Abstract
Of 11 fatty acids and monoglycerides tested against Campylobacter jejuni, the 1-monoglyceride of capric acid (monocaprin) was the most active in killing the bacterium. Various monocaprin-in-water emulsions were prepared which were stable after storage at room temperature for many months and which retained their microbicidal activity. A procedure was developed to manufacture up to 500 ml of 200 mM preconcentrated emulsions of monocaprin in tap water. The concentrates were clear and remained stable for at least 12 months. They were active against C. jejuni upon 160- to 200-fold dilution in tap water and caused a >6- to 7-log10 reduction in viable bacterial count in 1 min at room temperature. The addition of 0.8% Tween 40 to the concentrates as an emulsifying agent did not change the microbicidal activity. Emulsions of monocaprin killed a variety of Campylobacter isolates from humans and poultry and also killed strains of Campylobacter coli and Campylobacter lari, indicating a broad anticampylobacter activity. Emulsions of 1.25 mM monocaprin in citrate-lactate buffer at pH 4 to 5 caused a >6- to 7-log10 reduction in viable bacterial counts of Salmonella spp. and Escherichia coli in 10 min. C. jejuni was also more susceptible to monocaprin emulsions at low pH. The addition of 5 and 10 mM monocaprin emulsions to Campylobacter-spiked chicken feed significantly reduced the bacterial contamination. These results are discussed in view of the possible utilization of monocaprin emulsions in controlling the spread of food-borne bacteria from poultry to humans.
Food-borne illness caused by the microbial contamination of poultry is a major public health problem in developed and developing countries. Campylobacter jejuni is the most common cause of food-borne infection. There are approximately 2.1 to 2.4 million cases of human campylobacteriosis each year in the United States, and C. jejuni causes 46% of all laboratory-confirmed cases of bacterial gastroenteritis. C. jejuni infections are followed in prevalence by Salmonella (28%), Shigella (17%), and Escherichia coli O157 (5%) infections (1). Because of the high rate of diarrheal illness caused worldwide by food-borne bacteria and the severity of the infections, both in human suffering and economic loss, it is important to develop means to control the transmission of the bacteria from food, particularly poultry, to humans. One approach is to prevent infection in broiler chickens by eliminating Campylobacter or Salmonella from their drinking water by the addition of chemicals such as chlorine (22) or organic acids at low pH (7). Another approach is to reduce the levels of bacterial colonization in the intestinal tracts of broilers by the addition of chemicals to their feeds (11, 20), by competitive inhibition with other bacterial strains (2), or by phage therapy (19). Many attempts to treat poultry carcasses in the slaughterhouse with antibacterial chemicals have been made, but with various results (12, 22). In spite of all these efforts, the contamination of raw poultry and related food products remains a serious health problem. New and more efficient methods to decontaminate or prevent the contamination of food such as raw meat and poultry by pathogens such as Campylobacter, Salmonella, and E. coli would therefore be desirable.
The microbicidal activities of lipids, particularly fatty acids and their 1-monoglycerides, have been tested against a number of bacterial species (8, 14, 15). More recently, bacteria such as Chlamydia trachomatis, Neisseria gonorrhoeae, Helicobacter pylori, Staphylococcus aureus, and streptococci of groups A and B were found to be rapidly killed by fatty acids and monoglycerides, although with a considerable difference in activity profiles (3, 4, 5, 6). However, most of the bacterial species tested, either gram negative or gram positive, were found to be particularly susceptible to the 1-monoglyceride of capric acid (monocaprin [MC]). Lauric acid and monolaurin have been found to inhibit Listeria monocytogenes, but monocaprin was not tested (21). In the present work, the susceptibility of C. jejuni to a series of fatty acids and monoglycerides was tested and monocaprin was found to be particularly active in killing this bacterium. Concentrated emulsions of monocaprin in water were prepared which kill Campylobacter species rapidly and in large numbers after dilution in tap water and which significantly reduce the number of viable bacteria in chicken feeds. Upon dilution in acidified water, the concentrates also effectively kill Salmonella spp. and E. coli, but a previous study has shown that these gram-negative bacteria are resistant to inactivation by lipids at neutral pH (6, 16). These results are discussed in relation to a possible use of concentrated emulsions of monocaprin to control food-borne infections at various points along the food chain from farm to fork.
MATERIALS AND METHODS
Bacteria.
Two strains of C. jejuni, namely, a standard strain from the American Type Culture Collection, Rockville, MD (ATCC 33560), and a recent clinical isolate (isolate 1) from a patient with campylobacteriosis and confirmed as a strain of C. jejuni at the Department of Microbiology, National University Hospital, Reykjavik, Iceland, were used to compare the microbicidal activities of various lipids against this bacterium. Both strains were grown on blood agar plates and were incubated in a gas jar with a microaerobic atmosphere (gas generating kit, Campylobacter system BR 060A; Oxoid, Ltd., Hampshire, United Kingdom) at 37°C. Twenty-two strains of C. jejuni isolated from the skin, feces, and cecal contents of chickens; four isolates from the skin of ducks; and one from the skin of a turkey were tested against emulsions of monocaprin in water. Furthermore, monocaprin emulsions were tested against Campylobacter coli and Campylobacter lari, two strains of each. All strains isolated from poultry were obtained from the Environment and Food Agency of Iceland and from the Institute for Experimental Pathology, University of Iceland at Keldur, and confirmed to be C. jejuni. Ten human strains of C. jejuni isolated from patients with campylobacteriosis were used, five from Iceland and five from patients who had contracted the infection in foreign countries, i.e., India, Kenya, Poland, Latvia, and Spain. In addition, two strains of E. coli, i.e., strain O157 obtained from the ATCC and a recent clinical isolate from a urine sample; two recent clinical isolates of Salmonella enterica serovar Typhimurium; and one clinical isolate of Salmonella enterica serovar Enteritidis were used. All clinical isolates were obtained from the Department of Microbiology, National University Hospital, Reykjavik, Iceland.
Lipids.
The fatty acids and 1-monoglycerides (purest grade) used for the comparative studies were purchased from Sigma Chemical Co., St. Louis, MO. The MC used as the active ingredient in the stable microbicidal emulsions was obtained from Danisco A/S, Copenhagen, Denmark, as emulsifier TS-PH 003 glycerol monocaprate (pharmaceutical grade), which met the specification requirements set by USP24/NF19 (United States Pharmacopeia—National Formulary [www.usp.org/USPNF]) for mono- and diglycerides. Stock solutions (1 M) of all lipids were made in pure ethanol (Merck).
Emulsifying agents.
The following polysorbates were used as emulsifiers: polyoxyethylene sorbitan monolaurate (Tween 20 [TW20]), polyoxyethylene sorbitan monopalmitate (TW40), polyoxyethylene sorbitan monostearate (TW60), polyoxyethylene sorbitan monooleate (TW80), and polyoxyethylene sorbitan trioleate (TW85). They were all purchased from ICN Biomedicals, Inc., Aurora, Ohio.
Assay of microbicidal activity of various lipids against C. jejuni.
Blood agar plates were inoculated with C. jejuni and incubated for 2 days as described above. Plates showing abundant bacterial growth were harvested, and the bacteria were collected with a cotton swab and suspended in ESP 80A aerobic broth (AccuMed International, Inc.). Lipid stock solutions were diluted in aerobic broth to the desired concentration by vortexing at the highest speed for 1 min. The bacterial suspension was dispensed into test tubes and mixed with equal amounts of a lipid sample. Bacterial suspensions mixed with broth with or without 2% ethanol were used as controls. The mixtures were incubated at 37°C for 10 min or at room temperature for 1 min and were then assayed by making 10-fold dilutions in saline and streaking 10 μl of 10−2 to 10−6 dilutions and 100 μl of a 10−1 dilution on blood agar plates with a pipette tip. The Campylobacter colonies were counted after incubation of the plates for 48 h in a microaerobic atmosphere, as described above. The viable bacterial counts (log10 CFU) of lipid-bacterium mixtures were subtracted from the bacterial counts (CFU) of the control mixtures without ethanol, and the difference was used as a measure of the microbicidal activities of the lipids.
Preparation of emulsions of MC in water.
Test tubes containing sterile distilled water were heated in a water bath at 60 to 70°C, and a 1 M MC (Danisco) solution in ethanol was added to the water under constant vortexing at the highest speed for 1 min to make MC emulsions with concentrations of 20 mM or lower. Tween surfactants were added to the mixtures and vortexed briefly, whereupon the MC emulsions became clear.
Preparation of concentrated emulsions of MC in water.
Sterile tap water in volumes up to 500 ml was heated to a temperature of 60 to 70°C, and a 1 M solution of MC (Danisco) in ethanol was added under constant forceful stirring for 1 min in order to make 50, 100, and 200 mM emulsions. In contrast to the emulsions containing less than 20 mM MC, these emulsions became clear at room temperature. Alternatively, Tween surfactants were added to the concentrated monocaprin emulsions and stirred briefly.
Assay of the activities of MC emulsions.
The activities of the emulsions were tested against Campylobacter in the same way as described above for the various lipids, except that bacterial suspensions were made in sterile tap water. Salmonella spp. and E. coli were tested as previously described (6), except that the bacteria were suspended in saline. Solutions containing Tween and/or ethanol were used as controls.
Addition of MC emulsion to chicken feed.
Pellets of commercial broiler feed (MR Feeds, Reykjavik, Iceland) were weighed (0.5 g) and mixed with equal amounts (by weight) of an MC-TW40 emulsion, either in tap water or in citrate-lactate buffer at pH 4.1. A suspension of C. jejuni, a chicken fecal isolate, in sterile tap water was added to the wet pellets in equal volumes and mixed well by shaking. The mixture was placed in a water bath at 37°C for 30 min and was then diluted 10-fold with water. After centrifugation at 2,000 rpm for 10 min in a Sorvall RT6000D centrifuge, the decimal dilutions of the supernatant were streaked onto Campy-Cefex plates (17) and colonies were counted after incubation for 2 days at 37°C in a microaerobic environment, as described above. Pellets mixed in the same way with TW40 but without MC were used as controls.
RESULTS
Activities of fatty acids and 1-monoglycerides against C. jejuni.
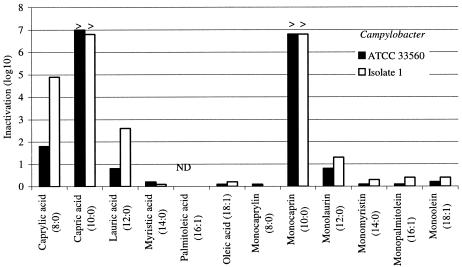
Medium-chain saturated and long-chain unsaturated fatty acids and their 1-monoglycerides were tested against C. jejuni strain ATCC 33560 and clinical isolate 1. They were all tested at final concentrations of 10 mM and were incubated with the bacteria at 37°C for 10 min. Figure 1 shows the reduction in bacterial counts (log10 CFU) after treatment with lipids, compared with that of the controls. Of the fatty acids tested, capric acid was the most active in killing the bacteria, reducing the bacterial count by ≥6.8 log10 in 10 min. MC was the most active of the monoglycerides, reducing the bacterial count by ≥6.8 log10. There was no difference in the bacterial counts of controls with or without ethanol, both of which varied between 8.8 and 9.0 log10 CFU per ml. To further compare the activities of MC and capric acid, they were tested at lower concentrations for either 10 or 1 min at room temperature. The activity of capric acid was completely lost at 2.5 mM in 10 min. In contrast, MC was fully active at 2.5 mM in 1 min and at 1.25 mM in 10 min. From these results, it was concluded that of the lipids tested, MC has the highest microbicidal activity against C. jejuni and kills the bacteria rapidly and in large numbers at concentrations as low as 1.25 mM.
FIG. 1.
Inactivation of two strains of Campylobacter jejuni mixed with 10 mM of fatty acids and monoglycerides for 10 min at 37°C. The bars represent the reduction of CFU (log10). At the top of the bars, > indicates that the reduction of the bacterial count was equal to or greater than the value indicated on the left axis. ND, not done.
Anticampylobacter activities of MC emulsions in water containing Tween surfactants as emulsifying agents.
Emulsions containing MC (Danisco) with Tween surfactants and prepared in small volumes by vortexing, as described in Materials and Methods, appeared clear at room temperature. They were tested against clinical isolate 1 of C. jejuni for 1 min to establish whether or not MC is active in the presence of Tween surfactants. Emulsions containing a final concentration of 5 mM MC with 0.025% (vol/vol) TW20, TW40, TW60, TW80, or TW85 in water remained stable and highly active against C. jejuni for at least 6 months. All of them reduced the viable bacterial counts by ≥6 log10 upon contact for 1 min, compared with those of the control solutions. Emulsions of 10 mM MC were tested without Tween and after the addition of Tween surfactants in various concentrations. TW20 or TW40 at a concentration of 0.25% did not affect the activity of MC, whereas 0.25% TW80 reduced the activity. None of the Tween surfactants were bactericidal at a concentration of 0.5% in the absence of MC. All emulsions tested against bacteria contained 1% or less of ethanol. These concentrations of ethanol in water had no effect on the viable counts of C. jejuni bacteria.
Anticampylobacter activities of preconcentrated MC emulsions with or without Tween surfactants.
Concentrated emulsions of MC in water were prepared as described in Materials and Methods. The concentrations of MC ranged from 50 to 200 mM, and the 50 mM emulsions were prepared either with or without the addition of 0.1% (vol/vol) concentrations of the different Tween surfactants. At room temperature, all the emulsions were clear and colorless except for the 50 mM emulsions, which had a bluish color. All of the emulsions caused reductions of ≥6 to 7 log10 in the number of viable C. jejuni bacteria, human isolate 1, after dilution in tap water at room temperature to a final concentration of 5 mM MC with or without Tween. The concentrated emulsions were stored at room temperature for 4 to 11 months and were then diluted in tap water and tested against C. jejuni with the same results. It is notable that not only the concentrates but also the diluted emulsions remained stable and active after storage at room temperature for at least 3 months. Concentrated MC emulsions without Tween were remarkably stable and remained clear after storage for at least 21 months at room temperature and retained their microbicidal activities. The 200 mM MC emulsions seemed of particular interest since they were highly active after 100-fold dilution in water. All further studies were therefore carried out with 200 mM MC concentrates with or without TW40, which was selected because of its favorable properties, such as its low cytotoxicity compared with that of TW20 (data not shown).
Standard emulsions of 200 mM MC in tap water with or without 0.8% TW40.
Concentrated (200 mM) MC emulsions were diluted in water to the desired concentrations with brief magnetic stirring and were tested for microbicidal activity against C. jejuni. The clear concentrated emulsions became slightly cloudy upon dilution, but the diluted emulsions remained stable and active for many months. Table 1 shows the anticampylobacter activities of diluted emulsions, with or without TW40, after the storage of MC concentrates for up to 12 months at room temperature and of MC-TW40 concentrates for up to 17 months. The emulsions were tested against a strain of C. jejuni isolated from chicken feces. Although some of the emulsions lost activity upon storage, and 10-min rather than 1-min treatments were needed to kill the bacteria, all the concentrates which were prepared in 250- to 500-ml quantities remained clear and remarkably active against C. jejuni in 160-fold to 200-fold final dilutions. This indicates that 80 to 100 liters of active emulsion can be made from 500 ml of a 200 mM MC concentrate, either with or without 0.8% TW40. The procedure designed to make stable, active anticampylobacter emulsions is well established and reproducible, as can be seen from the list of 200 mM MC concentrates with or without TW40 (Table 1) made at different times.
TABLE 1.
Anticampylobacter activities of dilutions of different preconcentrated MC emulsions with or without Tween 40 stored at room temperature for various lengths of time
| Preconcentrated emulsion | Time in storage (mo) | Concn after dilutiona | Reduction in bacterial count (log10 CFU)b |
|---|---|---|---|
| 200 mM MC | 12 | 1.25 mMc | ≥6.5d |
| 200 mM MC | 9.5 | 1.25 mM | ≥6.8e |
| 200 mM MC | 2 | 1.0 mMf | ≥6.2e |
| 200 mM MC-0.8% TW40 | 17 | 1.25, 0.005% | ≥6.5d |
| 200 mM MC-0.8% TW40 | 12.5 | 1.25, 0.005% | ≥6.8e |
| 200 mM MC-0.8% TW40 | 7 | 1.0, 0.004%f | ≥6.2d |
Final concentration(s) after the addition of bacteria (1:1). Concentrations of MC and TW40 are given in millimolar and percentages, respectively.
Compared with count of bacteria mixed 1:1 with the control solution.
After 160-fold dilution (1.25 mM [0.03%] MC).
After treatment for 10 min at room temperature.
After treatment for 1 min at room temperature.
After 200-fold dilution (1.0 mM [0.024%] MC).
Microbicidal activities of MC emulsions against isolates of C. jejuni from various sources and against C. coli and C. lari.
It is important to establish that MC emulsions have a broad anticampylobacter activity against strains isolated from a variety of sources. A total of 27 isolates from chickens, ducks, and a turkey and 10 human isolates, as described in Materials and Methods, were tested with 1.25 to 10 mM MC emulsions, which were incubated with the bacteria for either 1 or 10 min at room temperature. The viable bacterial counts of all the human isolates were reduced by ≥6 to 7 log10 in 1 min, and those of the poultry isolates were reduced by ≥4 to 7 log10 in 1 to 10 min. These results confirm a broad microbicidal activity of MC against C. jejuni isolated either from humans or from poultry. Similarly, C. coli and C. lari, two isolates of each, were tested with 10 mM MC emulsions, and their viable counts were reduced by ≥6 log10 in 1 min. This again confirms a broad anticampylobacter activity of MC emulsions.
Effects of acidified emulsions of MC and TW40 on Salmonella spp., E. coli, and C. jejuni.
It has been shown that Salmonella spp. and E. coli are not killed by fatty acids and monoglycerides at neutral pH, in contrast to Helicobacter pylori (6). However, it has been reported that E. coli is inactivated by monoglycerides in the presence of citric acid (16). It was therefore decided to test these bacteria with acidified MC emulsions. Sterile tap water was acidified by the addition of citrate-lactate buffer (0.06 M trisodium citrate-lactic acid) and adjusted to pH 4.1, 4.5, 5.0, or 5.5. A 200 mM emulsion of MC with 0.8% TW40 was diluted to a concentration of 2.5 mM MC with 0.01% TW40 in citrate-lactate buffers at pH 4.1, 4.5, 5.0, or 5.5 and to 10 mM MC with 0.04% TW40 at pH 5.5 and incubated at 37°C for 10 min with Salmonella spp. and E. coli. Emulsions in buffer at neutral pH were tested as controls. The reductions in viable cell counts of the bacteria compared with that of the control are shown in Table 2. Both Salmonella spp. and E. coli were effectively killed by 1.25 mM MC at pHs 4.1, 4.5, and 5.0, but not at pH 4.1 without MC, and to a small extent by 1.25 mM and 5 mM MC in buffer at pH 5.5. They were not killed by 5 mM MC at neutral pH. C. jejuni was killed by 0.625 mM MC at pH 4.1, but not at pH 4.1 without MC, and only to a small extent by 0.625 mM MC at neutral pH. There is apparently a synergistic effect of MC and acid on the bacteria, since they are not killed by MC at neutral pH or pH 4.1 in the absence of MC. In the experiments reported in Table 2, a concentrate of 200 mM MC with 0.8% TW40 was used to make the dilutions. After dilution in buffer with a pH as low as 4.1, the emulsions remained stable and active for at least several days. Concentrates of 200 mM MC without TW40 were equally active when used against the bacteria immediately after dilution in buffer but were not stable and precipitated out as crystals after storage for a few hours.
TABLE 2.
Antibacterial activities of emulsions of MC and TW40 at various pHs
| MC-TW40 concnb | pH | Reduction of CFU (log10)a
|
||
|---|---|---|---|---|
| Salmonella spp. | E. colic | C. jejuni | ||
| 1.25 mM-0.005% | 4.1 | ≥7.0 | ≥6.7 | ND |
| 1.25 mM-0.005% | 4.5d | ≥7.0 | ≥6.7 | ND |
| 1.25 mM-0.005% | 5.0 | 6.60 ± 0.84 | ≥6.7 | ND |
| 1.25 mM-0.005% | 5.5 | 1.90 ± 0.14 | 2.06 ± 0.07 | ND |
| 5 mM-0.02% | 5.5 | 0.78 ± 0.63 | 2.43 ± 0.13 | ND |
| 5 mM-0.02% | 7.2 | 0.30 ± 0.17 | 0.98 ± 0.19 | ND |
| 0.625 mM-0.0025% | 4.1 | ND | ND | ≥6.0e |
| 0.625 mM-0.0025% | 7.2 | ND | ND | 1.04 ± 0.76 |
| Acid controlf | 4.1 | 0.13 ± 0.06 | 0 | 0.10 ± 0.10 |
Compared with count of bacteria mixed 1:1 with control solution at pH 7. Values are means ± standard deviations of at least four determinations. Standard deviations were not available for those values shown without them. ND, not done.
Concentrations after the addition of bacteria (1:1).
Means are for both E. coli strains, each tested at least twice.
Bacteria were treated at room temperature for 10 min with the same results.
Activity against C. jejuni was tested for 10 min at room temperature.
Buffer without MC.
Killing of C. jejuni by MC emulsions added to chicken feed.
Pellets of broiler feed were mixed with MC-TW40 emulsions diluted either in sterile tap water or in 0.06 M citrate-lactate buffer at pH 4.1. The pH of the buffer increased to 5.5 after being mixed with feed, whereas the pH of the water remained at 7 after it was added to the feed. Table 3 shows that 10 mM and 5 mM MC emulsions mixed with feed killed C. jejuni at 37°C for 30 min. However, the microbicidal activity was less than that in water without feed, where a 1.25 mM MC emulsion causes a reduction in viable bacterial counts of ≥6 log10 in 1 min (Table 1). The data were analyzed by the Tukey-Kramer method of multiple comparisons among pairs of means, which showed that in feed mixed with MC in water, the microbicidal activity was significantly greater (P < 0.01) at 10 mM (0.25%) MC than at 5 mM MC, but in buffer at pH 5.5, there was not a significant difference in activity between 10 mM and 5 mM MC. The activity of the 5 mM MC concentration seemed to be slightly higher in the buffer than in water, but the difference was not significant. Feed mixed with water or buffer without MC did not significantly reduce the viable Campylobacter counts in 30 min at 37°C, compared to water alone.
TABLE 3.
Killing of C. jejuni in chicken feed mixed with MC-TW40 at different concentrations and pHs and incubated with the bacteria at 37°C for 30 min
| MC-TW40 added to feed | Viable bacterial count (log10 CFU/ml)a in:
|
|
|---|---|---|
| Tap water at pH 7.0 | Buffer at pH 5.5 | |
| 10 mM MC-0.04% TW40 | 2.15 ± 0.17 | 2.07 ± 0.12 |
| 5 mM MC-0.02% TW40 | 3.98 ± 0.35b | 3.13 ± 0.51c |
| 0 mM MC-0.04% TW40 | 6.37 ± 0.81 | 6.77 ± 0.46 |
The viable Campylobacter count in the control solution (0.04% TW40, 1% ethanol) with incubation at 37°C for 30 min was 7.07 ± 0.74 log10 CFU/ml. The viable Campylobacter counts were measured after incubation at 37°C for 30 min in a mixture of MC-TW40 in tap water and feed and in buffer and feed. Means ± standard deviations of three experiments are given. The data were analyzed by the Tukey-Kramer method of multiple comparisons among pairs of means.
This bacterial count is significantly (P < 0.01) higher than that at 10 mM MC, significantly lower than that in feed without MC, but not significantly different from that at 5 mM MC in buffer.
This bacterial count is significantly (P < 0.01) lower than that in the feed without MC but not significantly different from that in 10 mM MC in buffer.
DISCUSSION
MC has been found by previous work to be very active in killing a broad spectrum of bacteria and viruses (3, 4, 5, 6, 10, 18) and has now been shown to be extremely active in killing Campylobacter. Since this bacterium, particularly C. jejuni, is a common cause of food-borne infection in humans, it is considered worthwhile to explore the feasibility of using emulsions of monocaprin in water to control transmission of the bacteria from food, particularly poultry, to humans. Compared to other means which have been attempted for this purpose, MC seems to be an attractive choice since it is a breakdown product of medium-chain triglycerides which are common in various fats, both from animals, e.g., in milk, and from plants, such as coconut oil. Monoglycerides are therefore natural compounds which are harmless to the human body at concentrations which kill viruses and bacteria, e.g., in the stomach, and are classified as GRAS (generally recognized as safe) by the U.S. Food and Drug Administration (18a). In the present study, a method was developed to disperse MC in water in such a way that it forms lipid-in-water emulsions which are stable and remain strongly microbicidal against Campylobacter species for at least 12 months, either undiluted or after they have been diluted up to 200-fold in tap water. The emulsions are equally stable and active in killing Campylobacter whether or not they contain small amounts of an emulsifying agent, i.e., a Tween, in addition to the microbicidal lipid. Tween surfactants are nonionic surfactants which are commonly used in the food industry as oil-in-water emulsifying agents at concentrations ranging from 0.1 to 1.0% (wt/wt) or much higher than those used in the MC emulsions. Lipid-in-water emulsions containing MC as the microbicidal agent have been found to kill Campylobacter rapidly and in large numbers in contaminated water, where the viable bacterial counts are reduced by more than 6 log10 in 1 min. Diluted MC emulsions in water, with or without Tween surfactants, may therefore possibly be used to eliminate or reduce the number of viable Campylobacter bacteria in contaminated drinking water, e.g., the drinking water of chickens. Emulsions of MC significantly reduce the number of viable bacteria in Campylobacter-spiked chicken feed, although much less than in water. Most likely the MC molecules bind to proteins and/or lipids in the feed, which may partly block their bactericidal activity. However, the 2- to 4-log10 reduction in bacterial counts in MC-treated feeds (Table 3) suggests a possible use in feed disinfection or even in the reduction of bacterial counts in the intestinal contents of chickens colonized with the bacteria. These applications are being considered with the aim of finding means of diminishing Campylobacter infection in chickens. As a first step, preliminary studies indicate that the addition of MC emulsions to the drinking water and feed of broiler chickens has no adverse effect on their health or growth rate. The finding that MC emulsions containing TW40 kill Salmonella and E. coli in buffer at pH 5 or lower opens the possibility of utilizing MC emulsions in the control of food-borne infections caused by these bacteria. It is of interest that a number of studies have been done on the effect of organic acids, such as lactic acid, in reducing the number of Salmonella and C. jejuni bacteria on chicken carcasses (9, 13) or in diminishing intestinal colonization of Salmonella in chickens (11, 20). Since there seems to be a synergistic effect of MC and acid in killing these bacteria as well as Campylobacter, a combination of MC and organic acids might be a feasible approach, e.g., as an additive to feeds or for rinsing carcasses.
Acknowledgments
This work was supported by a grant from the Icelandic Research Council.
We thank Hjördís Hardardóttir and Sigfús M. Karlsson for human bacterial strains, Eggert Gunnarsson and Franklin Georgsson for campylobacter isolates, and Danisco A/S, Copenhagen, Denmark, for the generous gift of monocaprin.
REFERENCES
- 1.Altekruse, S. F., N. J. Stern, P. I. Fields, and D. L. Swerdlow. 1999. Campylobacter jejuni—an emerging foodborne pathogen. Emerg. Infect. Dis. 5:28-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrow, P. A., and K. Page. 2000. Inhibition of colonization of the alimentary tract in young chickens with Campylobacter jejuni by pre-colonization with strains of C. jejuni. FEMS Microbiol. Lett. 182:87-91. [DOI] [PubMed] [Google Scholar]
- 3.Bergsson, G., J. Arnfinnsson, S. M. Karlsson, Ó. Steingrímsson, and H. Thormar. 1998. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 42:2290-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergsson, G., Ó. Steingrímsson, and H. Thormar. 1999. In vitro susceptibilities of Neisseria gonorrhoeae to fatty acids and monoglycerides. Antimicrob. Agents Chemother. 43:2790-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergsson, G., J. Arnfinnsson, Ó. Steingrímsson, and H. Thormar. 2001. Killing of gram-positive cocci by fatty acids and monoglycerides. APMIS 109:670-678. [DOI] [PubMed] [Google Scholar]
- 6.Bergsson, G., Ó. Steingrímsson, and H. Thormar. 2002. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents 20:258-262. [DOI] [PubMed] [Google Scholar]
- 7.Chaveerach, P., D. A. Keuzenkamp, H. A. P. Urlings, L. J. A. Lipman, and F. van Knapen. 2002. In vitro study on the effect of organic acids on Campylobacter jejuni/coli populations in mixtures of water and feed. Poultry Sci. 81:621-628. [DOI] [PubMed] [Google Scholar]
- 8.Conley, A. J., and J. J. Kabara. 1973. Antimicrobial action of esters of polyhydric alcohols. Antimicrob. Agents Chemother. 4:501-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cudjoe, K. S., and G. Kapperud. 1991. The effect of lactic acid sprays on Campylobacter jejuni inoculated onto poultry carcasses. Acta Vet. Scand. 32:491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilmarsson, H., T. Kristmundsdóttir, and H. Thormar. 2005. Virucidal activities of medium- and long-chain fatty alcohols, fatty acids and monoglycerides against herpes simplex virus types 1 and 2: comparison at different pH levels. APMIS 113:58-65. [DOI] [PubMed] [Google Scholar]
- 11.Hinton, M., and A. H. Linton. 1988. Control of salmonella infections in broiler chickens by the acid treatment of their feed. Vet. Rec. 123:416-421. [DOI] [PubMed] [Google Scholar]
- 12.Hwang, C.-A., and L. Beuchat. 1995. Efficacy of selected chemicals for killing pathogenic and spoilage microorganisms on chicken skin. J. Food Prot. 58:19-23. [DOI] [PubMed] [Google Scholar]
- 13.Izat, A. L., M. Colberg, R. A. Thomas, M. H. Adams, and C. D. Driggers. 1990. Effects of lactic acid in processing waters on the incidence of salmonellae on broilers. J. Food Qual. 13:295-306. [Google Scholar]
- 14.Kabara, J. J., D. M. Swieczkowski, A. J. Conley, and J. P. Truant. 1972. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 2:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabara, J. J. 1978. Fatty acids and derivatives as antimicrobial agents—a review, p. 1-14. In J. J. Kabara (ed.), Symposium on the pharmacological effect of lipids. The American Oil Chemists' Society, Champaign, Ill.
- 16.Shibasaki, I., and N. Kato. 1978. Combined effects on antibacterial activity of fatty acids and their esters against gram-negative bacteria, p. 15-21. In J. J. Kabara (ed.), Symposium on the pharmacological effect of lipids. The American Oil Chemists' Society, Champaign, Ill.
- 17.Stern, N. J., B. Wojton, and K. Kwiatek. 1992. A differential-selective medium and dry ice-generated atmosphere for recovery of Campylobacter jejuni. J. Food Prot. 55:514-517. [DOI] [PubMed] [Google Scholar]
- 18.Thormar, H., and G. Bergsson. 2001. Antimicrobial effects of lipids, p. 157-173. In S. G. Pandalai (ed.), Recent developments in antiviral research, vol. 1. Transworld Research Network, Trivandrum, India. [Google Scholar]
- 18a.U.S. Food and Drug Administration. 1999. Code of Federal Regulations. Title 21, vol. 3, part 184, section 184.1505, p. 505. U.S. Government Printing Office, Washington, D.C. [Google Scholar]
- 19.Wagenaar, J. A., M. A. P. Van Bergen, M. A. Mueller, T. M. Wassenaar, and R. M. Carlton. 2005. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 109:275-283. [DOI] [PubMed] [Google Scholar]
- 20.Waldroup, A., S. Kaniawati, and A. Mauromoustakos. 1995. Performance characteristics and microbiological aspects of broilers fed diets supplemented with organic acids. J. Food Prot. 58:482-489. [DOI] [PubMed] [Google Scholar]
- 21.Wang, L.-L., and E. A. Johnson. 1992. Inhibition of Listeria monocytogenes by fatty acids and monoglycerides. Appl. Environ. Microbiol. 58:624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White, P. L., A. R. Baker, and W. O. James. 1997. Strategies to control salmonella and campylobacter in raw poultry products. Rev. Sci. Tech. Off. Int. Epizoot. 16:525-541. [DOI] [PubMed] [Google Scholar]