 4 days resulted in increased numbers and the maximum size of clumps on the nets. This stringy biomaterial stuck strongly to the polyethylene and was retrieved for subsequent analysis using 1-ml syringes.
Phase-contrast microscopy, FISH, and phylogenetic studies of the milky, slime-like biomaterial.
Phase-contrast microscopy revealed that this slimy biomaterial from the subsurface consisted almost exclusively of small microbes (diameter, approximately 0.7 μm) with a coccoid morphology. These cocci were arranged three-dimensionally at defined distances from each other and were embedded in a polymeric substance. The architecture was very similar to microcolonies of the SM1 euryarchaeon seen in the interiors of single pearls within string-of-pearls communities (32). Using an SM1-specific euryarchaeal probe (SMARCH714) and a mixture of three archaeon-specific probes, coccoid archaeal cells, which made up at least 95% of the total microbial population, were identified (data not shown). All archaeal cells within the biofilm showed a positive hybridization signal with the SM1-specific euryarchaeal probe.
The identity and predominance of the SM1 euryarchaeon within the biofilm were confirmed by RFLP analysis, 16S rRNA gene sequence analysis, and sequencing of the 16S-23S rRNA intergenic spacer region. All analyzed recombinants showed identical RFLP patterns. All sequences obtained were identical to the SM1 euryarchaeal sequences derived from string-of-pearls communities (31, 32).
Only a few bacteria (up to 5% of the total biofilm cells) with different morphologies were enclosed within the biofilm and were identified by FISH. This observation indicated, in combination with our archaeal phylogenetic studies, that we had discovered the first archaeal “monospecies” biofilm in nature, which was formed by the SM1 euryarchaeon.
Since sulfate-reducing bacteria were detected during phylogenetic studies of microbial samples from the Sippenauer Moor (21), the sulfate reducer-specific oligonucleotide SRB385 (2) was used in quantitative FISH studies to access the phylogeny of the biofilm-enclosed bacteria. Most of these cells (∼85% of the bacterial population) showed a positive hybridization signal and can therefore be assigned to the δ-proteobacteria.
Quantitative collection of euryarchaeal SM1 biofilms.
In order to quantify the amount of euryarchaeal SM1 biofilms coming from the subsurface and to obtain sufficient biomaterial for biochemical and genetic studies, a total of 12 polyethylene nets clamped to plastic frames were placed on top of each other within a tube and submersed in the drill hole up to 75 cm deep (Fig. 1B and 2A). Using this method, we were able to harvest, in a fast and reproducible manner, about 1.5 to 2.0 g (wet weight) of euryarchaeal SM1 biofilm within 4 days, which is equivalent to 7 × 109 to 9 × 109 cells. This biomaterial preferentially attached to the five lowermost nets, while the upper nets contained only a small percentage of the total trapped euryarchaeal SM1 biofilm material. However, it was possible to harvest visible, smaller amounts (up to 30 μl per drop) of biofilm (equivalent to about 1 × 108 to 2 × 108 cells) from the net after just 10 min of exposure. The presence of visible biofilm drops, each consisting of at least 108 cells, already after such a short period of time indicates that the biofilms did not grow on the nets but were washed up from deeper underground layers and randomly attached to the polyethylene nets.
Field observations and sampling during a period of about 1 year showed that harvesting could be performed reliably and periodically without significant variations in biofilm quantity or quality.
Physiological and biochemical investigations of subsurface-derived SM1 biofilms.
About 95% of the SM1 euryarchaeal cells in biofilms from the subsurface were viable (Live/Dead Baclight staining), independent of their location within the biofilm. A comparison of the wet and dry weights of euryarchaeal SM1 biofilms revealed a water content of 99.6%. The pH of the interior of the biofilm was 7 to 7.5. Biochemical analyses of the EPS indicated the presence of carbohydrates and proteins in a total ratio of 1:1.5. The DNA contents of the EPS extractions were very low, indicating that intracellular substances did not contribute to these results (19). Due to the proteinaceous character of the EPS, treatment with very low concentrations of proteinase K (15 U/0.1 g of SM1 biofilm) allowed almost complete degradation. This gentle enzymatic procedure did not affect cell stability or viability and allowed the release of single, nonadhesive biofilm cells.
Genetic studies.
The genome size of the SM1 euryarchaeon was determined by PFGE. High-molecular-weight DNA could not be extracted from the agarose-embedded SM1 euryarchaeal cells by standard cell lysis methods: after PFGE, only small DNA fragments (50 to 100 kbp) were observed. An adapted cell lysis protocol was therefore developed, and this allowed the detection of a single band after PFGE, indicating a total genome size of about 3 Mbp. The SM1 genome could not be digested by standard restriction enzymes, suggesting a natural modification of the DNA (e.g., methylation). Since it has been shown that determinations of genome sizes of undigested and restricted DNAs are comparable (36), the determined genome size of about 3 Mbp for the SM1 euryarchaeon is considered accurate. Genomic DNA extracted from the SM1 biofilm showed a G+C content of 35.0 mol%; this agrees with the G+C content determined for the Sippenauer Moor SM1 population grown in situ on polyethylene nets (34.5 mol%) (22).
Ultrastructural studies of subsurface-derived SM1 biofilms.
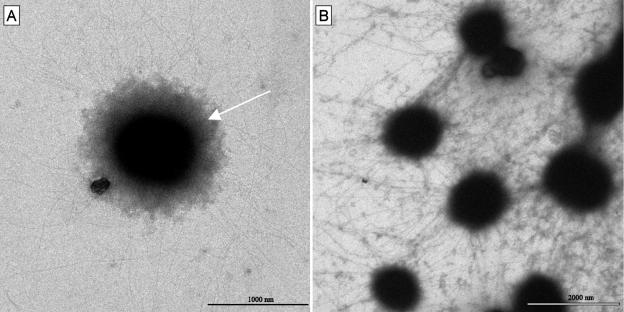
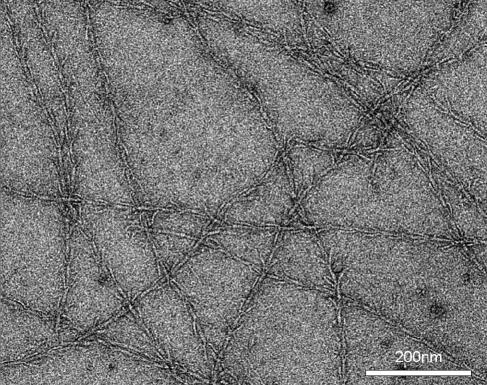
To gain insights into the SM1 biofilm cellular architecture and EPS, transmission electron microscopy (TEM) was performed. Each small coccoid archaeal cell (diameter, 0.4 to 1.0 μm) was embedded in a matrix of highly complex cell appendages comparable to the recently described SM1 hami (20) (Fig. 3A). The hami of neighboring cells were entangled and formed a large, dense web (Fig. 3B and 4). Ultrastructural TEM analysis of the hami revealed a unique architecture with a hook-and-prickle region with the same dimensions and proportions as the hami of the Sippenauer Moor SM1 net population (22) (Fig. 4). Comparative TEM studies showed that proteinase K at low concentrations digested the hami and led to the release of biofilm cells. In contrast to the SM1 cells within native pearls or net populations, most of the cells showed an electron-dense corona in addition to the hami structures (Fig. 3A), which might indicate the existence of an additional polysaccharide matrix component.
FIG. 3.
Electron micrographs of SM1 cells from a negatively stained biofilm. (A) Single SM1 cell with hami and an electron-dense corona surrounding the cell (arrow). (B) Entangled web of SM1 hami within the biofilm.
FIG. 4.
Electron micrograph of negatively stained SM1 hami within the biofilm.
CLSM studies.
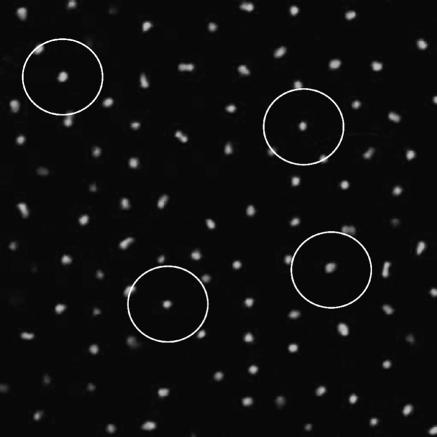
To determine the three-dimensional arrangement and structure of the subsurface-derived SM1 biofilm, confocal laser scanning microscopy was performed, as this method allows for the observation of deeper layers of biomaterial without interference (9, 24). As already indicated by phase-contrast microscopy, CLSM studies showed a constant and regular three-dimensional arrangement of the archaeal cells, with each cell being approximately 4 μm from its neighbors (Fig. 5). It is likely that this constant distance is caused by the hami of neighboring cells, which have an average length of 2 μm. The bacteria that are associated with the archaeal biofilm were distributed throughout the biofilm layers and arranged in small microcolonies of 3 to 20 cells. Several gaps were observed within the biofilm, resembling the so-called “water channels” which are common in natural biofilms (12). Live/Dead Baclight staining indicated that the majority of the cells showed an active physiological state, independent of their location within the biofilm. Accordingly, all cells showed a strong hybridization signal, indicating active reproduction machinery.
FIG. 5.
Confocal laser scanning micrograph of a layer of the SM1 biofilm. The FISH-stained SM1 cells (ARCHmix) show a constant and regular three-dimensional arrangement. Each cell has a distance of about 4 μm to its neighbors. The circles have a diameter of 8 μm.
The SM1 biofilm did not contain any bubbles or inclusions. Furthermore, the matrix was very clear, such that optical cuts to a depth of 50 μm could be performed without problems.
Occurrence of SM1 biofilms in other biotopes.
To investigate the possible occurrence of euryarchaeal SM1 biofilms in different springs of the Sippenauer Moor, we placed polyethylene nets as deeply as possible within the spring holes and in areas with low dissolved oxygen concentrations. However, since the architecture of the Sippenauer Moor springs is quite different from that of the Islinger Mühlbach spring, in most cases it was not possible to place the nets at depth (most springs emanate between stones or the spring holes are very small and not accessible). Nevertheless, after 1 to 2 days of incubation in a few spring holes and areas with low oxygen concentrations and no visible white biomats, milky, slimy biomaterial was attached to the polyethylene nets. A combination of macroscopic and microscopic observations, FISH studies, 16S rRNA gene sequencing, and 16S-23S ribosomal intergenic spacer region sequence analysis clearly showed that this biomaterial once again consisted almost exclusively of the SM1 euryarchaeon. Furthermore, the biofilm architecture, composition, and ultrastructure were very similar to those of the SM1 biofilms from Islinger Mühlbach.
DISCUSSION
The SM1 biofilm has been identified as a new community of SM1 euryarchaea that is different from previously described string-of-pearls structures (Table 1). Short-term experiments clearly showed that the euryarchaeal SM1 biofilms did not grow on the nets but were washed up from the subsurface and randomly attached to the polyethylene nets. This is in striking contrast to other microbial string-of-pearls communities, for which growth on polyethylene nets in sulfidic surface waters has clearly been demonstrated (22).
TABLE 1.
Comparison of different life forms of the SM1 euryarchaeon
| Characteristic | Description for indicated life form
|
||
|---|---|---|---|
| Microbial string-of-pearls community (21, 32) | SM1 net population (22) | SM1 biofilm (this study) | |
| Biotope | Sulfidic surface water at Sippenauer Moor (SM) and Islinger Mühlbach (IM) | Sulfidic surface water at Sippenauer Moor and Islinger Mühlbach | Subsurface (?) at Sippenauer Moor and Islinger Mühlbach |
| SM1 cell viability | >95% | After physical separation; >90% | >95% |
| Microbial structure | SM1 euryarchaeal microcolony surrounded by bacterial filaments, mainly Thiothrix (SM) or IMB1 ɛ-proteobacterium (IM) | Similar to the natural string-of-pearls community; after physical separation, single, coccoid SM1 euryarchaeal cells | Slimy, milky appearance; predominantly SM1 euryarchaeon (up to 95%) |
Since an anaerobic (sulfate-reducing) metabolism has been envisaged for the nonmethanogenic SM1 euryarchaeon (21), it might be the case that SM1 encounters anaerobic conditions suitable for partner-independent growth in the subsurface while requiring a specific interaction with Thiothrix sipK4 or the IMB1 ɛ-proteobacterium for growth in oxygenic surface waters (Table 1). The ability of archaea to form biofilms has rarely been reported for laboratory cultures, e.g., Thermococcus litoralis or Archaeoglobus fulgidus, and this only after artificial induction with special media, pH or temperature changes, or treatments with chemicals (17, 30).
In nature, archaeal biofilms have hitherto not been detected, and archaea have only been identified as minor components of multispecies biofilms in diverse habitats (5, 7, 41). Furthermore, natural biofilms consisting largely of only one species are rare (37, 41). Therefore, SM1 represents the first archaeon for which the formation of a naturally occurring “monospecies” biofilm has been demonstrated. In contrast to the previously described string-of-pearls communities, in which the ratio of archaea to bacteria was about 1:1, the SM1 biofilm is dominated by the archaeal component. The high purity of the biofilm suggests a competition between SM1 euryarchaea and bacteria in which the archaea prevail.
One possibility is that the bacteria are outcompeted by inhibiting molecules such as archaeocines (26) and/or by fast competitive growth of the SM1 euryarchaeon. The majority of the biofilm-enclosed bacteria belong to the sulfate-reducing group of δ-proteobacteria, which is in contrast to the sulfide-oxidizing metabolism of the bacterial partners within the string-of-pearls communities (21, 31). Since sulfate reduction has been proposed for the SM1 euryarchaeon (21), this result may support the theory of a (nutrient) competition between the SM1 euryarchaon and enclosed bacteria within the SM1 biofilm.
A new in situ biofilm trapping system was developed which allowed the quantitative harvesting of the uncultured, cold-loving SM1 euryarchaeon at high purity. This method also enabled periodic sampling and was the basis for a variety of detailed biological analyses and genomic studies of the SM1 euryarchaeon, e.g., revealing a genome size of about 3 Mbp (Table 2). The biochemical and ultrastructural study of the SM1 biofilms showed the existence of EPS enclosing the SM1 euryarchaeal cells and maintaining them separately at constant distances. The EPS consisted of polysaccharides and proteins, which is typical for bacterial biofilms (11). The protein component of the EPS was mainly formed by the filamentous hami, which built up an entangled web between the cells (20) and contributed significantly to the biofilm structure. Most likely, the regular, three-dimensional arrangement of the SM1 euryarchaeal cells within the biofilm is due to the hami, whose average length of about 2 μm results in a cell-cell distance of 4 μm. The hami could be responsible for initial attachment to surfaces and biofilm initiation, as observed for diverse pilus- and flagellum-carrying bacteria (27, 28). The detection of an electron-dense corona surrounding the biofilm cells suggested the existence of a polysaccharide capsule (13); the excretion of (poly)saccharides such as glycogen or mannose has already been demonstrated for diverse mesophilic and thermophilic archaea (3, 15, 25, 33).
TABLE 2.
Biological properties of SM1 euryarchaeal biofilm
| Parameter | Description for SM1 euryarchaeal biofilm or value |
|---|---|
| Visual appearance | Milky, slimy, small drops |
| Harvesting | Harvesting was done by providing polyethylene nets as deep as possible in the sulfidic spring bore hole; attachment of visible, small biofilm drops was seen already after 10 min; quantitative harvesting after 4 days resulted in 1.5 to 2 g (wet weight) |
| Microbial composition | ∼95% SM1 euryarchaea, ∼5% bacteria (mainly representatives of the δ-proteobacteria) |
| Structural features | Cells surrounded by filamentous hami and an additional, dense EPS; hami were degradable by proteinase K |
| EPS composition | Carbohydrates and proteins present at a ratio of 1:1.5 |
| Water content | ∼99.6% |
| pH | 7-7.5 |
| Genome size (SM1 biofilm cells) | ∼3 Mbp |
| GC content (SM1 biofilm cells) | 35.0 mol% |
Most likely, the polysaccharides surrounding the SM1 cells are responsible for the formation of the slimy matrix and, together with the proteins, for the extraordinarily high water content of the biofilm of 99.6% (11). Nevertheless, nutrient flow seemed to be possible even within deeper layers of the biofilm: cell activity and viability, as indicated by CLSM studies combined with live/dead staining, were confirmed by strong FISH hybridization signals. The latter correlated with the content of cellular rRNA, a good marker of metabolic activity (39, 40). Nevertheless, the SM1 biofilm appears to be subject to a maturation and aging process in its subsurface habitat (27). This includes detachment of parts of the biofilm by the subsurface water flow or enzymatic degradation of exopolymers (1), which then attach to the provided polyethylene nets in the drill hole.
Our results suggest that large, extended SM1 biofilms occur in the subsurface, where the stable, anaerobic milieu of deeper earth layers (38) provides optimal conditions for the partner-independent growth of the SM1 euryarchaeon. SM1 and its relatives have been found in diverse habitats around the world, such as rice fields and rice roots, a deep-sea hydrothermal vent system, gold mine fissure waters, and diverse sulfurous springs (31). This suggests a significant broad ecological role of the SM1 biofilms and perhaps an important role in biogeochemical activities and geomicrobiological processes such as environmental sulfate reduction. Furthermore, sulfate-reducing biofilms have become the focus of biotechnological research for their potential use in sulfate-reducing bioreactors for wastewater treatment (18).
This study describes the discovery and first insights into the organization, properties, and ecological significance of thus far unknown natural archaeal biofilms by using the described polyethylene net approach. The information obtained significantly extends our knowledge of naturally occurring archaeal life forms in low-temperature environments.
Acknowledgments
We thank Maria Sonnleitner for providing data, Reinhard Wirth for PFGE, and Reinhard Rachel for EM support.
Financial support from the Deutsche Forschungsgemeinschaft (Hur 711/2) is gratefully acknowledged.
REFERENCES
- 1.Allison, D. G., B. Riz, A. SanJose, and P. Gilbert. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179-184. [DOI] [PubMed] [Google Scholar]
- 2.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antón, J., I. Meseger, and F. Rodríguez-Valera. 1988. Production of an extracellular polysaccharide by Haloferax mediterranei. Appl. Environ. Microbiol. 54:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antunes, A., W. Eder, P. Fareleira, H. Santos, and R. Huber. 2003. Salinisphaera shabanensis gen. nov., sp. nov., a novel, moderately halophilic bacterium from the brine-seawater interface of the Shaban Deep, Red Sea. Extremophiles 7:29-34. [DOI] [PubMed] [Google Scholar]
- 5.Battin, T. J., A. Wille, B. Sattler, and R. Psenner. 2001. Phylogenetic and functional heterogeneity of sediment biofilms along environmental gradients in a glacial stream. Appl. Environ. Microbiol. 67:799-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann, C., M. Judex, H. Huber, and R. Wirth. 1998. Estimation of genome sizes of hyperthermophiles. Extremophiles 2:101-108. [DOI] [PubMed] [Google Scholar]
- 7.Bond, P. L., S. P. Smriga, and J. F. Banfield. 2000. Phylogeny of microorganisms populating a thick, subaerial, predominantly lithotrophic biofilm at an extreme acid mine drainage site. Appl. Environ. Microbiol. 66:3842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burggraf, S., H. Huber, and K. O. Stetter. 1997. Reclassification of the crenarchaeal orders and families in accordance with 16S ribosomal RNA sequence data. Int. J. Syst. Bacteriol. 47:657-660. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell, D. E., D. R. Korber, and J. R. Lawrence. 1993. Analysis of biofilm formation using 2D vs 3D digital imaging. J. Appl. Bacteriol. Symp. 74(Suppl.):52S-66S. [Google Scholar]
- 10.Daims, H., A. Brühl, R. Amann, K.-H. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 11.Flemming, H.-C., J. Wingender, and C. Mayer. 1998. Extracellular polymeric substances—the material which forms the biofilm. Med. Fac. Landbouww. Univ. Gent 63:1129-1136. [Google Scholar]
- 12.Fletcher, M., and A. W. Decho. 2001. Biofilms. Encyclopedia Life Sci. [Online.] 10.1038/npg.els.0000342.
- 13.Grund, S. 1991. Slime capsule and fimbriae on Salmonella typhimurium var. cop.—electron microscopic study. J. Vet. Med. 38:3-16. [DOI] [PubMed] [Google Scholar]
- 14.Higgins, D. G., and P. M. Sharp. 1988. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene 73:237-244. [DOI] [PubMed] [Google Scholar]
- 15.König, H., R. Skorko, W. Zillig, and W. D. Reiter. 1982. Glycogen in thermophilic archaebacteria of the genera Sulfolobus, Thermoproteus, Desulfurococcus and Thermococcus. Arch. Microbiol. 132:297-303. [Google Scholar]
- 16.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, England.
- 17.LaPaglia, C., and P. L. Hartzell. 1997. Stress-induced production of biofilm in the hyperthermophile Archaeoglobus fulgidus. Appl. Environ. Microbiol. 63:3158-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lens, P., M. Vallero, G. Esposito, and M. Zandvoort. 2002. Perspectives of sulfate reducing bioreactors in environmental biotechnology. Rev. Environ. Sci. Biotechnol. 1:311-325. [Google Scholar]
- 19.Liu, H., and H. H. P. Fang. 2002. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 95:249-256. [DOI] [PubMed] [Google Scholar]
- 20.Moissl, C., R. Rachel, A. Briegel, H. Engelhardt, and R. Huber. 2004. The unique structure of archaeal “hami,” highly complex cell appendages with nano- grappling hooks. Mol. Microbiol. 56:361-370. [DOI] [PubMed] [Google Scholar]
- 21.Moissl, C., C. Rudolph, and R. Huber. 2002. Natural communities of novel archaea and bacteria with a string-of-pearls-like morphology: molecular analysis of the bacterial partners. Appl. Environ. Microbiol. 68:933-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moissl, C., C. Rudolph, R. Rachel, M. Koch, and R. Huber. 2003. In situ growth of the novel SM1 euryarchaeon from a string-of-pearls-like microbial community in its cold biotope, its physical separation and insights into its structure and physiology. Arch. Microbiol. 180:211-217. [DOI] [PubMed] [Google Scholar]
- 23.Nelson, D. C. 1989. Physiology and biochemistry of filamentous sulfur bacteria, p. 219-238. In H. G. Schlegel and B. Bowien (ed.), Autotrophic bacteria. Science Technical Publishers, Madison, Wis.
- 24.Neu, T. R., and J. R. Lawrence. 1997. Development and structure of microbial biofilms in river water studied by confocal laser scanning microscopy. FEMS Microbiol. Ecol. 24:11-25. [Google Scholar]
- 25.Nicolaus, B., M. C. Manca, I. Romano, and L. Lama. 1993. Production of an exopolysaccharide from two thermophilic archaea belonging to the genus Sulfolobus. FEMS Microbiol. Lett. 109:203-206. [Google Scholar]
- 26.O'Connor, E. M., and R. F. Shand. 2002. Halocins and sulfolobicins: the emerging story of archaeal protein and peptide antibiotics. J. Ind. Microbiol. Biotechnol. 28:23-31. [DOI] [PubMed] [Google Scholar]
- 27.O′Toole, G. A., and R. Kolter. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295-304. [DOI] [PubMed] [Google Scholar]
- 28.Pratt, L. A., and R. Kolter. 1999. Genetic analyses of bacterial biofilm formation. Curr. Opin. Microbiol. 2:598-603. [DOI] [PubMed] [Google Scholar]
- 29.Rabenhorst, L. 1848. Die Algen Sachsens, resp. Mitteleuropas Algen Europas Dec. 1-104 (101-204), no. 1-1000. Exsikkate und Begleittext, Dresden, Germany.
- 30.Rinker, K. D., and R. M. Kelly. 1996. Growth physiology of the hyperthermophilic archaeon Thermococcus litoralis: development of a sulfur-free defined medium, characterization of an exopolysaccharide, and evidence of biofilm formation. Appl. Environ. Microbiol. 62:4478-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudolph, C., C. Moissl, R. Henneberger, and R. Huber. 2004. Ecology and microbial structures of archaeal/bacterial strings-of-pearls communities and archaeal relatives thriving in cold sulfidic springs. FEMS Microbiol. Ecol. 50:1-11. [DOI] [PubMed] [Google Scholar]
- 32.Rudolph, C., G. Wanner, and R. Huber. 2001. Natural communities of novel archaea and bacteria growing in cold sulfurous springs with a string-of-pearls-like morphology. Appl. Environ. Microbiol. 67:2336-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sowers, K. R., and R. P. Gunsalus. 1988. Adaptation for growth at various saline concentrations by the archaebacterium Methanosarcina thermophila. J. Bacteriol. 170:998-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stahl, D. A., and R. Amann. 1991. Development and application of nucleic acid probes, p. 205-248. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, England.
- 35.Summit, M., and J. A. Baross. 2001. A novel microbial habitat in the mid-ocean ridge subseafloor. Proc. Natl. Acad. Sci. USA 98:2158-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun, L. V., J. M. Foster, G. Tzertzinis, M. Ono, C. Bandi, B. E. Slatko, and S. L. O'Neill. 2001. Determination of Wolbachia genome size by pulsed-field gel electrophoresis. J. Bacteriol. 183:2219-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland, I. W. 2001. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3-9. [DOI] [PubMed] [Google Scholar]
- 38.Vorobyova, E., V. Soina, M. Gorlenko, N. Minkovskaya, N. Zalinova, A. Mamkelashvili, D. Gilichinsky, E. Rivkina, and T. Vishnivetskaya. 1997. The deep cold biosphere: facts and hypothesis. FEMS Microbiol. Rev. 20:277-290. [Google Scholar]
- 39.Wagner, M., R. Amann, P. Kämpfer, B. Assmus, A. Hartmann, P. Hutzler, N. Springer, and K. H. Schleifer. 1994. Identification and in situ detection of gram-negative filamentous bacteria in activated sludge. Syst. Appl. Microbiol. 17:405-417. [Google Scholar]
- 40.Wallner, G., R. Amman, and W. Beisker. 1993. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometrie 14:136-143. [DOI] [PubMed] [Google Scholar]
- 41.Webster, N. S., L. D. Smith, A. J. Heyward, J. E. M. Watts, R. I. Webb, L. L. Blackall, and A. P. Negri. 2004. Metamorphosis of a scleractinian coral in response to microbial biofilms. Appl. Environ. Microbiol. 70:1213-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winogradsky, S. 1887. Ueber Schwefelbacterien. Botanische Zeitung 45:513-559. [Google Scholar]