Abstract
The intramolecular distribution of nitrogen isotopes in N2O is an emerging tool for defining the relative importance of microbial sources of this greenhouse gas. The application of intramolecular isotopic distributions to evaluate the origins of N2O, however, requires a foundation in laboratory experiments in which individual production pathways can be isolated. Here we evaluate the site preferences of N2O produced during hydroxylamine oxidation by ammonia oxidizers and by a methanotroph, ammonia oxidation by a nitrifier, nitrite reduction during nitrifier denitrification, and nitrate and nitrite reduction by denitrifiers. The site preferences produced during hydroxylamine oxidation were 33.5 ± 1.2‰, 32.5 ± 0.6‰, and 35.6 ± 1.4‰ for Nitrosomonas europaea, Nitrosospira multiformis, and Methylosinus trichosporium, respectively, indicating similar site preferences for methane and ammonia oxidizers. The site preference of N2O from ammonia oxidation by N. europaea (31.4 ± 4.2‰) was similar to that produced during hydroxylamine oxidation (33.5 ± 1.2‰) and distinct from that produced during nitrifier denitrification by N. multiformis (0.1 ± 1.7‰), indicating that isotopomers differentiate between nitrification and nitrifier denitrification. The site preferences of N2O produced during nitrite reduction by the denitrifiers Pseudomonas chlororaphis and Pseudomonas aureofaciens (−0.6 ± 1.9‰ and −0.5 ± 1.9‰, respectively) were similar to those during nitrate reduction (−0.5 ± 1.9‰ and −0.5 ± 0.6‰, respectively), indicating no influence of either substrate on site preference. Site preferences of ∼33‰ and ∼0‰ are characteristic of nitrification and denitrification, respectively, and provide a basis to quantitatively apportion N2O.
Over the past several decades, anthropogenic activity, primarily agriculture, has doubled the annual input of biologically reactive nitrogen into the environment (14). This surplus of reactive nitrogen has stimulated natural microbial activity, the largest source of the greenhouse gas nitrous oxide (N2O) (17, 26). Ammonia- and methane-oxidizing organisms produce N2O during the oxidation of hydroxylamine (NH2OH) to nitrite (NO2−). Ammonia-oxidizing bacteria also reduce NO2− to N2O and N2 under anoxic conditions by a process termed nitrifier denitrification (12, 22, 23). Nitrous oxide can also be produced and consumed by heterotrophic denitrifying organisms. In this case, N2O is produced and consumed by the stepwise reduction of nitrate (NO3−) to N2 (33).
The relative importance of nitrification and denitrification in N2O production has proven difficult to determine. Previous attempts to differentiate nitrification- and denitrification-mediated N2O production in soils using stable isotope approaches (4, 20, 30, 31, 32) relied on the observation that the fractionation factor associated with N2O production by denitrifiers (2) is substantially less than that associated with nitrification (34). The assumption was that N2O with a high δ15N value is indicative of denitrification, whereas a lower value reflects nitrification (4). However, this approach is problematic because the isotopic composition of the substrate (nitrate or ammonia) can vary temporally and spatially (20).
Yoshida and Toyoda (35) suggested that analyses of the intramolecular distributions of 15N in N2O (isotopomers) could offer additional information to more tightly constrain sources and sinks of this greenhouse gas. Quantification of the relative abundances of 15N in the central (α) and terminal (β) N atoms of the linear N2O molecule relies on the fragmentation of N2O+ to NO+ within the ion source of a mass spectrometer (5, 11, 29). The intramolecular distribution of 15N is often expressed as the site preference (SP = δ15Nα − δ15Nβ) (28).
Sutka et al. (27) demonstrated that N2O produced from NH2OH oxidation by Nitrosomonas europaea and Methylococcus capsulatus Bath had a higher SP than that produced by NO2− reduction. In the present study, we evaluated the SPs of N2O produced during nitrification and denitrification more comprehensively. In addition to N. europaea and M. capsulatus Bath, we examined N2O production from NH2OH by the ammonia oxidizer Nitrosospira multiformis and the methane oxidizer Methylosinus trichosporium. We also examined the SPs of N2O produced during ammonia oxidation in batch cultures of N. europaea and compared them to the results from the NH2OH oxidation experiments. In order to understand culture conditions that might influence N2O production by NO2− reduction rather than NH2OH oxidation, we evaluated the effects of the NO2− concentration and the surface area available for oxygen diffusion in concentrated cell suspensions of N. europaea. Nitrous oxide production by denitrifying organisms that lack N2O reductase, namely, Pseudomonas chlororaphis and Pseudomonas aureofaciens, was also studied to compare the values to those obtained in studies of Pseudomonas fluorescens and Pseudomonas denitrificans by Toyoda et al. (29). These SP data provide an essential foundation to apportion the production of N2O in field studies, such as those of Pérez et al. (20) and Yamulki et al. (32).
MATERIALS AND METHODS
Organisms and cultivation.
Nitrosomonas europaea (ATCC 19718) was maintained in ammonium mineral salts medium as described by Sutka et al. (27). The medium was autoclaved, cooled, adjusted to pH 7.5 to 7.7 with 5% (wt/vol) K2CO3, and inoculated with 5 ml of culture. Cultures were incubated at 25°C, and the pH was adjusted back to 7.5 to 7.7 as necessary (typically three to four times per week). Nitrosospira multiformis (ATCC 25196) was maintained in 75 ml of Nitrosolobus medium (ATCC medium 929) at 25°C, and the pH was adjusted to 7.5 to 7.7 with 5% (wt/vol) K2CO3. Methylosinus trichosporium (ATCC 49243) was maintained in modified nitrate mineral salts medium under a headspace of 30% (vol/vol) methane in air as described by Sutka et al. (27). Pseudomonas aureofaciens (ATCC 13985) and Pseudomonas chlororaphis (ATCC 43928) were maintained on tryptic soy agar (Difco, Detroit, MI) plates at 25°C. To prepare starter cultures for N2O production experiments, an isolated colony of P. aureofaciens or P. chlororaphis was used to inoculate 50 ml of citrate minimal medium (CMM) (1).
Preparation of cell suspensions for NH2OH oxidation experiments.
Cell suspensions of N. europaea (6.2 × 107 to 7.0 × 107 cells/ml) and N. multiformis (2.3 × 107 to 2.5 × 107 cells/ml) were prepared by combining three cultures (75-ml liquid volume) grown to late exponential phase. The cultures used for cell suspensions were tested for heterotrophic contamination by inoculating 3 ml of culture separately into 5 ml of nutrient broth (Difco) and tryptic soy broth (Difco) in 25-ml screw-cap tubes. In all cases, turbidity was not detected in the tubes after 30 days of incubation, indicating an absence of detectable contamination in the ammonia oxidizer cultures. Cell suspensions of M. trichosporium (7.4 × 107 to 1.9 × 108 cells/ml) were prepared from three cultures (50-ml liquid volume) grown to the late exponential phase of growth. N. europaea, N. multiformis, and M. trichosporium cells were concentrated by centrifugation at 10,000 × g for 10 min at 5°C. The cells were subsequently resuspended in 20 ml of 0.1 M potassium phosphate buffer (pH 7.5) and then reconcentrated by centrifugation. This process was completed twice to remove ammonium (NH4+), NO2−, and NH2OH. The final cell pellet was resuspended in 10 ml of 0.01 M potassium phosphate buffer. Experimental cultures to test the amount of N2O produced from NH2OH oxidation were prepared in 25-ml anaerobic culture tubes (Bellco, Vineland, NJ) by adding 2 ml of cell suspension and 300 μl of 0.01 M of NH2OH solution and stoppering the tubes under air.
Preparation of cultures for ammonia oxidation experiments.
Nitrous oxide production with NH4+ as the substrate was investigated in batch cultures of N. europaea (1.9 × 107 to 3.8 × 107 cells/ml after 6 days of growth). The experimental cultures consisted of 25 ml of ammonium mineral salts medium, as described by Sutka et al. (27), in 165-ml serum bottles stoppered under air. An additional aliquot of 5 ml of O2 was added to the headspace of the serum bottles to ensure oxic conditions throughout the course of incubation. The serum bottles were inoculated with 0.5 ml of N. europaea stock culture and incubated for 6 days on a rotating arm to facilitate equilibration of the liquid phase with the headspace gases. Headspace samples were obtained at the end of the 6-day incubation period and analyzed immediately.
Preparation of cell suspensions for NO2− and NO3− reduction experiments.
Cells of N. multiformis for NO2− reduction experiments were concentrated and resuspended in potassium phosphate buffer as described for the NH2OH oxidation experiments. The cultures were constructed by adding 2 ml of cell suspension (2.3 × 107 to 2.5 × 107 cells/ml), 300 μl of a 0.01 M NaNO2 solution, and 100 μl of a 0.01 M NH2OH solution. The headspace was purged for 5 min with N2 to hasten the onset of anoxia, and then the tubes were stoppered.
Cultures for cell suspensions of P. chlororaphis (1.2 × 109 to 4.9 × 109 cells/ml) and P. aureofaciens (4.4 × 109 cells/ml) were prepared by inoculating 0.1 ml of each seed culture into 50 ml of CMM with 10 mM NO3− in a 160-ml serum bottle purged with N2. After the cultures were grown to late exponential phase, two 50-ml cultures were combined and concentrated by centrifugation at 10,000 × g for 10 min at 5°C. Cells were resuspended in 20 ml of CMM without NO3−. The experimental cultures were prepared by adding 2 ml of the cell suspension to a 25-ml serum tube and purging the headspace for 5 min with N2. For the NO3− and NO2− reduction experiments, 300 μl of a 0.01 M NaNO3− or NaNO2 solution, respectively, was added to the cell suspension. The experiment to calculate isotopic enrichment factors for NO3− reduction to N2O by P. chlororaphis and P. aureofaciens was completed in 12-ml Exetainer vials (Labco, United Kingdom) with 2 ml of cell suspension and 300 ml of 0.01 M NaNO3−.
N2O concentration and isotopic analysis.
Headspace samples were obtained from the cell suspensions with gas-tight syringes (Hamilton, Reno, NV). Typical headspace sample sizes ranged from 100 to 2,000 μl. Prior to sampling, an equal volume of air (for NH2OH oxidation experiments) or N2 (for NO2− and NO3− reduction experiments) was injected into the headspace to maintain atmospheric pressure in the culture tubes. Multiple gas samples were obtained from the same tube, and N2O concentrations were corrected to take into account the dilution due to the addition of air or N2. Samples were immediately analyzed on a Trace Gas system interfaced with a multicollector IsoPrime mass spectrometer (GV Instruments, United Kingdom) as described by Sutka et al. (27). The isotopic composition of 15N and 18O in N2O is expressed in δ notation with respect to the air and Vienna Standard Mean Ocean Water (VSMOW) standards, as follows: δ = [(Rsample/Rstandard) − 1] × 1,000, where Rsample = 15N/14N and 18O/16O for the sample and Rstandard = 15N/14N and 18O/16O for the standard.
The isotopic composition of 15N at the β position was calculated after measurement of the δ-15Nbulk and δ-15Nα, as follows: δ-15Nbulk = (δ15Nα + δ15Nβ)/2.
Isotope values were corrected for the presence of 17O and rearrangement within the ion source by the approach indicated by Toyoda and Yoshida (28). The δ15N, δ15Nα, and δ18O values for the in-house N2O reference are 1.6, 14.9, and 41.7‰, respectively.
Relative importance of NO2− reduction and NH2OH oxidation to N2O production.
The relative importance of NH2OH oxidation and NO2− reduction can be influenced by the O2 and NO2− concentrations. In our experiments, we varied the surface area available for oxygen diffusion to either limit or promote oxygen availability in the liquid phase of concentrated N. europaea cell suspensions. A 1% (wt/wt) mixture of >98% 15N-enriched NaNO2− (Cambridge Isotope Laboratories, Andover, MA) and NaNO2− at its natural abundance level (1.5‰) was diluted to make a working solution with a final concentration of 0.01 M NO2− and a nitrogen isotopic composition of 0.99 ± 0.02 atom% (n = 3). The liquid surface area available for oxygen diffusion was varied by incubating 25-ml stoppered tubes either horizontally (ratio of liquid surface area to total liquid volume [S/V], 1.2 cm−1) or vertically (S/V,of 12.6 cm−1). The experimental cultures for the two incubation conditions consisted of 2 ml of concentrated N. europaea cell suspension amended with 300 μl of NH2OH and 100 μl of 15N-enriched NO2−. The experimental cultures were incubated statically for 120 min, and the headspace gas was then sampled and analyzed immediately for the concentration and δ15N value of N2O. The fraction of N2O derived from the 15N-enriched NO2− versus NH2OH oxidation was calculated based on isotope mass balance.
In experiments to examine the effect of NO2− concentration on the fraction of N2O derived from NO2− reduction versus NH2OH oxidation, concentrated N. europaea cell suspensions were prepared similarly to those used in the surface area experiments. In this case, the concentration of 15N-enriched NO2− varied from 0.05 mM to 0.6 mM, and 100 μl of 0.01 M NH2OH was added to each cell suspension. All incubations were conducted with a high S/V ratio (12.6 cm−1) for 90 min. Headspace gas samples were obtained at the end of the incubation period and immediately analyzed to obtain the concentration and δ15N value of N2O.
Abiological N2O production.
Control experiments were conducted to evaluate the abiological production of N2O from NH2OH and NO2−. In the first experiment, 2 ml of 0.1 M potassium phosphate buffer and 300 μl of a 0.01 M NH2OH solution were added to a 25-ml stoppered serum tube with an air headspace. The concentration of N2O attributed to abiological reactions involving NH2OH after 8 h of incubation at 25°C was 0.4 μM. A second experiment was constructed with 2 ml of 0.1 M phosphate buffer, 300 μl of a 0.01 M NaNO2− solution, and 100 μl of 0.01 M NH2OH in a 25-ml test tube with a headspace of N2. The headspace concentration of N2O was 0.8 μM in the second experimental control after 8 h of incubation. A killed-cell experiment was conducted with 2 ml of N. europaea cell suspension that had been autoclaved for 20 min at 120°C and 22 lb/in2. The killed-cell suspension was amended with 300 μl of a 0.01 M NH2OH solution with an air headspace. The N2O concentration was 0.1 μM in the killed-cell control after 8 h of incubation.
Statistical analysis.
A general linear mixed model with N2O as the covariate was used to determine if there was a trend between SP and N2O concentrations. We found no trend and used repeated-measure analysis of variance (RMANOVA) with a general linear mixed model to investigate differences in SP associated with taxa and reaction pathways within nitrifiers and denitrifiers. Specifically, we asked (i) if there was a difference in SP of N2O produced by individual taxa during NH2OH oxidation, (ii) if N2O produced by NH2OH oxidation and NO2− reduction by nitrifiers had different SPs, (iii) if the SP of N2O produced by NO3− reduction differed from that produced by NO2− reduction for the same denitrifier taxon, and (iv) if there was a difference in SP of N2O produced from NO3− and NO2− by denitrifiers. For RMANOVA, taxon was the fixed effect, time was the repeated measure, and replicate cultures were the subject units. A similar RMANOVA was used to determine if δ15N and δ18O values for N2O produced by NH2OH oxidation differed among taxa. All analyses were performed using SAS, version 8.0 (SAS Institute).
RESULTS
NH2OH oxidation.
The headspace N2O concentrations from NH2OH oxidation by N. europaea, N. multiformis, and M. trichosporium increased during time course experiments (Table 1). The average δ15N values of N2O produced by NH2OH oxidation by N. europaea (−0.3 ± 4.9‰), N. multiformis (−0.3 ± 2.9‰), and M. trichosporium (3.4 ± 1.9‰) were similar, as were the average δ18O values of 38.8 ± 2.9‰ for N. euro paea, 38.6 ± 2.5‰ for N. multiformis, and 39.7 ± 4.0‰ for M. trichosporium. We define an apparent fractionation for branched reactions as follows: δ15N(substrate) − δ15N(product), or Δ15N. The Δ15N associated with N2O production from NH2OH oxidation was −2.0‰ for N. europaea and N. multiformis and −5.7‰ for M. trichosporium (δ15N of NH2OH = −2.3‰). The average SPs of N2O produced during NH2OH oxidation were high for N. multiformis (32.5 ± 0.6‰), N. europaea (33.5 ± 1.2‰), and M. trichosporium (35.6 ± 1.4‰). The site preference for N. europaea did not differ from that for N. multiformis (P = 0.198) or M. trichosporium (P = 0.166). The significant difference observed between M. trichosporium and N. multiformis (P = 0.025) was related in part to the low standard deviation associated with the N. multiformis data.
TABLE 1.
Concentration, δ15N, δ18O, and site preference of N2O produced during NH2OH oxidation by concentrated cell suspensions of N. europaea, N. multiformis, and M. trichosporium
| Organism and replicatea | Time elapsed (min) | [N2O] (μM) | δ15N-N2O (‰) | δ18O-N2O (‰) | Site preference (‰) |
|---|---|---|---|---|---|
| Nitrosomonas europaea | |||||
| A | 304 | 10.8 | −5.5 | 35.8 | 34.5 |
| A | 390 | 10.5 | −5.5 | 35.5 | 34.6 |
| A | 442 | 10.9 | −4.8 | 37.0 | 31.1 |
| B | 135 | 12.6 | 3.8 | 40.8 | 33.1 |
| B | 315 | 15.2 | 4.6 | 42.5 | 31.9 |
| B | 395 | 16.6 | 5.1 | 42.3 | 32.1 |
| C | 289 | 6.8 | 0.6 | 38.4 | 33.7 |
| C | 429 | 6.8 | −1.6 | 38.3 | 37.5 |
| C | 507 | 6.7 | 0.9 | 38.5 | 33.1 |
| Avg | −0.3 | 38.8 | 33.5 | ||
| SD | 4.9 | 2.9 | 1.2 | ||
| Nitrosospira multiformis | |||||
| A | 260 | 7.5 | −3.6 | 35.6 | 33.1 |
| A | 369 | 8.8 | −3.9 | 35.5 | 32.1 |
| A | 422 | 9.3 | −3.5 | 36.1 | 34.2 |
| B | 215 | 7.5 | 1.3 | 40.0 | 31.4 |
| B | 270 | 8.5 | 0.9 | 39.7 | 30.7 |
| B | 315 | 8.8 | 0.9 | 39.9 | 33.6 |
| C | 411 | 8.6 | 1.7 | 38.0 | 32.7 |
| C | 490 | 9.9 | 1.6 | 41.2 | 31.8 |
| C | 573 | 10.1 | 1.6 | 41.8 | 32.8 |
| Avg | −0.3 | 38.6 | 32.5 | ||
| SD | 2.9 | 2.5 | 0.6 | ||
| Methylosinus trichosporium | |||||
| A | 165 | 5.7 | 1.4 | 35.1 | 37.3 |
| A | 185 | 5.7 | 1.4 | 35.4 | 35.0 |
| A | 205 | 6.3 | 1.3 | 35.6 | 37.5 |
| B | 95 | 12.7 | 5.2 | 39.2 | 34.9 |
| B | 155 | 19.5 | 5.0 | 40.4 | 33.3 |
| B | 195 | 23.3 | 4.7 | 41.1 | 35.1 |
| B | 290 | 30.8 | 4.2 | 42.6 | 35.0 |
| B | 330 | 30.9 | 4.2 | 41.8 | 35.0 |
| B | 450 | 38 | 3.9 | 45.7 | 34.4 |
| Avg | 3.4 | 39.7 | 35.6 | ||
| SD | 1.9 | 4.0 | 1.4 |
Replicates (A, B, or C) represent experiments conducted on separate days with different cultures sampled over time.
Ammonia oxidation.
The N2O concentrations in the headspaces of N. europaea batch cultures with ammonia as the substrate were 1.8 to 3.7 μM after 6 days of incubation (Table 2). The δ15N and δ18O values for N2O were −46.5 ± 0.4‰ and 23.5 ± 1.3‰, respectively (Table 2). The Δ15N associated with the production of N2O during ammonia oxidation was −46.9‰ [δ15N of (NH4)2SO4 = 0.4‰]. There was no difference between the SP of the N2O produced from ammonia oxidation by N. europaea (31.4 ± 4.2‰; Table 2) and that in the NH2OH oxidation experiments (P = 0.334) (Table 1).
TABLE 2.
Concentration, δ15N, δ18O, and site preference of N2O produced during ammonia oxidation by cultures of Nitrosomonas europaea for four independent experiments
| N2O concn (μM) | δ15N-N2O (‰) | δ18O-N2O (‰) | Site preference (‰) |
|---|---|---|---|
| 2.4 | −46.6 | 22.2 | 36.8 |
| 1.8 | −46.9 | 22.6 | 27.5 |
| 3.4 | −46.2 | 24.9 | 28.6 |
| 3.7 | −46.1 | 24.4 | 32.8 |
| Avg | −46.5 | 23.5 | 31.4 |
| SD | 0.4 | 1.3 | 4.2 |
Nitrifier denitrification.
The concentration of N2O in the headspace of Nitrosospira multiformis cell suspensions incubated with NO2− increased in each replicate (Table 3). The average δ15N and δ18O values for the N2O produced were −22.9 ± 0.6‰ and 10.8 ± 0.5‰, respectively. The average SP for this experiment (0.1 ± 1.7‰) was significantly different from those produced from NH2OH by N. multiformis, N. europaea, and M. trichosporium (P < 0.001 for all taxa).
TABLE 3.
Concentration, δ15N, δ18O, and site preference of N2O produced during NO2− reduction in concentrated cell suspensions of Nitrosospira multiformis
| Replicate | Time elapsed (min) | N2O concn (μM) | δ15N-N2O (‰) | δ18O-N2O (‰) | Site preference (‰) |
|---|---|---|---|---|---|
| A | 773 | 4.2 | −23.6 | 10.7 | −3.8 |
| A | 854 | 4.4 | −23.5 | 10.9 | 0.5 |
| A | 874 | 5.1 | −23.7 | 10.1 | 0.0 |
| A | 984 | 6.4 | −23.5 | 9.8 | −4.0 |
| B | 335 | 3.2 | −23.4 | 11.5 | 1.2 |
| B | 479 | 7.9 | −22.0 | 10.5 | 1.1 |
| B | 541 | 8.2 | −21.7 | 10.5 | 1.5 |
| C | 210 | 6.2 | −24.2 | 11.5 | 0.3 |
| C | 280 | 8.5 | −23.9 | 11.4 | 0.6 |
| C | 390 | 14.1 | −23.1 | 11.5 | 1.9 |
| C | 460 | 17.4 | −21.7 | 10.9 | 1.6 |
| C | 530 | 20.8 | −21.5 | 11.4 | 0.3 |
| Avg | −22.9 | 10.8 | 0.1 | ||
| SD | 0.6 | 0.5 | 1.7 |
Denitrification.
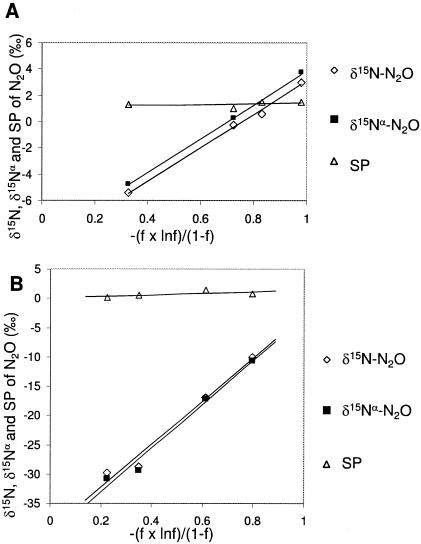
Nitrous oxide was produced by P. chlororaphis and P. aureofaciens with NO2− and NO3− as electron acceptors (Table 4). The SPs of N2O produced by P. chlororaphis and P. aureofaciens from NO3− were −0.5 ± 1.9‰ and −0.5 ± 0.6‰, respectively, and those of N2O produced from NO2− were −0.6 ± 1.9‰ and −0.5 ± 1.9‰, respectively. There was no significant influence of the inorganic nitrogen substrate on SP values for either taxon (P = 0.087 and 0.099, for P. chlororaphis and P. aureofaciens, respectively), indicating that the SP of N2O produced during denitrification is independent of the substrate (Table 4). The isotope enrichment factor for a unidirectional reaction relating the isotopic composition of the product to the substrate (ɛp/s) is described as follows (16): δ15N(product) = δ15N(substrate) − ɛp/s[(f × ln f)/(1 − f)], where f = [NO2−]/[NO2−]initial. The slope of the relationship of −(f × ln f)/(1 − f) to δ15N, δ15Nα, or the SP of accumulated N2O from NO2− is equated with ɛp/s during nitrifier denitrification (16). The values for ɛp/s for δ15N and δ15Nα associated with P. chlororaphis were 12.7‰ and 12.9‰, respectively (Fig. 1A). For P. aureofaciens, ɛp/s for N2O production from NO3− reduction is 36.7‰, with a value of 37.4‰ for δ15N and δ15Nα, respectively (Fig. 1B). The SP for N2O did not change appreciably during the course of the reaction. This is demonstrated by the slope, which cannot be distinguished from zero based on our analytical precision (slope = 0.3 and 1.3 for P. chlororaphis and P. aureofaciens, respectively) (Fig. 1).
TABLE 4.
Concentration and site preference of N2O produced by concentrated cell suspensions of P. chlororaphis and P. aureofaciens in time course samples with NO2− and NO3− as substrates
| Organism and substrate | Sampling point | Time elapsed (min) | [N2O] (μM) | Site preference (‰) |
|---|---|---|---|---|
| Pseudomonas chlororaphis | ||||
| NO3− | 1 | 55 | 21.1 | 2.5 |
| 2 | 75 | 40.5 | 3.7 | |
| 3 | 95 | 57.2 | −0.3 | |
| Avg | −0.5 | |||
| SD | 1.9 | |||
| NO2− | 1 | 50 | 6.6 | −2.5 |
| 2 | 110 | 12.2 | 0.9 | |
| 3 | 190 | 22.6 | −1.9 | |
| 4 | 230 | 29.3 | 1.2 | |
| Avg | −0.6 | |||
| SD | 1.9 | |||
| Pseudomonas aureofaciens | ||||
| NO3− | 1 | 234 | 7.1 | −1.2 |
| 2 | 461 | 16.5 | −0.2 | |
| 3 | 554 | 25.1 | 0.0 | |
| Avg | −0.5 | |||
| SD | 0.6 | |||
| NO2− | 1 | 186 | 6.2 | −1.8 |
| 2 | 233 | 16.8 | 0.4 | |
| 3 | 268 | 21.8 | 1.8 | |
| 4 | 324 | 25.8 | −2.2 | |
| Avg | −0.5 | |||
| SD | 1.9 |
FIG. 1.
δ15N, δ15Nα, and SPs of N2O produced in concentrated cell suspensions of P. chlororaphis (A) and P. aureofaciens (B), with NO3− as the substrate. The isotopic enrichment factors for δ15N, δ15Nα, and site preference were 12.7, 12.9, and 0.3, respectively, for cell suspensions of P. chlororaphis (A) and 36.7, 37.4, and 1.3, respectively, for P. aureofaciens (B).
Relative importance of NO2− reduction and NH2OH oxidation on N2O production.
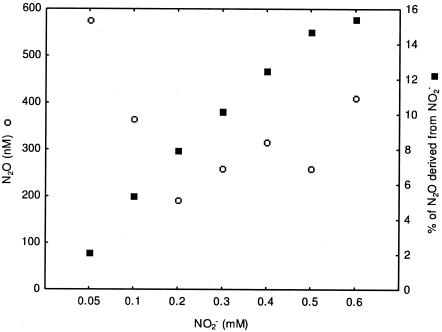
Concentrated N. europaea cell suspensions in the presence of NO2− and NH2OH with a high liquid S/V ratio produced four times more N2O than those with a low S/V ratio (15.0 μM and 3.4 μM, respectively). The N2O in the low-S/V-ratio experiment was more highly enriched in 15N than that produced in the high-S/V-ratio experiment (283‰ and 114‰, respectively). Relative to the high-S/V-ratio condition, isotope mass balance indicated that the production of N2O from NO2− reduction was approximately two times greater in the low-S/V-ratio experiment (6.8 and 16.6%, respectively).
The concentration of N2O in cultures of N. europaea incubated with a high S/V ratio initially decreased for NO2− concentrations between 0.05 and 0.2 mM and then increased for concentrations between 0.2 and 0.6 mM NO2− (Fig. 2). The percentage of N2O produced from the reduction of 15N-enriched NO2− increased nearly linearly, from 2.0 to 15.3%, over the NO2− concentration range from 0.05 to 0.6 mM. Since the S/V ratio controls the diffusion of O2, our results are consistent with those of an earlier study for N. europaea that found little or no effect of pO2 on N2O production if NO2− concentrations were >0.05 mM (1).
FIG. 2.
N2O concentration (○) and % of N2O derived from NO2− reduction versus NH2OH oxidation (▪) in concentrated cell suspensions of N. europaea with various NO2− concentrations (0.05 to 0.6 mM of 15N-enriched NO2−).
δ15N-N2O versus δ18O-N2O.
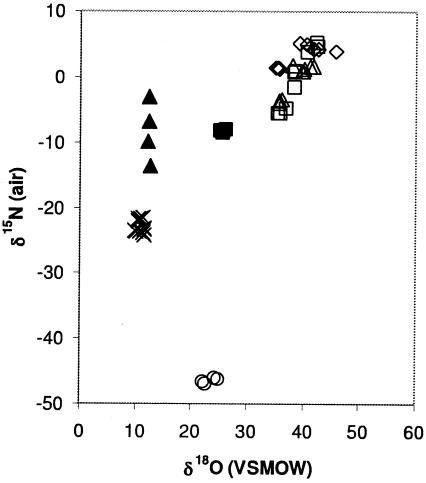
The bulk nitrogen isotopic composition of N2O was nonetheless distinct for N2O produced from NH2OH oxidation and NO2− reduction by N. multiformis (Fig. 3). N2O produced during NH2OH oxidation by N. europaea, N. multiformis, and M. trichosporium could not be distinguished on the basis of δ18O and δ15N values (Fig. 3). The oxygen isotope ratios of N2O produced by NO2− reduction in P. aureofaciens and P. chlororaphis differed by 12‰ (Fig. 3). Nitrite reduction by N. multiformis produced N2O with a δ15N value that was different from that produced by P. aureofaciens and P. chlororaphis (Fig. 3). Nitrous oxide produced by N. europaea with NH4+ as a substrate could be distinguished from that produced by denitrification and nitrifier denitrification on the basis of δ15N values (Fig. 3).
FIG. 3.
δ18O and δ15N values (reported relative to air and VSMOW standards, respectively) for N2O produced by Nitrosomonas europaea with NH4+ as a substrate (○), Nitrosomonas europaea with NH2OH as a substrate (□), Nitrosospira multiformis with NH2OH as a substrate (▵), Methylosinus trichosporium with NH2OH as a substrate (⋄), Nitrosospira multiformis with NO2− as a substrate (×), Pseudomonas aureofaciens with NO2− as a substrate (▴), and Pseudomonas chlororaphis with NO2− as a substrate (▪).
DISCUSSION
Potential for NO2− reduction in N. europaea cell suspensions.
By varying the liquid S/V ratio of N. europaea cultures, we showed that increased oxygen diffusion decreases the importance of NO2− reduction relative to NH2OH oxidation in N2O production. The experiments demonstrated that there was an increase in the relative importance of NO2− reduction relative to NH2OH oxidation in N2O production when there was a low S/V ratio in N. europaea cultures. However, even under conditions of high liquid S/V ratios, the production of N2O from NO2− reduction was increased by elevating the concentration of NO2− in the sample. Anderson et al. (1) found that NO2− concentrations of >0.05 mM can stimulate NO2− reduction. More recently, Beaumont et al. (3) found that NirK was expressed aerobically in response to increasing concentrations of NO2−, demonstrating the potential for aerobic denitrification by nitrifiers. We suggest that the variation in the SPs of N2O produced during nitrification could have been the result of a contribution of NO2− reduction to N2O production stimulated by low oxygen concentrations or an increase in NO2− concentration in the liquid phase of the concentrated cell suspensions, as described by Sutka et al. (27).
Bulk δ15N and δ18O as a basis to differentiate N2O production during nitrification and denitrification.
Distinctions in the bulk δ15N and δ18O values for N2O from different sources provide a basis for evaluating sources of N2O in the troposphere (9, 19, 24). Nonetheless, the tendency is for production pathways to produce N2O with a wide range of isotope values such that source apportionment is difficult. Our results further document a wide range of isotope values for N2O produced both within replicate cultures carrying out the same process and between microbial production pathways. For example, during the reduction of NO2− by P. aureofaciens, the δ15N value for N2O became more depleted in 15N as N2O was produced (Fig. 3).
The evaluation of δ18O data for N2O is particularly challenging because isotope pathways reflect not only the source of atomic O but also the tendency for intermediate compounds of N2O production to exchange O with water. Ostrom et al. (18) proposed that the observed shifts in the δ18O value of N2O with depth in the ocean reflects a predominance of N2O derived from NH4+ oxidation, with the preponderance of N2O from NO2− reduction within a comparatively narrow depth interval. Our results are consistent with this dual-source interpretation, as N2O produced by nitrifier denitrification was markedly depleted in 18O relative to that produced by NH4+ oxidation by N. europaea and NO2− reduction by N. multiformis (Fig. 3). Despite the distinction in δ18O values between NH2OH oxidation and nitrifier denitrification, a variation of approximately 12‰ was evident between cultures of two denitrifiers (P. chlororaphis and P. aureofaciens) carrying out NO2− reduction. This indicates that there may not be a uniform oxygen isotope signature for N2O production by denitrifiers. The substrate, NO2− (δ15N = 1.5‰), was identical in the two Pseudomonas experiments; therefore, isotopic variation is likely due to the exchange of oxygen atoms between intermediates and water, as discussed by Casciotti et al. (7). Schmidt et al. (25) discussed the difficulty of using isotopic values to differentiate between nitrification and denitrification. The current study confirms the challenges of using bulk nitrogen and oxygen isotopes as indications of the biogenic source. However, site-specific isotope characterization can differentiate between nitrification and denitrification.
Site preferences of N2O produced by ammonia- and methane-oxidizing organisms.
The majority of information on the genetics and biochemical pathways of ammonia-oxidizing bacteria derives from studies of N. europaea. However, the genus Nitrosomonas is not as dominant in soils and waters as other nitrifiers such as Nitrosospira (6, 15, 24). In this study, the average SPs of N2O produced during NH2OH oxidation by N. multiformis and N. europaea were similar (33.5 ± 1.2‰ and 32.5 ± 0.6‰, respectively). In addition, the SPs of N2O produced in N. europaea batch cultures with NH4+ and in concentrated cell suspensions were similar (31.4 ± 4.2‰ and 33.5 ± 1.2‰, respectively). This similarity is particularly startling given that the substrates were similar in δ15N yet had large differences in bulk δ15N values (Tables 1 and 2). This result indicates that bulk N isotope fractionation during nitrification occurs mainly during the conversion of NH4+ to NH2OH, and furthermore, that the SP is constant even though differences are evident in bulk δ15N fractionation. Thus, despite variations in substrates and physiological differences between the genera Nitrosomonas and Nitrosospira, SP values of 32 to 35‰ for N2O produced by NH2OH and NH4+ oxidation can be applied to ammonia-oxidizing organisms as a whole.
Methanotrophs are divided into three groups (types I, II, and X) on the basis of phylogeny and ecology (13). The SP of N2O produced by NH2OH oxidation in cultures of M. trichosporium (35.6 ± 1.4‰) in this study was similar to that we previously reported for M. capsulatus Bath (30.8 ± 5.9‰) (27). In addition, the SPs of N2O produced during NH2OH oxidation by N. multiformis and N. europaea were similar to those of N2O produced by M. trichosporium and M. capsulatus Bath. The results indicate that large SPs of 32 to 35‰ are characteristic of nitrification, regardless of whether it is catalyzed by a methane or NH4+ oxidizer.
Site preference of N2O produced during denitrification.
The SP reported here for N2O produced by denitrification and nitrifier denitrification (∼0‰) (Table 3) is similar to the value of −5‰ reported by Toyoda et al. (29), who used cultures of Pseudomonas denitrificans. However, Toyoda et al. (29) found that the SP of N2O produced by Pseudomonas fluorescens was approximately 24‰. They suggested that variations in SP resulted from the production of N2O by abiological reactions within the culture, because the N2O production rates were low in the P. fluorescens experiments and NO2− may have accumulated to high concentrations (29). Consequently, the SP value of 24‰ may be anomalous and not characteristic of N2O production by denitrifying bacteria. Since this study included P. chlororaphis, which possesses a cd1-type NO2− reductase (31), and P. aureofaciens, which has a Cu-containing NO2− reductase (8), and there was no difference in the SPs for the N2O produced, we can conclude that the type of NO2− reductase does not influence the SP during denitrification. Consequently, our results and those of Toyoda et al. (29) indicate a consistent SP of approximately 0‰ for N2O production by denitrifiers, regardless of the enzyme involved.
Site preference and field studies.
Our study demonstrates that the SP of N2O produced by denitrification, whether catalyzed by Pseudomonas cultures or NH4+ oxidizers (nitrifier denitrification), is approximately 33‰ lower than that produced by nitrification. Furthermore, our work and that of others (27, 29) demonstrate that in contrast to bulk isotope values, SP is conservative and independent of the substrate isotopic composition, which lays a foundation for the use of isotopomers to evaluate origins in field studies. Yamulki et al. (31) observed a change in SP, from between 5 and 9‰ to approximately −2‰, over a 24-h period in urine-amended grassland soil that was attributed to a shift in the relative importance of production from nitrification to denitrification. Based on isotope mass balance and our SP values of 33‰ and 0.1‰ for nitrification and denitrification, respectively, we estimate that 84% of N2O production was from denitrification in the first time period, with 100% being from denitrification in the second time period. These results confirm the initial suggestions of Yamulki et al. (31) and provide a quantification of the importance of nitrification and denitrification.
Although N2O reduction is a process that could influence SP, preliminary evidence from soil mesocosm experiments indicates that N2O reduction results in a negligible change in SP for the majority of a reaction (21). In addition, Firestone and Tiedje (10) observed that N2O consumption lagged behind production as a consequence of a delay in the synthesis of reducing enzymes. The impact of N2O reduction on SP is likely minimal in studies of episodic fluxes that are stimulated by the onset of anoxic conditions, such as the Yamulki study (31).
Concluding remarks.
The SP of N2O is a robust and quantitative indicator of the microbial origins of this important greenhouse gas. In contrast to traditional bulk stable isotope analyses, the SP is not affected by isotopic fractionation. This approach may now provide important insights into management activities directed toward curtailing N2O emissions.
Acknowledgments
This research was supported by a grant from the National Science Foundation (DEB 0316908).
This work benefited greatly from the comments provided by three anonymous reviewers.
REFERENCES
- 1.Anderson, I. C., M. Poth, J. Homstead, and D. Burdige. 1993. A comparison of NO and N2O production by the autotrophic nitrifier Nitrosomonas europaea and the heterotrophic nitrifier Alcaligenes faecalis. Appl. Environ. Microbiol. 59:3525-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barford, C. C., J. P. Montoya, M. A. Altabet, and R. Mitchell. 1999. Steady-state nitrogen isotope effect of N2 and N2O production in Paracoccus denitrificans. Appl. Environ. Microbiol. 65:989-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaumont, H. J. E., S. I. Lens, W. N. M. Reijnders, H. V. Westerhoff, and R. J. M. van Spanning. 2004. Expression of nitrite reductase in Nitrosomonas involves NsrR, a novel nitrite-sensitive transcription repressor. Mol. Microbiol. 54:148-158. [DOI] [PubMed] [Google Scholar]
- 4.Boontanon, N., S. Ueda, P. Kanatharana, and E. Wada. 2000. Intramolecular stable isotope ratios of N2O in the tropical swamp forest in Thailand. Naturwissenschaften 87:188-192. [DOI] [PubMed] [Google Scholar]
- 5.Brenninkmeijer, C. A. M., and T. Röckmann. 1999. Mass spectrometry of the intramolecular nitrogen isotope distribution of environmental nitrous oxide using fragment-ion analysis. Rapid Commun. Mass Spectrom. 13:2028-2033. [DOI] [PubMed] [Google Scholar]
- 6.Bruns, M. A., M. R. Fries, J. M. Tiedje, and E. A. Paul. 1998. Functional gene hybridization patterns of terrestrial ammonia-oxidizing bacteria. Microb. Ecol. 36:293-302. [DOI] [PubMed] [Google Scholar]
- 7.Casciotti, K. L., D. M. Sigman, M. G. Hastings, J. K. Bohlke, and A. Hilkert. 2002. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal. Chem. 74:4905-4912. [DOI] [PubMed] [Google Scholar]
- 8.Coyne, M. S., A. Arunakumari, B. A. Averill, and J. Tiedje. 1989. Immunological identification and distribution of dissimilatory heme cd1 and nonheme copper nitrite reductases in denitrifying bacteria. Appl. Environ. Microbiol. 55:2924-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dore, J. E., B. N. Popp, D. M. Karl, and F. J. Sansone. 1998. A large source of atmospheric nitrous oxide from subtropical North Pacific surface waters. Nature 396:63-66. [Google Scholar]
- 10.Firestone, M. K., and J. M. Tiedje. 1979. Temporal change in nitrous oxide and dinitrogen from denitrification following onset of anaerobiosis. Appl. Environ. Microbiol. 38:673-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman, L., and J. Bigeleisen. 1950. Oxygen and nitrogen isotope effects in the decomposition of ammonium nitrate. J. Chem. Phys. 18:1325-1331. [Google Scholar]
- 12.Goreau, T. J., W. A. Kaplan, S. C. Wofsky, M. B. McElroy, F. W. Valois, and S. W. Watson. 1980. Production of NO2− and N2O by nitrifying bacteria at reduced concentrations of oxygen. Appl. Environ. Microbiol. 40:526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson, R., and T. Hanson. 1996. Methanotrophic bacteria. Microbiol. Rev. 60:439-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IPCC. 2001. Climate change 2001: the scientific basis. Cambridge University Press, Cambridge, United Kingdom.
- 15.Jiang, Q.-Q., and L. R. Bakken. 1999. Nitrous oxide production and methane oxidation by different ammonia-oxidizing bacteria. Appl. Environ. Microbiol. 65:2679-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariotti, A., J. C. Germon, P. Hubert, P. Kaiser, R. Letolle, A. Tardieux, and P. Tardieux. 1981. Experimental determination of nitrogen kinetic isotope fractionation: some principles. Illustration for the denitrification and nitrification processes. Plant Soil 62:413-430. [Google Scholar]
- 17.Mosier, A., C. Kroeze, C. Nevison, O. Oenema, S. Seitzinger, and O. van Cleemput. 1998. Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle—OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology. Nutr. Cycl. Agroecosyst. 52:225-248. [Google Scholar]
- 18.Ostrom, N. E., L. O. Hedin, J. C. VonFischer, and G. P. Robertson. 2002. Nitrogen transformation and NO3− removal at a soil-stream interface: a stable isotope approach. Ecol. Appl. 12:1027-1043. [Google Scholar]
- 19.Pérez, T., S. E. Trumbore, S. C. Tyler, P. A. Matson, I. Ortiz-Monasterio, T. Rahn, and D. W. T. Griffith. 2001. Identifying the agricultural imprint on the global N2O budget using stable isotopes. J. Geophys. Res. 106:9869-9878. [Google Scholar]
- 20.Pérez, T., S. E. Trumbore, S. C. Tyler, E. A. Davidson, M. Keller, and P. B. de Camargo. 2000. Isotopic variability of N2O emissions from tropical forest soils. Global Biogeochem. Cycles 14:525-535. [Google Scholar]
- 21.Pitt, A. J. 2003. M.S. thesis. Michigan State University, East Lansing.
- 22.Poth, M., and D. D. Focht. 1985. 15N kinetic analysis of N2O production by Nitrosomonas europaea: an examination of nitrifier denitrification. Appl. Environ. Microbiol. 49:1134-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poth, M. 1986. Dinitrogen production from nitrite by a Nitrosomonas isolate. Appl. Environ. Microbiol. 52:957-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prosser, J. I. 2002. Molecular and functional diversity in soil micro-organisms. Plant Soil 244:9-17. [Google Scholar]
- 25.Schmidt, H.-L., R. A. Werner, N. Yoshida, and R. Well. 2004. Is the isotopic composition of nitrous oxide an indicator for its origin in nitrification or denitrification? A theoretical approach from referred data and microbiological and enzyme kinetic aspects. Rapid Commun. Mass Spectrom. 18:2036-2040. [DOI] [PubMed] [Google Scholar]
- 26.Stein, L. Y., and Y. L. Yung. 2003. Production, isotopic composition, and atmospheric fate of biologically produced nitrous oxide. Annu. Rev. Earth Planet. Sci. 31:329-356. [Google Scholar]
- 27.Sutka, R. L., N. E. Ostrom, P. H. Ostrom, H. Gandhi, and J. A. Breznak. 2003. Nitrogen isotopomer site preference of N2O produced by Nitrosomonas europaea and Methylococcus capsulatus Bath. Rapid Commun. Mass Spectrom. 17:738-745. [DOI] [PubMed] [Google Scholar]
- 28.Toyoda, S., and N. Yoshida. 1999. Determination of nitrogen isotopomers of nitrous oxide on a modified isotope ratio mass spectrometer. Anal. Chem. 71:4711-4718. [Google Scholar]
- 29.Toyoda, S., M. H. Mutobe, H. Yamagishi, N. Yoshida, and Y. Tanji. 2005. Fractionation of N2O isotopomers during production by denitrifier. Soil Biol. Biochem. 37:1535-1545. [Google Scholar]
- 30.Wrage, N., J. Lauf, A. del Prado, M. Pinto, S. Pietrzak, S. Yamulki, O. Oenema, and G. Gebauer. 2004. Distinguishing sources of N2O in European grasslands by stable isotope analysis. Rapid Commun. Mass Spectrom. 18:1201-1207. [DOI] [PubMed] [Google Scholar]
- 31.Yamulki, S., I. Wolf, R. Bol, B. Grant, R. Brumme, E. Veldkamp, and S. C. Jarvis. 2000. Effects of dung and urine amendments on the isotopic content of N2O released from grasslands. Rapid Commun. Mass Spectrom. 14:1356-1360. [DOI] [PubMed] [Google Scholar]
- 32.Yamulki, S., S. Toyoda, N. Yoshida, E. Veldkamp, B. Grant, and R. Bol. 2001. Diurnal fluxes and the isotopomer ratios of N2O in a temperate grassland following urine amendment. Rapid Commun. Mass Spectrom. 15:1263-1269. [DOI] [PubMed] [Google Scholar]
- 33.Ye, R. W., M. R. Fries, S. G. Bezborodnikov, B. A. Averill, and J. M. Tiedje. 1993. Characterization of the structural gene encoding a copper-containing nitrite reductase and homology of this gene to DNA of other denitrifiers. Appl. Environ. Microbiol. 59:250-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida, N. 1988. 15N-depleted N2O as a product of nitrification. Nature 335:528-529. [Google Scholar]
- 35.Yoshida, N., and S. Toyoda. 2000. Constraining the atmospheric N2O budget from intramolecular site preference in N2O. Nature 405:330-334. [DOI] [PubMed] [Google Scholar]